Abstract
Brain-derived neurotrophic factor (BDNF) plays a pivotal role in brain development and synaptic plasticity. It is synthesized as a precursor (pro-BDNF), sorted into the secretory pathway, transported along dendrites and axons, and released in an activity-dependent manner. Mutant Huntingtin with expanded polyglutamine (polyQ) and the V66M polymorphism of BDNF reduce the dendritic distribution and axonal transport of BDNF. However, the mechanism underlying this defective transport remains unclear. Here, we report that Huntingtin-associated protein-1 (HAP1) interacts with the prodomain of BDNF and that the interaction was reduced in the presence of polyQ-expanded Huntingtin and BDNF V66M. Consistently, there was reduced coimmunoprecipitation of pro-BDNF with HAP1 in the brain homogenate of Huntington disease. Pro-BDNF distribution in the neuronal processes and its accumulation in the proximal and distal segments of crushed sciatic nerve and the activity-dependent release of pro-BDNF were abolished in HAP1−/− mice. These results suggest that HAP1 may participate in axonal transport and activity-dependent release of pro-BDNF by interacting with the BDNF prodomain. Accordingly, the decreased interaction between HAP1 and pro-BDNF in Huntington disease may reduce the release and transport of BDNF.
Keywords: Neurobiology, Neurological Diseases, Neuroscience, Neurotrophic Factor, Protein-Protein Interactions, BDNF, HAP1, Huntington Disease, Axonal Transport, Secretion
Introduction
Neurotrophins play important roles in the proliferation, differentiation, and survival of neurons during development and in the maintenance of normal functions of the mature nervous system by activating their respective tyrosine kinase receptors TrkA, TrkB, and TrkC and the common receptor p75NTR (1–6). Neurotrophins are synthesized as precursors (proneurotrophins), which are either cleaved intracellularly by furin (2, 7, 8) and released as mature forms (9), or cleaved extracellularly by several proteases, including prohormone convertases, tissue-activated plasminogen/plasmin, MMP-3, and MMP-7 (8, 10, 11). Recently, it has been shown that unprocessed, the nerve growth factor precursor and the brain-derived neurotrophic factor precursor (pro-BDNF)3 bind both Sortilin and p75NTR with a high affinity and preferentially activate p75NTR, leading to apoptosis (12–15).
Although the retrograde neurotrophic hypothesis is well recognized, accumulating evidence indicates that neurotrophins such as BDNF and neurotrophin-3 are also trafficked anterogradely within dendrites and axons, released in an activity-dependent manner, and uptaken by second- or third-order target neurons (16–19). The anterogradely transported and released BDNF regulates neuronal survival, differentiation, dendritic morphology, and synaptic plasticity (17, 20–22). Both Sortilin and carboxypeptidase E play important roles in post-translational Golgi sorting of BDNF to the regulated secretory pathway and activity-dependent release by interacting with the prodomain (23) and the mature domain, respectively (24). Recently, we showed that pro-BDNF, like mature BDNF, is also transported anterogradely and retrogradely within axons of sensory neurons (25). However, how pro-BDNF and mature BDNF are transported within dendrites and axons remain to be investigated. A single nucleotide polymorphism in the BDNF gene (BDNFmet) at codon 66 in the prodomain results in the reduction of BDNF transport and activity-dependent secretion (26). The mutation was associated with reduction of hippocampal volume, impairment of episodic learning (27, 28), and a number of neurological disorders (29–31).
The polyglutamine (polyQ) expansion in Huntingtin (Htt) and knocking down of Htt-associated protein-1 (HAP1) and p150Glued can reduce BDNF transport and lead to the degeneration of striatal neurons (26, 32). Htt is a scaffold protein predominantly found in the cytoplasm where it associates with various vesicular structures and molecular motors to form a cargo complex and may play a role in intracellular trafficking (32, 33). The expanded (>37) glutamine stretch repeat in the Htt N terminus causes abnormally assembled protein complexes. HAP1 is distributed throughout the brain and spinal cord and is involved in axonal transport of BDNF (34). However, the mechanism for this transport remains to be investigated. It is also unclear how mutant Htt interferes with the transport of BDNF. Furthermore, whether mutant Htt interferes with the transport of mature BDNF or pro-BDNF remains to be investigated (32). Here, we demonstrate that HAP1 associates with the prodomain of BDNF and is required for the intracellular trafficking, axonal transport, and activity-dependent secretion of pro-BDNF. Moreover, polyQ-expanded Htt and V66M BDNF decrease the association of the prodomain of BDNF with HAP1.
EXPERIMENTAL PROCEDURES
Animals and Human Brain Tissue
All procedures involving animals were approved by the Animal Welfare Committee of Flinders University and undertaken according to the guidelines of the National Health and Medical Research Council of Australia. HAP1 knock-out mice were generated previously (35). All animals were kept under standardized barrier breeding conditions (12-h light/12-h dark cycle) with free access to water and food. Brain samples from HD cases (2 males, age 75 and 73) and control cases (2 males, age 68 and 73) were obtained from the Flinders University Brain Bank and approved by Flinders Human Ethic Committee. The CAG repeat length was identified using the following primer: CTACGAGTCCCTCAAGTCCTTCCAGC and GACGCAGCAGCGGCTGTGCCTG. PCR genotyping of HAP1 knock-out mice were carried out as described (35).
Preparation of Pro-BDNF (Full Length), the Prodomain of BDNF (Prodomain Fragment 1–130), and V66M Prodomain BDNF Recombinant Proteins
For preparation of pET100/D-TOPO pro-BDNF, pET100/D-TOPO prodomain, and pET100/D-TOPO-V66M prodomain, the cDNA fragments of rat pro-BDNF (749 bp), the prodomain (423 bp), and the V66M prodomain (423 bp) were amplified by PfuTurbo DNA polymerase (Stratagene) from AApro-BDNF (amino acids 129 and 130, RR to AA point mutation to generate pro-BDNF furin-resistant recombinant protein) or V66M pro-BDNF-EGFP constructs (gifts from Dr. Masami Kojima, Research Institute for Cell Engineering, National Institute of Advanced Industrial Science and Technology, Ikeda, Osaka, Japan) by the following PCR primers (GeneWorks Pty Ltd): prodomain (forward) 5′-CACCACCATCCTTTTCCTTACTATG, and prodomain (reverse) 5′-CTAGCGCCGAACCCTCATAGA; pro-BDNF (reverse) 5′-CTACCTTCCCCTTTTAATGGT-3′. Pro-BDNF fragment, wild type, and V66M prodomains were amplified by using corresponding primers. The amplified fragments were subcloned in-frame into pET100/D-TOPO by following the instruction manual of Champion pET Directional TOPO expression kit (Invitrogen), and the sequences of the final constructs were verified by DNA sequencing.
The plasmids pET100/D-TOPO-AApro-BDNF, pET100/ D-TOPO-prodomain BDNF, and pET100/D-TOPO-V66M prodomain were transformed into Escherichia coli BL21, and the proteins were expressed according to the protocols provided by the manufacturer (Invitrogen). Briefly, after overnight culture in isopropyl β-d-thiogalactopyranoside, the bacteria were harvested, and the pellet was resuspended in binding buffer, lysed, and sonicated. Inclusion bodies were then collected, washed, and solubilized in 8 m urea solution. The proteins were purified using nickel column chromatography. The final protein concentration was assayed using BCATM protein assay kit (Pierce). For the assay of full-length pro-BDNF, supernatant lysate was used without urea such that naturally refolded protein was affinity-purified on the nickel column. The mature BDNF recombinant protein expressed from E. coli was obtained from PeproTech (Rocky Hill, NJ). All recombinant proteins were characterized by SDS-PAGE with Coomassie Blue staining and Western blot analysis. An example of the prodomain SDS gel is shown in supplemental Fig. S1. Mature BDNF was a gift from Regeneron.
