Abstract
Plasmid R6K, which contains 3 replication origins called α, γ, and β, is a favorable system to investigate the molecular mechanism(s) of action at a distance, i.e. replication initiation at a considerable distance from the primary initiator protein binding sites (iterons). The centrally located γ origin contains 7 iterons that bind to the plasmid-encoded initiator protein, π. Ori α, located at a distance of ∼4 kb from γ, contains a single iteron that does not directly bind to π but is believed to access the protein by π-mediated α-γ iteron-iteron interaction that loops out the intervening ∼3.7 kb of DNA. Although the cis-acting components and the trans-acting proteins required for ori γ function have been analyzed in detail, such information was lacking for ori α. Here, we have identified both the sequence elements located at α and those at γ, that together promoted α activity. The data support the conclusion that besides the single iteron, a neighboring DNA primase recognition element called G site is essential for α-directed plasmid maintenance. Sequences preceding the iteron and immediately following the G site, although not absolutely necessary, appear to play a role in efficient plasmid maintenance. In addition, while both dnaA1 and dnaA2 boxes that bind to DnaA protein and are located at γ were essential for α activity, only dnaA2 was required for initiation at γ. Mutations in the AT-rich region of γ also abolished α function. These results are consistent with the interpretation that a protein-DNA complex consisting of π and DnaA forms at γ and activates α at a distance by DNA looping.
Keywords: DNA-binding Protein, DNA Primase, DNA-Protein Interaction, DNA Replication, DNA Synthesis, DNA Looping, DnaA Protein, G Site, Pi Protein, Primase
Introduction
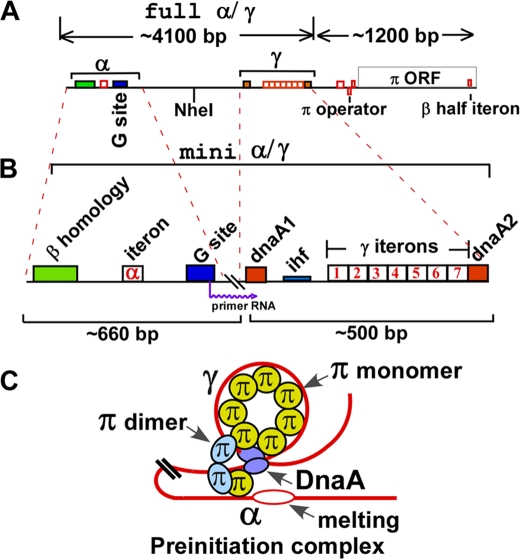
Action at a distance, which activates transcription, controls development, causes immunoglobulin switching, and other important DNA transactions by intrachromosomal and interchromosomal long range, protein-mediated DNA site-site interactions, has emerged as a topic of considerable current interest (1–6). The plasmid R6K is an excellent system to study action at a distance as it applies to activation of a replication origin at a considerable distance away from the primary initiator protein binding sites. The plasmid contains 3 replication origins called α, γ, and β. The various structural features of ori α and ori γ are shown in Fig. 1. The complete natural ori α sequence consists of a ∼3.7-kb DNA sequence at the left that is joined to downstream ori γ sequence near the left of the dnaA1 box (Fig. 1A). Ori γ extends from the left of dnaA1 box and ends just after the dnaA2 box (Fig. 1B). Ori α has an obligatory requirement for γ in cis to be functional (7). The mini α-γ replicon contains a deletion that fuses 660 bp of the α sequence from the left margin to that of γ near the left of dnaA1 box and removes the intervening sequence (Fig. 1B). The mini α is fully functional in plasmid maintenance (8).
FIGURE 1.
Schematic depiction of α-γ and mini α-γ replicons and a model of a preinitiation complex at α. A, schematic diagram showing the full α-γ sequence, the locations of the various iterons and half-iterons are shown. B, mini α-γ replicon formed by retaining 660 bp from the left end and deleting the remainder of the sequence all the way to, but not including the dnaA1 box of γ. C, model of a preinitiation complex formed at ori α, showing the 6 iterons of γ postulated to be wrapped around the monomeric π protein molecules, the single α iteron bound to a monomeric π and paired with the γ iteron (also bound to a monomeric π with the iteron pair stabilized by a dimeric π), and possibly a pair of DnaA proteins bound to the dnaA1 and dnaA2 boxes and containing a melted region.