Preparation of BDNF-binding Proteins from the Rat Brain Homogenate, Two-dimensional DIGE, Two-dimensional SDS-PAGE, and Mass Spectrometry Analysis
Rat brains samples were homogenized in RIPA buffer (150 mm NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40, 50 mm Tris, 1 mm EDTA) containing protease inhibitors (protease inhibitor mixture, Roche Applied Science), and the crude homogenate was centrifuged for 20 min at 5,000 × g. The supernatant was added to the columns of the prodomain and mature BDNF, which were immobilized on CNBr-activated SepharoseTM 4B beads based on the manufacturer's instructions (Amersham Biosciences). The columns were washed using gravity flow with washing buffer A (56 mm NaH2PO4, 144 mm Na2PO4, 2 m NaCl, pH 7.2, plus 1% Tween 20) and then with washing buffer B (56 mm NaH2PO4, 144 mm Na2PO4, 1 m NaCl, pH 7.2) to remove the detergent (Tween 20). The elution buffer (buffer C, 0.1 m glycine-HCl, pH 2.5) was then added to the columns. The elution fractions were collected, and the pH was adjusted immediately to 7.2 with 1 m Tris base. The proteins were dialyzed using distilled water at 4 °C. Each protein preparation was cleaned by using PlusOne two-dimensional clean-up kit (GE Healthcare) and quantified by using PlusOne Two-dimensional Quant kit (GE Healthcare).
For sample labeling, 50 μg of protein fraction from each column was labeled with 400 pmol of cyanine dyes, Cy3 for the prodomain-binding proteins, and Cy5 for the mature BDNF-binding proteins according to a standard protocol. The labeled samples were mixed to allow the gel to contain 50 μg each of Cy3- and Cy5-labeled samples, respectively. For the first dimension separation, the labeling mixture was applied to ImmobilineTM DryStrips (13 cm, pH 3–11 linear) by cup loading with a total running time of 55 kV-h of isoelectric focusing. The second dimension was carried out with 10% SDS-polyacrylamide gels, and gel images were subsequently acquired at the recommended wavelengths by using a TyphoonTM Variable Mode imager (GE Healthcare).
For identification of the separated proteins, 100 μg of prodomain-binding proteins were separated in two-dimensional SDS-PAGE as above. After the two-dimensional electrophoresis, the gel was stained with SYPRO Ruby. The spots on the gel were analyzed using a PE Biosystems MALDI-TOF Voyager- DE STR mass spectrometer. For the data base searches, the peptides were selected in the mass range of 750–900 kDa and evaluated using the ExPasy server.
Pulldown Assay, Subcellular Fractionation, and Immunoprecipitation of Human Brain Samples
Five hundred ng of recombinant protein (pro-BDNF, the prodomain, mature BDNF) were incubated with 4 μg of HAP1-GST proteins together with glutathione-Sepharose beads at 4 °C for 2 h without or with the presence of an equal quantity of PC12 cell lysate transfected with Htt-23Q or Htt-103Q (gift from E. S. Schweitzer, Departments of Physiological Science and Neurology, Brain Research Institute, UCLA) or with the same quantity of normal or HD human brain lysates. The beads were washed three times and boiled in loading buffer to release the bound proteins. Resolved by SDS-PAGE, the proteins were transferred to a polyvinylidene difluoride membrane (Amersham Biosciences) and detected by immunoblotting using rabbit anti-HAP1 antibody (gift from M. DiFiglia) and rabbit anti-prodomain (25, 36).
Subcellular fractions of wild type and HAP1−/− mice cortex were prepared essentially as described (37, 38). The SDS-polyacrylamide gels were loaded with equal amounts of proteins from each fraction (65 μg per lane) to conduct Western blotting using sheep anti-HAP1 (raised against recombinant HAP1) and rabbit anti-pro-BDNF.
For immunoprecipitation experiments, human brains were lysed in RIPA buffer (150 mm NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40, 50 mm Tris, 1 mm EDTA) containing protease inhibitors (protease inhibitor mixture, Roche Applied Science), and the crude homogenate was centrifuged for 20 min at 5,000 × g. The supernatants were pre-cleared by incubation with an excess of protein A + G-agarose. The protein lysates (1 mg) were incubated with 2 μg of HAP1 antibody immobilized in 25 μl of protein A-Sepharose beads (Amersham Biosciences) for 2 h at 4 °C. The immunoprecipitation products and exogenous prodomain and mature BDNF as positive controls were detected by Western blotting using rabbit anti-prodomain antibody (36), mouse anti-mature BDNF, rabbit anti-sortilin (Abcam), and or a sheep anti-HAP1 antibody.
Sciatic Nerve Crush
All the experiments were done on postnatal day 1. When the mouse was motionless on ice, an incision 0.5 cm long was made in the left thigh, and the left sciatic nerve was exposed and crushed at the mid-thigh level for 10 s with a pair of fine forceps. The skin was closed, and animals were kept on a warm blanket for recovery. After recovery, the neonates were returned to their mother. Six hours after sciatic nerve crush, puppies were anesthetized on ice again and killed by perfusion through the heart with 4% paraformaldehyde. The sciatic nerve was dissected and processed for immunohistochemistry.
Pro-BDNF Immunohistochemistry
Antibodies to pro-BDNF were generated by immunization of rabbits with synthetic peptide, corresponding to the 14 amino acids of the prodomain sequence of pro-BDNF (MTILFLTMVISYFG), which were conjugated to keyhole limpet hemocyanin. This antibody recognizes endogenous pro-BDNF, recombinant pro-BDNF, and recombinant prodomain. Its immunoreaction specificity was verified after affinity purification by reaction with the immunizing antigen and characterized by Western blotting and immunohistochemistry with the negative staining of brain sections of BDNF−/− mice as reported (36). The immunohistochemistry for pro-BDNF on sciatic nerve and cultured neurons was performed as described previously (25, 36).
ELISA
Constitutive and regulated secretion of pro-BDNF from cultured cortical neurons was examined as described (23). The pro-BDNF protein concentrations in the respective medium samples were determined using the pro-BDNF ELISA system with the recombinant prodomain as a standard.
Polystyrene plates (number 3590, Costar, Cambridge, MA) were coated with monoclonal prodomain antibody (PB17-2A, 1:3000) in 0.1 m carbonate coating buffer, pH 9.6, and incubated for overnight at 4 °C. The wells were blocked with 5% skim milk in 1% Tween 20/phosphate-buffered saline (PBST) for 2 h at room temperature. After washes, the collected media were added to the wells in triplicate. Plates were incubated overnight at 4 °C. After an additional wash with PBST, 100 μl of horseradish peroxidase-labeled monoclonal antibody to pro-BDNF (PB5E-2) was added to the wells (20 μg/ml) and incubated for 2 h at room temperature. After washing with PBST, the plates were developed in 3,3′,5,5′-tetramethylbenzidine liquid substrate system (Sigma). Standards and samples were performed in triplicate, and each group contains six independent samples. After collecting condition media, cells were fixed and examined under fluorescence microscopy to determine the transfection efficiency. Each sample had a transfection efficiency of ∼80%.
Statistical Analysis
Values from multiple samples were be expressed as S.E. and compared by Student's t test between pairs.
RESULTS
HAP1 Associates with Pro-BDNF, the Prodomain, but Not Mature BDNF
To investigate mechanisms underlying the axonal transport of BDNF, we attempted to identify the proteins that interact with BDNF. To do this, we first generated recombinant prodomain and mature BDNF (Fig. 1A) for affinity purification of interacting proteins from the rat brain. Proteins bound to the prodomain and mature BDNF were labeled by cyanine dyes Cy3 and Cy5, respectively, and resolved by two-dimensional DIGE. The results showed that the proteins interacting with mature BDNF from the rat brain are obviously different from those interacting with the prodomain (Fig. 1B), as most proteins detected from the two sets of samples are not colocalized and displayed different colors.
FIGURE 1.
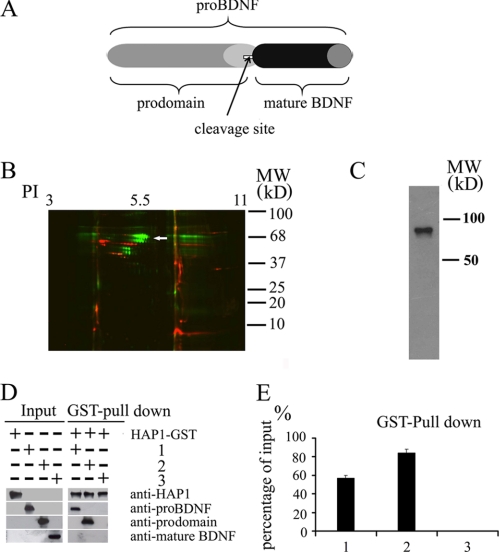
Association of HAP1 with pro-BDNF and the prodomain in vitro. A, domain structure of pro-BDNF, prodomain, and mature BDNF. Pro-BDNF is the unprocessed full-length form after synthesis and is cleaved to produce mature BDNF. The prodomain is the fragment of 1–130 amino acids of pro-BDNF. B, binding proteins were collected by passing rat brain lysate through affinity columns containing the recombinant prodomain and mature BDNF. DIGE two-dimensional gel was used to reveal all proteins bound to the recombinant prodomain (green) and mature BDNF (red) affinity columns. When sequenced by MALDI-TOF, one of these proteins was shown to be HAP1 (white arrow). PI, isoelectric point. C, affinity-purified prodomain-binding proteins was probed with rabbit anti-HAP1 antibody. The antibody recognized a band (around 75 kDa) in the prodomain-binding proteins. D, HAP1 GST fusion proteins were immobilized on glutathione-agarose beads and incubated with pro-BDNF (1), prodomain (2), and mature BDNF (3). Bound proteins were detected by Western blotting with the indicated antibodies. E, analysis of percentage of pulldown proteins. The staining intensity of each band on the blots in D was quantified by the ImageJ program (National Institutes of Health). The percentage of input was calculated using input proteins as 100%. The data are presented as mean ± S.E. (n = 3).
To further identify which proteins interact with the prodomain, the protein samples isolated from the prodomain affinity column were subjected to two-dimensional SDS-PAGE, and the protein spots were determined by MALDI-TOF Voyager-DE STR mass spectrometer and data base search. One of these proteins was HAP1. To confirm the result, the prodomain-binding proteins were analyzed by Western blotting using anti-HAP1 rabbit antibody. Western blot confirmed that HAP1 was present in the protein fraction isolated from the prodomain column (Fig. 1C).
To strengthen the finding that HAP1 associates with pro-BDNF and to test whether HAP1 directly interacts with BDNF fragments, we performed GST pulldown experiments. The pulldown assay showed that the HAP1 fragment (280–445 amino acids) could bind both the prodomain and full-length pro-BDNF but not mature BDNF (Fig. 1D). Quantitative analysis showed 56% pro-BDNF and 84% prodomain were pulled down by HAP1-GST (Fig. 1E).
Mutant Huntingtin Decreases the Association of HAP1 with Pro-BDNF
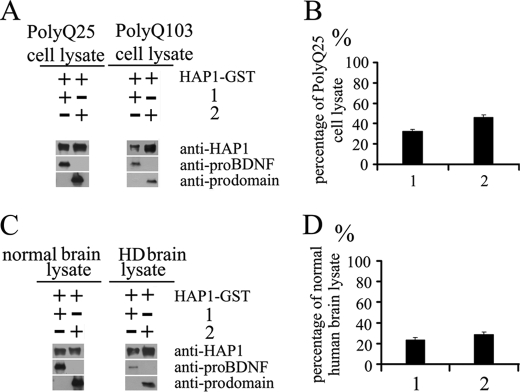
Growing evidence has shown that mutant Htt affects the axonal transport of BDNF (32, 34, 39, 40), but the mechanisms by which mutant Htt impairs the transport of BDNF remains unclear. It is known that mutant Htt binds more tightly to HAP1 than does wild type (WT) Htt (32, 41, 42). We hypothesize that mutant Htt may interfere with the interaction between HAP1 and the prodomain. To test the hypothesis, we conducted the GST-pulldown assay in the presence of wild type or mutant Htt. The PC12 neuronal cell lines that overexpress wild type Htt with polyQ25 or mutant Htt with polyQ103 (43) were homogenized. An equal amount of total proteins in each cell lysate was incubated with HAP1-GST and pro-BDNF or the prodomain. The pulldown of pro-BDNF and the prodomain in the presence of polyQ103 PC12 cell lysates was significantly decreased as compared with the samples in the presence of WT Htt (polyQ25) cell lysate (Fig. 2A). Quantitative analysis showed that the pull down of proteins in the presence of mutant Htt was 32.5% for pro-BDNF and 46% for prodomain in the presence of WT Htt (Fig. 2B).
FIGURE 2.
Mutant htt in PC12 cell lysate and HD brain lysate decrease the efficiency of the interaction of HAP1 with pro-BDNF. HAP1 GST fusion protein was immobilized on glutathione-agarose beads and incubated with pro-BDNF (1) and the prodomain (2) in the presence of polyQ25- or polyQ103-containing Htt-transfected PC12 cell lysate or in the presence of normal or HD human brain lysate. Binding proteins were detected by immunoblotting. A, quantity of proteins pulled down in the presence of polyQ25-htt PC12 cell lysate or polyQ103-htt PC12 cell lysate is shown in the blot. B, percentages of proteins in the presence of polyQ103-htt PC12 cell lysate were calculated using the polyQ25-htt PC12 cell lysate as 100% references. C, quantity of proteins pulled down in the presence of normal brain lysate or HD brain lysate is shown in the blot. D, percentages of proteins in the presence of HD brain lysate were calculated using normal brain lysate as 100%. The blot intensity of each band was quantified by the NIH ImageJ program. The data are presented as mean ± S.E., from three separate experiments.
Next, we examined the interaction of GST-HAP1 and pro-BDNF or HAP1 and prodomain in the presence of cell lysates of normal human brain (28 CAGs and 32 CAGs) or Huntington disease human brain (94 CAGs and 82 CAGs heterozygous HD) by the pulldown experiments. The result showed that the pulldown of pro-BDNF and the prodomain in the presence of HD human brain cell lysates was significantly decreased compared with the normal human brain cell lysates (Fig. 2C). The quantitative data showed the pulldown of pro-BDNF in the presence of HD brain lysate was decreased to 23.6% and the prodomain to 28.7% of normal brain lysates (Fig. 2D). This finding is in agreement with the transfected PC12 cell lysates, although less protein amount from the brain lysates than from PC12 cells was added in the incubation mixture.
Pro-BDNF, HAP1, and Sortilin Are Associated in the Human Brain, and the Association Is Reduced in Huntington Disease Brain
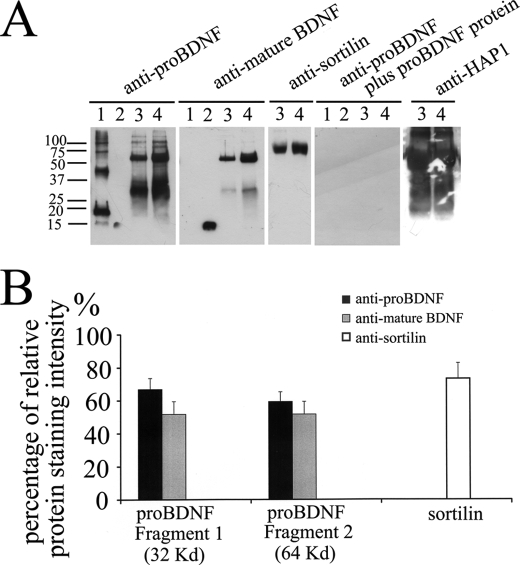
To further confirm the interaction between HAP1 and pro-BDNF, we immunoprecipitated HAP1-binding proteins from human brain homogenates with an antibody to HAP1. We also tested whether the association is altered in the brain homogenate from HD. As shown in Fig. 3, there are several species of pro-BDNF detected in the HAP1-immunoprecipitated sample at molecular masses of 32 and 64 kDa of normal human brain. The bands did not appear when the membrane was probed with the antibodies pre-absorbed with recombinant pro-BDNF protein. The amount of pro-BDNF in the complex immunoprecipitated from the HD brain was significantly reduced (Fig. 3, A and B). Quantification results showed that amount of pro-BDNF immunoprecipitated from HD brain was 66.9% (32-kDa band) and 51.7% (64-kDa band) of the normal brain, when probed with rabbit anti pro-BDNF, and was 59.3% (32 kDa) and 51.6% (64 kDa) of normal brain when probed with monoclonal anti-mature BDNF antibody (Fig. 3B). For positive controls, we also loaded the prodomain and mature BDNF, respectively. As shown in Fig. 3A, the pro-BDNF antibody, which against the prodomain, did not recognize mature BDNF, and the mature BDNF antibody did not recognize the prodomain. However, both antibodies recognized the endogenous full-length pro-BDNF. The multiple bands in Fig. 3A, lane 1, are likely dimers and oligomers of the prodomain. The SDS gel showed that the fresh purified prodomain was a single band with 95% purity and formed oligomers in 1 week at 4 °C (supplemental Fig. S1A), but Western blot analysis confirmed that the purified prodomain monomer forms dimers and oligomers with the time (1 week) at 4 °C in water (supplemental Fig. S1, A and B). We detected minor bands (Fig. 3A, lanes 3 and 4) with the higher molecular weight detected by the pro-BDNF antibody but not by the mature BDNF antibody. These bands disappeared when the antibody was pre-incubated with an excessive amount of recombinant pro-BDNF.