The α region contains, from the left, a sequence of 41 bp that is homologous to that present in ori β and is therefore called the β-homology region (βHR),2a spacer sequence of 45 bp, the α iteron of 23 bp, and a spacer of 54 bp followed by the putative primase recognition site (G site) of 31 bp (9, 10). There are an additional 444 bp of DNA starting after the G site and extending up to the γ sequence. The replication origin β, that is not discussed here, contains the γ sequence plus all of the sequences extending through the π open reading frame (ORF) that includes a half-iteron located near the 3′-end of the π ORF (Fig. 1B) (11, 12). The features of ori γ have been extensively analyzed (13) and it contains from left to right, the dnaA1 box, an AT-rich area embedded in what is a binding site for the host-encoded DNA-bending protein IHF, 7 iterons, and the dnaA2 box that is contiguous with the 7th iteron. The sequence immediately preceding the π ORF contains an 8th iteron and two half-iterons that form inverted repeats, and together constitute the operator element of the π ORF (11, 12, 14, 15). As the name suggests, the dnaA boxes bind to the host-encoded DnaA initiator protein (16). Our goal was to determine which of these elements were necessary for α function.
Previous attempts to define the structural features of ori α were fraught with two problems. First, in the plasmid constructs used (17), the obligatory ori γ sequence, which was thought to be silent, actually retained activity. Therefore, one could not be certain whether the plasmid derivatives were being replicated from ori α or from ori γ. Second, the DNA sequences and the binding proteins, that were reported to be distorting the DNA and to be essential for α function (18, 19) were found by another group to be components of the neighboring origin of conjugative transfer (oriT) and do not seem to be a part of the α-γ replicon (20, 21).
A putative DnaG primase recognition site of R6K was identified by cloning the plasmid restriction fragments in a M13 vector and selecting for those inserts that rescued the phage replicon and allowed it to replicate in a DnaG-dependent mode (10). However, whether these sites were involved in any way with vegetative replication of the plasmid or its conjugative transfer was not known.
To investigate the mechanistic aspects of replication initiation at α located at a distance of ∼4000 bp away from the 7 γ iterons at γ, which are the primary binding sites of π, it was necessary to fully characterize the relevant sequences of α required for its function. It was also necessary to identify γ sequences necessary for plasmid maintenance driven by α. With these objectives in mind, and considering some of the uncertainties with regard to the existing information about essential elements of an authentic ori α, we proceeded to identify the sequences of both α and γ needed for α function. At this time, there is no mutation of a cis element of the replicon available that selectively inactivates ori γ but retains ori α activity. Therefore, we decided to use a mutation in a trans-acting factor namely the integration host factor (IHF) that is a DNA-bending protein and worked out conditions that either masked or discouraged background replication initiations from the ori γ sequence, in a α-γ replicon. These conditions allowed us to measure α activity without the interference from the backround of initiations at γ. Here we present the results of these investigations. Data support the interpretation that a protein-DNA complex consisting of the γ sequence and at least DnaA and π proteins were needed to support α-directed plasmid maintenance. The α iteron and the G site were essential elements needed for ori α function. Furthermore, two-dimensional gel analysis of replication intermediates of the replicon showed that replication bubbles were initiated within the same small (660 bp) restriction fragment that contained the α-proximal sequences necessary for the origin activity.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Oligonucleotides
Escherichia coli strain DH5α was used for all the cloning experiments. HN356 (IHF+) and HN840 (ihfΔ) cells were used for in vivo replication assays. The list of oligonucleotides used is given in Table 1, and the sequence will be provided upon request. All the strains were grown at 37 °C (unless otherwise stated). All of the chemicals were purchased from Sigma. The custom oligonucleotides were purchased from Integrated DNA Technology Inc.
TABLE 1.