FIGURE 3.
HAP1-pro-BDNF complex is altered in HD. A, rabbit anti-HAP1 antibody was immobilized on protein A beads and incubated with normal human brain or HD brain lysates. Blots were detected with anti-pro-BDNF, anti-mature BDNF, anti-sortilin, or anti-pro-BDNF plus the immunizing peptide, or anti-HAP antibodies. Lane 1, prodomain standard; lane 2, mature BDNF standard; lane 3, HAP1 immunoprecipitation from HD human brain; lane 4, HAP1 immunoprecipitation from normal human brain. B, percentages of relative protein staining intensity of HD human brain lysate compared with normal human brain lysate (the area of particles that stands for the relative staining intensity was quantified by the ImageJ program). The data are presented means ± S.E. of three separate experiments.
It is well known that HAP1 is associated with vesicles as demonstrated by electron microscopic studies and by sucrose gradient fractionations (44, 45). HAP1 may play roles in trafficking of pro-BDNF by interacting with sortilin, which also interacts with the prodomain of BDNF (23). To test the possibility, we probed the HAP1 immunoprecipitated complex from the human brain with a sortilin antibody. We found that sortilin was present in the HAP1-associated complex from the human brain (Fig. 3A). Interestingly, less sortilin (73%) was immunoprecipitated from the brain of HD than from the control (Fig. 3B).
Effects of the V66M Mutation on the Interaction between HAP1 and the BDNF Prodomain
20–30% of humans have an allele of the V66M polymorphism in the prodomain of the BDNF gene (46). The polymorphism is known to cause the reduction of axonal BDNF transport and activity-dependent release, leading to a reduction in the volume of the hippocampus and development of various mental disorders (23, 47, 48). The mechanism underlying the reduction of BDNF transport is not known. Here, we examined whether the V66M mutation affects the interaction between HAP1 and the prodomain. As shown in Fig. 4, A and B, compared with the WT prodomain, the amount of V66M prodomain pulled down by HAP1 was significantly reduced (84 versus 60%). The reduction in the interaction between HAP1 and V66M prodomain was further reduced by incubation in the presence of expanded polyQ Htt in PC12 cell lysates (46 versus 19%) or in HD brain lysates (28.7 versus 13%). These results indicate that the V66M mutation in the prodomain can reduce the interaction with HAP1, which may cause the reduction in pro-BDNF transport and release.
FIGURE 4.
Effect of the V66M mutation on the interaction between HAP1-A and BDNF prodomain. A, input of the WT prodomain and V66M prodomain is shown in panel a. HAP1 GST fusion proteins were immobilized on glutathione-agarose beads and incubated with the WT prodomain or the V66M prodomain (panel b). In separate reaction vials, the reactions as described in panel b were carried in the presence of polyQ25- (panel c) or polyQ103 Htt (panel d)-transfected PC12 cell lysate or in the presence of normal (panel e) or Huntington disease (panel f) human brain lysate. The quantities of binding proteins were detected by immunoblotting as indicated. B, quantification of the percentage of proteins pulled down at different conditions. Bar 1(panels b/a), comparison of the percentages of the WT and V66M prodomains; bar 2 (panels d/c), the comparison of the percentages of WT and V66M prodomains pulled down in the presence of polyQ103 PC12 cell lysate; bar 3 (panels f/e), comparison of the percentages of WT and V66M prodomains pulled down in the presence of HD brain lysate. The staining intensity of each band was quantified by the ImageJ program, and data are presented as means ± S.E. of three separate experiments.
Colocalization of Pro-BDNF and HAP1 in Transfected Cells and Neurons
To see whether HAP1 is colocalized with pro-BDNF, we carried out three different experiments (Fig. 5). First, we cotransfected two different sets of plasmids conjugated with different fluorescent markers in PC12 cells. Through Live Cell Image, HAP1-A (green fluorescence) was highly colocalized with pro-BDNF (83.2%) and the prodomain (76.8%), as indicated by yellow fluorescence (Fig. 5, A and B, rows 1 and 2). The results on colocalization of HAP1-B with pro-BDNF and the prodomain were similar to those of HAP1-A (supplemental Fig. S2). Second, we performed immunolocalization of endogenous pro-BDNF and HAP1 in cultured cortical neurons. As shown in Fig. 5C, pro-BDNF is highly colocalized with HAP1 (75.0%) in cultured cortical neurons in both cell bodies and in neurites in wild type mice. Third, we tested the specificity of our antibodies on neurons from HAP1−/− mice. We found that no HAP1 is detected in neurons from HAP1−/− mice. Interestingly, pro-BDNF is only present in the cell body but not neurites in HAP1−/− mice (Fig. 5C), suggesting that HAP1 may play a role in trafficking of pro-BDNF to axons and dendrites.
FIGURE 5.
Colocalization of HAP1-A with BDNF subdomains in PC12 cells. A, PC12 cells were transfected with plasmids HAP1-A/EGFP (green) in combination with either full-length pro-BDNF-Ds-Red (row 1) or prodomain-Ds-Red (2) (row 2) (red), respectively. Confocal images were taken and merged (yellow). Yellow dots indicate vesicles with both HAP1-A and BDNF subdomains. The snapshot represents the rate of colocalization quantitatively analyzed by the quantitative analysis software on a Leica SP5 confocal microscope. B, quantitative data showed pro-BDNF and prodomain highly colocalized with HAP1-A. The data are presented as mean ± S.E. (n = 20). C, double labeling of pro-BDNF and HAP1 in cultured cortical neurons from WT and HAP1−/− mice. Pro-BDNF is stained in red and HAP1 in green. No HAP1 was stained in HAP1−/− neurons. Pro-BDNF and HAP1 are highly colocalized in the cytoplasm and neuritis in WT neurons. In HAP1−/− neurons, pro-BDNF is only present in soma. Phase contrast images showed cell bodies and neurites of WT and HAP1−/− neurons.