List of primers
| Name of the primer | Purpose/use |
|---|---|
| GKNOCKF and GKNOCKR | To introduce NotI site |
| CTGMUTF and CTGMUTR | To mutate primase recognition sequence |
| IHFMUTF and IHFMUTR | To mutate IHF site in γ origin |
| dna2XhoF and dna2XhoF | To introduce XhoI site after VIIth iteron |
| Alfa5F and Alfa5R | To introduce 5-bp insertion between α iteron and G site |
| Alfa10F and Alfa10R | To introduce 10-bp insertion between α iteron and G site |
| Alpha F and Alpha R | To amplify α-γ fragment |
| Amp F and Amp R | To amplify pUC18 ampicillin gene |
Site-directed Mutagenesis and Plasmid Construction
Mini αγ (pAM738αγ) was used as template for carrying out deletions, insertions, and nucleotide changes at various positions of γ and α origin by site-directed mutagenesis using the primers listed in Table 1. The oligonucleotide pairs carrying the nucleotide sequence to be inserted/deleted or to be mutated were used to carryout PCR using Pfu polymerase (Stratagene) following the manufacturer's instructions. PCR products were treated with DpnI for 4 h, purified, and transformed into DH5α. DNA was isolated from the transformants growing on ampicillin (50 μg/ml) selection plates, and the mutations were confirmed by DNA sequencing.
M13 recombinant DNA carrying the wt or mutated DnaG sites were constructed by first cloning the mini α region into M13mp18 DNA and then mutating the G site by site-directed mutagenesis as described above. For the construction of pMS1 plasmid, the α-γ region from RSF 1040 (22) and the ampicillin gene from pUC18 were amplified by PCR using the “Ultra Pfu” DNA polymerase (Stratagene). The PCR product was digested with DpnI for 4–5 h and purified. The PCR product of ampicillin was digested by EcoRI and ligated with the EcoRI cut, dephosphorylated α-γ fragment. The ligation mixture was transformed into cells carrying a chromosomally integrated π ORF to supply π protein in trans. Plasmids were isolated from the transformants growing on ampicillin (50 μg/ml) and checked by restriction digestion and sequencing.
Plasmid Isolation and Purification of Replication Intermediates on BND Cellulose
A single colony of the cells carrying mini α-γ and pMS1 plasmids was inoculated into LB medium containing ampicillin (50 μg/ml) and grown overnight at 37 °C. The cultures were inoculated into fresh LB medium at 1:100 dilutions and grown further until the optical density at A600 reached 0.45. Cells were harvested, and plasmids isolated by a modified SDS-NaCl method (23). Briefly, the cell pellet from 1 liter of culture was resuspended in 10 ml of 10% sucrose in 50 mm Tris, pH 8.0, solution. To this, 2 ml of 10 mg/ml lysozyme in 10 mm Tris, pH 8.0 was added followed by the addition of 8 ml of 0.25 m EDTA. After mixing and incubation at room temperature for 10 min, 4 ml of 10% SDS was added and mixed gently. Finally 6 ml of 5 m NaCl was added and mixed gently, and the mixture was incubated overnight at 4 °C. The supernatant collected after ultracentrifugation at 30,000 rpm for 20 min was mixed with 0.6 volume of isopropyl alcohol and incubated at room temperature for 15 min. The pellet obtained after centrifugation at 5000 rpm for 20 min was dissolved in 1× TE (10 mm Tris, pH 8.0 and 1 mm EDTA, pH 8.0) buffer. An equal volume of 5 m lithium chloride was added to it to precipitate high molecular weight RNA; the supernatant obtained after centrifugation was mixed with 0.6 volume of isopropyl alcohol and after incubation at room temperature for 20 min was centrifuged again. The pellet was washed with 70% ethanol and dissolved in 1× TE. It was treated with Raise for 2 h at 37 °C and then with proteinase K to digest RNA and protein, respectively. After a 2× phenol/chloroform extraction, the DNA was precipitated with ethanol and sodium acetate and dissolved in 1× TE buffer.
Replication intermediates from these plasmids were isolated using a BND cellulose column. The mini α-γ plasmid pAM738αγ was digested with BamHI, and the pMS1 plasmid was digested with EcoRI and HindIII. The products were precipitated with ethanol and dissolved in NET buffer (10 mm Tris, pH 8.0, 1 mm EDTA, pH 8.0, and 1 m NaCl) and loaded onto BND cellulose columns that were pre-equilibrated with the NET buffer. The columns were washed five times with one column volume of NET buffer. Replication intermediates were eluted with the NET buffer containing 1.8% caffeine. The eluted replication intermediates were precipitated with one volume of isopropyl alcohol at −20 °C for 1–2 h and recovered by centrifugation at 10,000 rpm for 90 min. The DNA pellet was air dried and dissolved in 1× TE buffer. DNA was precipitated again with ethanol and sodium acetate and dissolved in minimum volume of 1× TE.
Two-dimensional Brewer-Fangman Gels
Two-dimensional gel electrophoresis was carried out as described (24). Briefly replication intermediates purified from a 1.5-liter culture were first resolved by electrophoresis for 40–44 h at 30 V without ethidium bromide at room temperature, and then the electrophoresis was carried out in a second dimension in 1.2% agarose gels in the presence of ethidium bromide for 20–24 h at 130 V at 4 °C. After Southern blotting (23), the blots were baked under vacuum and hybridized with a random primer-labeled α region-specific DNA probe. Signals were detected using a phosphorimager.
Proteins and Enzymes
SSB was bought from commercial sources (USB, Cleveland, OH). Polymerase III* was reconstituted from the core complex consisting of the α, θ, and ϵ purified from a recombinant overproducer plasmid (a gift from Dr. Mike O'Donnell, Rockefeller University (25)), The τ clamp loading complex consisting of the τ, δ, δ′, χ, and ψ subunits were purified from individual overproducing plasmids and reconstituted (26). We also purified all nine subunits of pol III* from a single overproducer plasmid pHOC3.2.1 (a gift from Drs. A. Pritchard and Charles McHenry, UCHSC, Denver, CO). IHF and DnaG proteins were purified as described (27, 28).
In Vitro Replication
Using M13G ori1 and the recombinant SS DNA of M13 containing the template strand of the R6K G site and its mutant forms, in vitro replication reactions were carried out with purified DNA pol III holoenzyme, SSB, and DnaG as described (10, 29). Briefly, the reaction was carried out in a 50-μl reaction mixture that contained 40 mm HEPES, KOH, pH 7.5, 4 mm dithiothreitol, 4% w/v sucrose, 8 mm magnesium acetate, 40 mm potassium glutamate, rifampicin 25 μg/ml, GTP, UTP, and CTP (200 μm each) 0.8 mm ATP, dATP, dGTP, dCTP (each at 50 μm) with 100–150 cpm [α-32P]dTTP (80–120 cpm/pmol), 0.2 μg of SS M13 DNA, 2 μg of SSB, and 100 ng of pol III holoenzyme, 75 ng of β-subunit, and 40 ng of primase. After 10 min of incubation at 30 °C, the reaction was stopped by adding 10% trichloroacetic acid, and acid-insoluble radioactivity was counted after being trapped on glass fiber filters.
RESULTS
Identification of the Minimal ori α Sequence Necessary for Plasmid Maintenance
Our goal was to identify (i) sequence elements in the mini α and (ii) those present in the γ segment that were necessary for plasmid maintenance by ori α. Because a functional ori γ sequence is needed in cis to elicit α activity, it was necessary to define in vivo conditions under which plasmid maintenance by γ was greatly reduced or eliminated so that α activity could be distinctly measured without the interfering γ activity. It has been shown that replication initiated from ori γ is very inefficient in the absence of IHF; so much so that the intracellular plasmids that are tagged with an ampR marker are maintained at very low copy number in the ihfΔ host cells that did not form colonies on LB plates containing 40–50 μg of ampicillin/ml (15, 30). The effect of 100 μg/ml ampicillin was even more drastic on colony formation in the absence of IHF, even for plasmids containing a functional α. IHF appears to be needed for initiation at γ because it bends the DNA and thereby promotes interactions of proteins located at binding sites on the opposite sides of the bend. Because the absence of IHF severely inhibited propagation of ori γ, any derivative of a α-γ replicon that allowed the ihfΔ host cells to form colonies on 40–50 μg/ml ampicillin containing Luria-Bertani (LB) plates was taken as preliminary evidence for initiation mediated by the α sequence present in the plasmid. Using this criterion, we proceeded to make specific deletions of the α region that were connected to the obligatory γ sequence as described below.