Transport of Endogenous Pro-BDNF in Wild Type and HAP1−/− Neurons
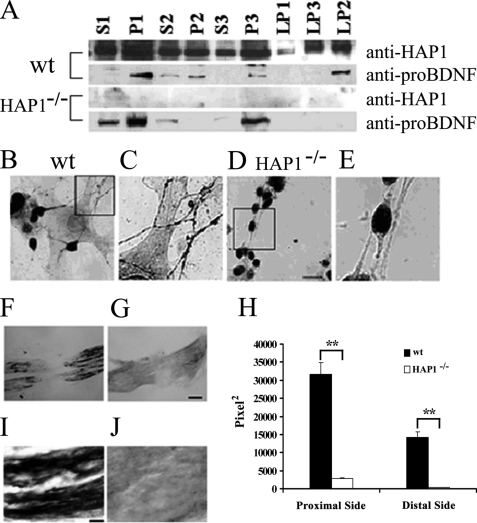
To further investigate the significance of the interactions between HAP1 and pro-BDNF, we fractionated the brain lysate of wild type and HAP1−/− mice with sucrose gradients to examine the distribution in organelles as reported previously (37, 38). The pro-BDNF antibody used to probe fractions was thoroughly characterized previously (25, 36). In wild type and HAP1−/− mice brain lysate, pro-BDNF shown as a single band at 32 kDa was present in both the crude nuclear fractions (P1) and membranous fractions in high speed pellets (P3), as well as in medium speed supernatant (S2). Further subfractionations of the P2 fraction showed that in wild type mice brain lysate, pro-BDNF is enriched in the subcellular vesicular fraction (LP2) (0.8–1.2 m sucrose gradient fraction). Interestingly, no pro-BDNF was detected in LP2 from HAP1−/− mice, although a larger amount of pro-BDNF was present in other subcellular fractions, such as S1, P1, S2, and P3 (Fig. 6A). These results suggest that the deletion of HAP1 affects the distribution of pro-BDNF in the synaptosomal fraction (LP2) and that HAP1 plays a role in the transport of pro-BDNF-containing vesicles into nerve terminals. To test this hypothesis, we performed pro-BDNF immunohistochemistry on cultured cortical neurons of wild type and HAP1−/− mice. We found that pro-BDNF punctate particles were distributed in both cell bodies and processes of neurons from wild type mice (Fig. 6, B and C) but were only present in cell bodies of neurons from HAP1−/− mice (Fig. 6, D and E). We counted neurons with pro-BDNF-ir neuronal processes from triplicate cultures of wild type and mutant animals. The data showed that 98% of cortical neurons from wild type mice contained pro-BDNF in neuronal processes, whereas no neuron from mutant mice contained pro-BDNF in their processes. However, punctate pro-BDNF particles were present in neuronal soma (Fig. 6, D and E, and Fig. 5C) or proximal neurite stalks, suggesting that pro-BDNF is present in the secretory vesicles and HAP1 is not required for pro-BDNF sorting from Golgi to secretory vesicles. It is worthwhile to point out that the pattern of staining for HAP1 and pro-BDNF in neurons at a physiological condition is different from that in transfected PC12 cells (Fig. 5, A versus C). The main difference is that no stigmoid bodies (large cytoplasmic aggregates) have been detected in primary cultured neurons. It is likely that the overexpression of HAP1 caused the sigmoid body that recruited pro-BDNF in large aggregates. This also supports our conclusion that HAP1 interacts with pro-BDNF in vivo.
FIGURE 6.
HAP1 is required for the transport of pro-BDNF. A, subcellular fractions of sucrose gradient from P1 neonatal wild type (wt) and HAP1−/− mouse cortex were detected by Western blot using sheep anti-pro-BDNF and rabbit anti-HAP1. Equal amounts of protein (65 μg) were loaded in each lane. B, pro-BDNF immunohistochemistry of primary cultured cortical neurons from P1 neonatal WT mouse. C, higher magnification view of the field highlighted in B. D, pro-BDNF immunohistochemistry of primary cultured cortical neurons from P1 neonatal HAP1−/− mouse (scale bar, 15 μm). E, higher magnification view of the field highlighted in D. F, photomicrograph of a crushed sciatic nerve section stained for pro-BDNF from a P1 neonatal WT mouse. G, photomicrograph of a crushed sciatic nerve section stained for pro-BDNF from a P1 neonatal HAP1−/− mouse. H, quantitative data on the relative staining of pro-BDNF in the sciatic nerve. y axis stands for the relative amount quantified by the ImageJ program. The data are presented as mean ± S.E., n = 3. **, p < 0.01, Student's t test, scale bar, 15 μm. I, high magnification of micrograph of the proximal segment of crushed nerve stained for pro-BDNF from a P1 neonatal WT mouse; scale bar, 60 μm. J, high magnification of micrograph of the proximal segment of crushed nerve stained for pro-BDNF from a P1 neonatal HAP1−/− mouse.
The in vitro results were further confirmed by our in vivo experiments in sciatic nerve, as BDNF is synthesized and anterogradely transported by sensory neurons (16). Sciatic nerve crush in wild type postnatal day 1 mice resulted in the accumulation of pro-BDNF immunoreactivity on both the proximal and distal sides of the crush site (Fig. 6F), consistent with our previous studies (16, 25). In contrast, no pro-BDNF immunoreactivity accumulated on either side of the crushed sciatic nerve in HAP1−/− mice (Fig. 6, G and J). On the proximal side of crushed sciatic nerve from wild type mice, the staining area was 31,715 ± 3125 pixel2. In contrast, the same proximal side of crushed sciatic nerve from the HAP1−/− mice was 2752 ± 133 pixel2 (p < 0.001) The stained area from the distal side of the crushed sciatic nerve was 14,282 ± 1635 pixel2 for WT mice and 288 ± 52 pixel2 for HAP1−/− mice (Fig. 6H). No pro-BDNF immunoreactivity was detected in control sections incubated with normal rabbit IgG or antibody pre-absorbed with immunized peptide or in brain sections of BDNF knock-out mice (16, 25).
HAP1 Plays a Critical Role in Activity-dependent Secretion of Pro-BDNF
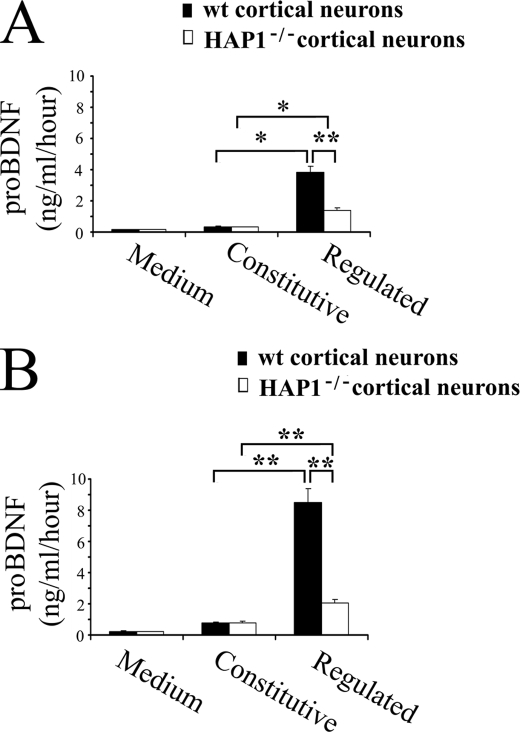
To assess whether HAP1 affects regulated and constitutive secretory pathways, we performed two series of experiments on the constitutive and regulated secretion in primary cultured cortical neurons from HAP1−/− and wild type mice (Fig. 7A) and in primary cultured cortical neurons transfected with the pro-BDNF plasmid (Fig. 7B). The secreted pro-BDNF was then detected by ELISA. Two monoclonal antibodies used for the ELISA were raised against the prodomain. The ELISA was set up to detect pro-BDNF (supplemental Fig. S3). In the case of nontransfected HAP1−/− and wild type mice cortical neurons, a similar quantity of pro-BDNF was secreted in a basal release condition (0.32 ng/ml/h from HAP1−/− versus 0.35 ng/ml/h from WT). In contrast, there was a significant difference between HAP1−/− and wild type mice cortical neurons (1.4 ng/ml/h from HAP1−/− versus 3.82 ng/ml/h from WT) in high potassium stimulated samples. A similar result was obtained from cortical neurons transfected with the pro-BDNF plasmid. Equal but increased amounts of pro-BDNF (0.77 ng/ml/h from HAP1−/− mice and 0.80 ng/ml/h from WT mice) were secreted from cortical neurons at the basal release condition. In contrast, the release of pro-BDNF from HAP1−/− mice was significantly impaired as compared with WT mice after stimulation with high potassium (8.52 ng/ml/h from HAP1−/− versus 2.08 ng/ml/h from WT). These results suggest that HAP1 is required for the activity-dependent secretion of pro-BDNF.
FIGURE 7.
Role of HAP1 in the constitutive and activity-dependent secretion of pro-BDNF from culture cortical neurons. A, experiment was performed on naive cortical neurons cultured for 48 h. Neurons were conducted under constitutive and depolarization secretion conditions. All collected media were subjected for quantification of pro-BDNF with an ELISA developed in our laboratory. B, experiment was performed in the same conditions as above except that neurons were transfected with the pro-BDNF plasmid. All results are presented as a mean ± S.E. determined from analysis of six independent experiments (*, p < 0.05; **, p < 0.01, n = 6, Student's t test).
DISCUSSION
BDNF is sorted into the regulated secretory pathway, anterogradely transported to presynaptic nerve terminals, released in an activity-dependent manner, and plays a critical role in synaptic plasticity (49, 50). The activity-dependent secretion of BDNF is critical for various forms of synaptic plasticity and the long term regulation of synaptic structure and function. In this study, we have provided mechanistic insight into how BDNF is transported anterogradely along axons.
HAP1 Directly Binds to Pro- BDNF
Defects in BDNF transport and secretion may underlie a wide range of nervous system diseases, such as epilepsy, Rett syndrome, neurodegenerative diseases, and neuropsychiatric diseases (32, 51–55). However, how BDNF-containing vesicles are transported is not known. Substantial evidence suggests that the anterograde transport of BDNF is dependent on the prodomain sequence. Sortilin interacts with the prodomain and sorts pro-BDNF into the regulated pathway (23). Nerve growth factor is normally released constitutively from dendrites of neurons (56–58), but nerve growth factor chimeric molecule (the prodomain of BDNF fused with mature nerve growth factor) can be sorted into the regulated pathway and released in an activity-dependent manner (23). The neuronal transport of BDNF is likely mediated by its interacting proteins. In this study, the DIGE experiments and Western blot results demonstrated that HAP1 associates with the prodomain. Further interaction studies using GST pulldown assays with recombinant proteins confirmed that the prodomain associates with a HAP1 fragment (amino acids 280–445).
Mutant Htt Affects the Association of HAP1 with Pro-BDNF
A large body of evidence suggests that mutant Htt abnormally interacts with HAP1. One of the pathophysiological mechanisms underlying neuronal death in HD is that mutant Htt down-regulates the expression and transport of BDNF. Several studies have already indicated that wild type Htt is an anti-apoptotic protein that could enhance intracellular transport of BDNF, but mutant Htt causes retardation of BDNF transport along microtubules (32, 59) and impairs the post-Golgi trafficking of WT BDNF but not V66M BDNF (60). However, how mutant Htt impairs the axonal transport and post-Golgi trafficking of BDNF is not known. We showed that mutant Htt significantly reduced the association of HAP1 with pro-BDNF (Fig. 2), suggesting that mutant Htt may disrupt pro-BDNF transport by interfering with the interaction between the prodomain and its cargo-carrying molecule HAP1. Further supporting this notion is the evidence that HAP1 in the brain of HD patients binds less pro-BDNF than that in the normal human brain (Fig. 3). It should be pointed out that mutant htt also affects the transcription of BDNF (40). Thus, the reduction in BDNF pulldown from HD brain could also be due to reduced expression of pro-BDNF.
V66M Polymorphism Reduces Its Association with HAP1
In human, 20–30% individuals carry the mutation of a valine to methionine substitution at codon 66 in the prodomain (46). The previous studies showed that the V66M BDNF polymorphism may associate with several neurodegenerative diseases (30, 61). The V66M mutation leads to the reduction in activity-dependent secretion and impairs the intracellular trafficking of BDNF without affecting the constitutive release of BDNF (26, 61). This mutation in human also causes the reduction in hippocampal volume (28) and affects hippocampal processing of episodic learning and memory (27). The phenomenon suggests that the impairment of activity-dependent secretion of BDNF is responsible for dysfunction in hippocampal plasticity and for a number of human psychological disorders (62). However, how the mutation results in reduction in activity-dependent secretion and transport is not clear. In this study, we showed that the mutation form of the prodomain reacts with HAP1 with less efficiency compared with the wild type form. Our data suggest that the mutation may reduce its association with HAP1. We also showed that the combination of V66M and mutant Htt further reduces the association of the prodomain with HAP1.
BDNF intracellular distribution can also be affected by its mRNA localization and trafficking. The BDNF mRNA transcript with long 3′-untranslated region tends to go to dendrites, whereas the transcript with short 3′-untranslated region is restricted to the soma (63). A recent study shows that the dendritic targeting of the long 3′-untranslated region transcript is regulated by Translin, an RNA-binding protein implicated in mRNA trafficking, and the G196A polymorphism, which results in V66M substitution and impairs the interaction between translin and BDNF oligonucleotides (64). Whether HAP1 also regulates BDNF mRNA dendritic targeting remains to be determined.
HAP1 May Play a Role in Intraneuronal Trafficking, Axonal Transport, and Activity-dependent Release of BDNF
HAP1 is an Htt-associated protein and interacts with the dynactin subunit, p150Glued (65), which is the accessory protein of dynein motor (66–68), and the kinesin light chain (69), which is a subunit of the kinesin motor (68, 70). As it is known that the dynein motor carries cargos retrogradely and the kinesin motor carries cargos anterogradely along axons (68), HAP1 may be the BDNF cargo-carrying molecule for these motors. Several lines of evidence support the idea that HAP1 is a BDNF cargo-carrying molecule. Cotransfection studies showed that HAP1 is highly colocalized with the prodomain of BDNF. Endogenous pro-BDNF is also highly colocalized with HAP1 in cultured neurons. Biochemical analysis of brain homogenate from wild type mice showed that BDNF is present in all precipitated components with different spin forces and is associated with the fraction of vesicles in which HAP1 is also present. However, in HAP1 knock-out mice, the normal distribution pattern of BDNF is altered (Fig. 6), and BDNF is not detectable in the vesicle fraction (LP2). Also, BDNF is not in the pellet of moderate speed centrifugation (P2) but was increased in P1, S1, and P3 fractions in the absence of HAP1. Immunohistochemical data showed that pro-BDNF was present in the neuritic processes (dendrites and axons) of most cortical neurons from wild type mice but not in those of neurons from HAP1 mutant mice. The data suggest that BDNF-containing vesicles fail to reach dendrites and axons when HAP1 is absent.
In support of the above idea, the transport of endogenous pro-BDNF in the sciatic nerve in HAP1 knock-out mice is abolished. Sciatic nerve is an ideal model to examine BDNF transport, as the BDNF is highly expressed in primary sensory neurons and transported anterogradely and retrogradely within the sciatic nerve (16, 25). Our data showed that the inactivation of the HAP1 gene completely blocks the transport of endogenous pro-BDNF in the sciatic nerve and in the processes of cortical neurons. These data indicate that HAP1 may be essential for the transport of pro-BDNF. Interestingly, both anterograde and retrograde transports are abolished in these animals, suggesting that HAP1 may not only be essential for carrying BDNF forward by the kinesin motor through interacting with its prodomain but also important for carrying the receptor-mediated internalized cargos of pro-BDNF to the cell body.
Previous studies showed that HAP1 is associated with secretory vesicles (44) and plays a role in protein trafficking and vesicular transport (34). It is likely that HAP1 is present both inside and outside vesicles. Biochemical and electron microscopic study of adult mouse basal forebrain and striatum showed that HAP1 is preferentially enriched in membrane fractions and localized to membrane-bound organelles, including large endosomes, tubulovesicular structures, and budding vesicles in neurons. HAP1 was also strongly associated with large dense organelles and within vesicles in dendrites (44, 45). Pro-BDNFs may directly interact with HAP1 within the vesicles and/or indirectly interact with pro-BDNF via sortilin, which is the type I transmembrane receptor for pro-BDNF (13, 15) and also interacts with pro-BDNF within vesicles (23). We provided evidence by HAP1 immunoprecipitation from the human brain that HAP1 is associated with sortilin, and the association was reduced in the HD brain (Fig. 3).
Consistent with the axonal transport data, the activity-dependent release of pro-BDNF is largely abolished in HAP1−/− mice, whereas the constitutive release is not affected. Our data suggest that the activity-dependent release but not the constitutive release of pro-BDNF may require the function of HAP1. As BDNF is critical for the survival and differentiation of many groups of neurons in the brain, the impairment of axonal transport and activity-dependent release of BDNF in HAP1−/− mice may underlie the death of neurons in the hypothalamus in these animals (35, 71) and explain the function of HAP1-A in neurite outgrowth (72, 73).
In summary, our findings suggest a new mechanism underlying the intraneuronal trafficking, axonal transport, and release of pro-BDNF via association with HAP1. Deletion of HAP1 diminishes the intraneuronal trafficking, axonal transport, and activity-dependent release of pro-BDNF. We also showed that mutant Htt and V66M polymorphism reduce the association of HAP1 with pro-BDNF. Thus, we conclude that HAP1 may play a role in the transport and release of pro-BDNF and that the reduced association of HAP1 with the prodomain may underlie the decreased transport and release of BDNF in HD and V66M polymorphism.
Acknowledgments
We thank Dr. M. DiFiglia (Harvard Medical School) for the gift of HAP1 antibody; Dr. M. Kojima (National Institute of Advanced Industrial Science and Technology, Ikeda, Japan) for providing wild type and V66M BDNF plasmids; Regeneron for mature BDNF; Dr. E. S. Schweitzer (Departments of Physiological Science and Neurology, Brain Research Institute, UCLA) for polyQ23 or polyQ103 stable expression PC12 cell lines; Jinxian Mi and Jinhua Zhong for technical assistance on purification of recombinant proteins and production of antibodies; Dr. Tim Chatway for the advice on two-dimensional gel; Dr. Damien Keating for editing the manuscript; and Dr. Hong-yun Li for critical comments during the study.
This work was supported in part by National Health and Medical Research Council Grants 375110 and 480423.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- BDNF
- brain-derived neurotrophic factor
- Htt
- Huntingtin
- ELISA
- enzyme-linked immunosorbent assay
- MALDI-TOF
- matrix-assisted laser desorption ionization time-of-flight
- WT
- wild type
- HD
- Huntington disease
- GST
- glutathione S-transferase.
REFERENCES
- 1.Chao M. V. (1992) Neuron 9, 583–593 [DOI] [PubMed] [Google Scholar]
- 2.Goodman L. J., Valverde J., Lim F., Geschwind M. D., Federoff H. J., Geller A. I., Hefti F. (1996) Mol. Cell. Neurosci. 7, 222–238 [DOI] [PubMed] [Google Scholar]
- 3.Huang E. J., Reichardt L. F. (2001) Annu. Rev. Neurosci. 24, 677–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu B. (2003) Neuron 39, 735–738 [DOI] [PubMed] [Google Scholar]
- 5.Poo M. M. (2001) Nat. Rev. Neurosci. 2, 24–32 [DOI] [PubMed] [Google Scholar]
- 6.Bibel M., Barde Y. A. (2000) Genes Dev. 14, 2919–2937 [DOI] [PubMed] [Google Scholar]
- 7.MacQueen G. M., Ramakrishnan K., Croll S. D., Siuciak J. A., Yu G., Young L. T., Fahnestock M. (2001) Behav. Neurosci. 115, 1145–1153 [DOI] [PubMed] [Google Scholar]
- 8.Mowla S. J., Farhadi H. F., Pareek S., Atwal J. K., Morris S. J., Seidah N. G., Murphy R. A. (2001) J. Biol. Chem. 276, 12660–12666 [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T., Rauskolb S., Polack M., Klose J., Kolbeck R., Korte M., Barde Y. A. (2008) Nat. Neurosci. 11, 131–133 [DOI] [PubMed] [Google Scholar]
- 10.Heymach J. V., Jr., Shooter E. M. (1995) J. Biol. Chem. 270, 12297–12304 [DOI] [PubMed] [Google Scholar]
- 11.Pang P. T., Teng H. K., Zaitsev E., Woo N. T., Sakata K., Zhen S., Teng K. K., Yung W. H., Hempstead B. L., Lu B. (2004) Science 306, 487–491 [DOI] [PubMed] [Google Scholar]
- 12.Lee R., Kermani P., Teng K. K., Hempstead B. L. (2001) Science 294, 1945–1948 [DOI] [PubMed] [Google Scholar]
- 13.Nykjaer A., Lee R., Teng K. K., Jansen P., Madsen P., Nielsen M. S., Jacobsen C., Kliemannel M., Schwarz E., Willnow T. E., Hempstead B. L., Petersen C. M. (2004) Nature 427, 843–848 [DOI] [PubMed] [Google Scholar]
- 14.Kenchappa R. S., Zampieri N., Chao M. V., Barker P. A., Teng H. K., Hempstead B. L., Carter B. D. (2006) Neuron 50, 219–232 [DOI] [PubMed] [Google Scholar]
- 15.Teng H. K., Teng K. K., Lee R., Wright S., Tevar S., Almeida R. D., Kermani P., Torkin R., Chen Z. Y., Lee F. S., Kraemer R. T., Nykjaer A., Hempstead B. L. (2005) J. Neurosci. 25, 5455–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X. F., Rush R. A. (1996) Neuroscience 74, 945–953 [DOI] [PubMed] [Google Scholar]
- 17.Conner J. M., Lauterborn J. C., Gall C. M. (1998) Rev. Neurosci. 9, 91–103 [DOI] [PubMed] [Google Scholar]
- 18.Tonra J. R. (1999) Microsc. Res. Tech. 45, 225–232 [DOI] [PubMed] [Google Scholar]
- 19.von Bartheld C. S., Wang X., Butowt R. (2001) Mol. Neurobiol. 24, 1–28 [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Zhou X. F. (2002) Neuroscience 112, 967–975 [DOI] [PubMed] [Google Scholar]
- 21.Kafitz K. W., Rose C. R., Thoenen H., Konnerth A. (1999) Nature 401, 918–921 [DOI] [PubMed] [Google Scholar]
- 22.Butowt R., von Bartheld C. S. (2001) J. Neurosci. 21, 8915–8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z. Y., Ieraci A., Teng H., Dall H., Meng C. X., Herrera D. G., Nykjaer A., Hempstead B. L., Lee F. S. (2005) J. Neurosci. 25, 6156–6166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou H., Kim S. K., Zaitsev E., Snell C. R., Lu B., Loh Y. P. (2005) Neuron 45, 245–255 [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Wu L. L., Song X. Y., Luo X. G., Zhong J. H., Rush R. A., Zhou X. F. (2006) Eur. J. Neurosci. 24, 2444–2452 [DOI] [PubMed] [Google Scholar]
- 26.Egan M. F., Kojima M., Callicott J. H., Goldberg T. E., Kolachana B. S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., Weinberger D. R. (2003) Cell 112, 257–269 [DOI] [PubMed] [Google Scholar]
- 27.Hariri A. R., Goldberg T. E., Mattay V. S., Kolachana B. S., Callicott J. H., Egan M. F., Weinberger D. R. (2003) J. Neurosci. 23, 6690–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pezawas L., Verchinski B. A., Mattay V. S., Callicott J. H., Kolachana B. S., Straub R. E., Egan M. F., Meyer-Lindenberg A., Weinberger D. R. (2004) J. Neurosci. 24, 10099–10102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventriglia M., Bocchio Chiavetto L., Benussi L., Binetti G., Zanetti O., Riva M. A., Gennarelli M. (2002) Mol. Psychiatry 7, 136–137 [DOI] [PubMed] [Google Scholar]
- 30.Momose Y., Murata M., Kobayashi K., Tachikawa M., Nakabayashi Y., Kanazawa I., Toda T. (2002) Ann. Neurol. 51, 133–136 [DOI] [PubMed] [Google Scholar]
- 31.Chen Z. Y., Jing D., Bath K. G., Ieraci A., Khan T., Siao C. J., Herrera D. G., Toth M., Yang C., McEwen B. S., Hempstead B. L., Lee F. S. (2006) Science 314, 140–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauthier L. R., Charrin B. C., Borrell-Pagès M., Dompierre J. P., Rangone H., Cordelières F. P., De Mey J., MacDonald M. E., Lessmann V., Humbert S., Saudou F. (2004) Cell 118, 127–138 [DOI] [PubMed] [Google Scholar]
- 33.Gunawardena S., Goldstein L. S. (2004) J. Neurobiol. 58, 258–271 [DOI] [PubMed] [Google Scholar]
- 34.Li X. J., Li S. H. (2005) Trends Pharmacol. Sci. 26, 1–3 [DOI] [PubMed] [Google Scholar]
- 35.Li S. H., Yu Z. X., Li C. L., Nguyen H. P., Zhou Y. X., Deng C., Li X. J. (2003) J. Neurosci. 23, 6956–6964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X. F., Song X. Y., Zhong J. H., Barati S., Zhou F. H., Johnson S. M. (2004) J. Neurochem. 91, 704–715 [DOI] [PubMed] [Google Scholar]
- 37.Sharp A. H., Loev S. J., Schilling G., Li S. H., Li X. J., Bao J., Wagster M. V., Kotzuk J. A., Steiner J. P., Lo A., et al. (1995) Neuron 14, 1065–1074 [DOI] [PubMed] [Google Scholar]
- 38.Gray E. G., Whittaker V. P. (1962) J. Anat. 96, 79–88 [PMC free article] [PubMed] [Google Scholar]
- 39.Li X. J., Li S. H., Sharp A. H., Nucifora F. C., Jr., Schilling G., Lanahan A., Worley P., Snyder S. H., Ross C. A. (1995) Nature 378, 398–402 [DOI] [PubMed] [Google Scholar]
- 40.Zuccato C., Ciammola A., Rigamonti D., Leavitt B. R., Goffredo D., Conti L., MacDonald M. E., Friedlander R. M., Silani V., Hayden M. R., Timmusk T., Sipione S., Cattaneo E. (2001) Science 293, 493–498 [DOI] [PubMed] [Google Scholar]
- 41.Block-Galarza J., Chase K. O., Sapp E., Vaughn K. T., Vallee R. B., DiFiglia M., Aronin N. (1997) Neuroreport 8, 2247–2251 [DOI] [PubMed] [Google Scholar]
- 42.Engelender S., Sharp A. H., Colomer V., Tokito M. K., Lanahan A., Worley P., Holzbaur E. L., Ross C. A. (1997) Hum. Mol. Genet. 6, 2205–2212 [DOI] [PubMed] [Google Scholar]
- 43.Aiken C. T., Tobin A. J., Schweitzer E. S. (2004) Neurobiol. Dis. 16, 546–555 [DOI] [PubMed] [Google Scholar]
- 44.Gutekunst C. A., Li S. H., Yi H., Ferrante R. J., Li X. J., Hersch S. M. (1998) J. Neurosci. 18, 7674–7686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin E. J., Kim M., Velier J., Sapp E., Lee H. S., Laforet G., Won L., Chase K., Bhide P. G., Heller A., Aronin N., Difiglia M. (1999) J. Comp. Neurol. 403, 421–430 [PubMed] [Google Scholar]
- 46.Shimizu E., Hashimoto K., Iyo M. (2004) Am. J. Med. Genet. B Neuropsychiatr. Genet. 126, 122–123 [DOI] [PubMed] [Google Scholar]
- 47.Bath K. G., Lee F. S. (2006) Cogn. Affect. Behav. Neurosci. 6, 79–85 [DOI] [PubMed] [Google Scholar]
- 48.Hashimoto K. (2007) BioEssays 29, 116–119 [DOI] [PubMed] [Google Scholar]
- 49.Katz L. C., Shatz C. J. (1996) Science 274, 1133–1138 [DOI] [PubMed] [Google Scholar]
- 50.Lu B. (2004) Prog. Brain Res. 146, 137–150 [DOI] [PubMed] [Google Scholar]
- 51.Binder D. K., Croll S. D., Gall C. M., Scharfman H. E. (2001) Trends Neurosci. 24, 47–53 [DOI] [PubMed] [Google Scholar]
- 52.Spires T. L., Grote H. E., Varshney N. K., Cordery P. M., van Dellen A., Blakemore C., Hannan A. J. (2004) J. Neurosci. 24, 2270–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrer I. (1999) Rev. Neurol. 29, 515–521 [PubMed] [Google Scholar]
- 54.Howells D. W., Porritt M. J., Wong J. Y., Batchelor P. E., Kalnins R., Hughes A. J., Donnan G. A. (2000) Exp. Neurol. 166, 127–135 [DOI] [PubMed] [Google Scholar]
- 55.Zuccato C., Tartari M., Crotti A., Goffredo D., Valenza M., Conti L., Cataudella T., Leavitt B. R., Hayden M. R., Timmusk T., Rigamonti D., Cattaneo E. (2003) Nat. Genet. 35, 76–83 [DOI] [PubMed] [Google Scholar]
- 56.Gu F., Crump C. M., Thomas G. (2001) Cell. Mol. Life Sci. 58, 1067–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hibbert A. P., Morris S. J., Seidah N. G., Murphy R. A. (2003) J. Biol. Chem. 278, 48129–48136 [DOI] [PubMed] [Google Scholar]
- 58.Mowla S. J., Pareek S., Farhadi H. F., Petrecca K., Fawcett J. P., Seidah N. G., Morris S. J., Sossin W. S., Murphy R. A. (1999) J. Neurosci. 19, 2069–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Humbert S., Saudou F. (2005) J. Soc. Biol. 199, 247–251 [DOI] [PubMed] [Google Scholar]
- 60.del Toro D., Canals J. M., Ginés S., Kojima M., Egea G., Alberch J. (2006) J. Neurosci. 26, 12748–12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Z. Y., Patel P. D., Sant G., Meng C. X., Teng K. K., Hempstead B. L., Lee F. S. (2004) J. Neurosci. 24, 4401–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu B. (2003) Learn. Mem. 10, 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.An J. J., Gharami K., Liao G. Y., Woo N. H., Lau A. G., Vanevski F., Torre E. R., Jones K. R., Feng Y., Lu B., Xu B. (2008) Cell 134, 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiaruttini C., Vicario A., Li Z., Baj G., Braiuca P., Wu Y., Lee F. S., Gardossi L., Baraban J. M., Tongiorgi E. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 16481–16486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li S. H., Gutekunst C. A., Hersch S. M., Li X. J. (1998) J. Neurosci. 18, 1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schroer T. A. (2004) Annu. Rev. Cell Dev. Biol. 20, 759–779 [DOI] [PubMed] [Google Scholar]
- 67.Vallee R. B., Williams J. C., Varma D., Barnhart L. E. (2004) J. Neurobiol. 58, 189–200 [DOI] [PubMed] [Google Scholar]
- 68.Gunawardena S., Goldstein L. S. (2004) J. Neurobiol. 58, 258–271 [DOI] [PubMed] [Google Scholar]
- 69.McGuire J. R., Rong J., Li S. H., Li X. J. (2006) J. Biol. Chem. 281, 3552–3559 [DOI] [PubMed] [Google Scholar]
- 70.Hirokawa N., Takemura R. (2004) Curr. Opin. Neurobiol. 14, 564–573 [DOI] [PubMed] [Google Scholar]
- 71.Sheng G., Chang G. Q., Lin J. Y., Yu Z. X., Fang Z. H., Rong J., Lipton S. A., Li S. H., Tong G., Leibowitz S. F., Li X. J. (2006) Nat. Med. 12, 526–533 [DOI] [PubMed] [Google Scholar]
- 72.Rong J., McGuire J. R., Fang Z. H., Sheng G., Shin J. Y., Li S. H., Li X. J. (2006) J. Neurosci. 26, 6019–6030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y., Chin L. S., Levey A. I., Li L. (2002) J. Biol. Chem. 277, 28212–28221 [DOI] [PubMed] [Google Scholar]