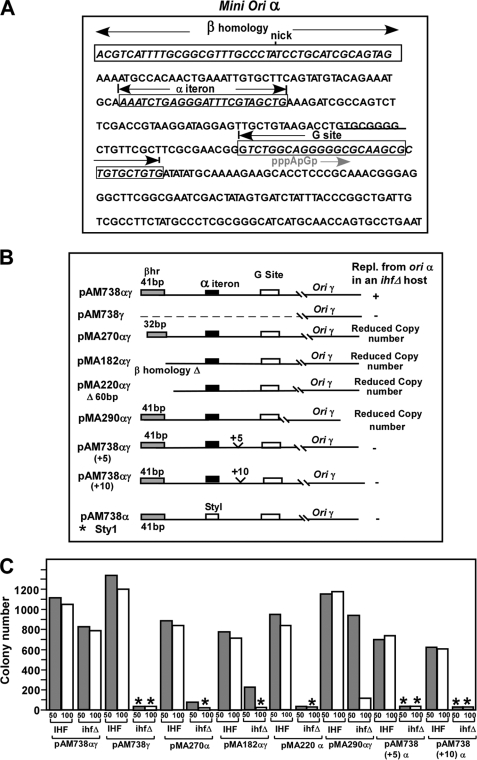
The nucleotide sequence of the mini α fragment is shown in Fig. 2A. The βHR, the α iteron, and the G sites are shown as boxed sequences. The nick corresponds to one of 2 oriTs present in R6K (21). The putative primer location starting with pppApG etc. is shown in gray. The various deletions of the mini α segments are shown in Fig. 2B. The construct marked with the asterisk was reported before (8). All plasmids carried the ampR marker.
FIGURE 2.
Diagrams showing the locations of various deletions of the α sequence of a mini α-γ replicon and their relative efficiencies with regard to plasmid maintenance in vivo in IHF+ and ihfΔ hosts. A, nucleotide sequence of the mini α region, showing the βHR, iteron, and G site that are boxed, the presumptive start point of DnaG-catalyzed primer RNA, and the nick site belonging to the closely located oriT. B, schematic representations of the various deletions and their effect on α-γ plasmid maintenance. C, quantitative analysis of plasmid maintenance in replicons deleted for various sequences about ori α in both IHF+ and the ihfΔ hosts on 50 and 100 μg/ml ampicillin plates. The asterisks indicate that no colonies were formed by host cells containing the particular plasmid derivatives.
The number of colonies generated by transforming IHF+ and ihfΔ host with equal amounts of DNA from each plasmid construct at both 50 and 100 μg/ml ampicillin are shown in Fig. 2C. The data represent the averages of three independent experiments. Each set of experiments included a plating efficiency control that determined the ratio of the number of colonies generated by equal amounts of pUC19 plasmid DNA transformed into the IHF+ strain divided by that in the ihfΔ host under identical conditions. This ratio was usually close to one and was used to normalize the transformation data. The data marked with asterisks in Fig. 2C indicate that the colony numbers were zero in those cases. The plasmid pAM738αγ, as expected, was propagated in the ihfΔ host. In contrast, the pAM738γ plasmid lacking the α sequence, also as expected, was not maintained in the same host. These served as positive and negative controls for the in vivo experiments, respectively. Deletion of 10 bp from the 5′-end of the β homology region, or a partial or complete deletion of βHR caused significant loss of plasmid maintenance in the absence of IHF. Deletion of all sequences from mini α, downstream of the 3′-end of the G site generated the plasmid pMA290αγ that showed significantly reduced copy number in ihfΔ cells.
We extracted the plasmid DNA from cells containing an internal standard (pACYC 174) and confirmed the low copy phenotype by measuring the ratio of the plasmid DNA and that of the internal standard after resolving the DNA by electrophoresis in agarose gels (not shown). Insertions of either 5 or 10 bp inbetween the α iteron and the G site, constructed in an attempt to study the possible effect of change the helical phase between the two sites, abolished plasmid maintenance in ihfΔ cells. Replacement of the α iteron by a StyI restriction site also eliminated plasmid propagation in the ihfΔ host. From these results, we conclude that the iteron, the G site (see below), and the sequence between the two were essential elements for replication control at α. The βHR and the sequence (∼150 bp) following the G site were needed for optimal plasmid propagation in the absence of IHF. It is possible that the sequences starting immediately downstream of the 3′-end of the iteron and extending up to the G site are all part of the extended G site (31), and, therefore, this sequence could not be altered without causing loss of plasmid maintenance.
The G Site Is an Essential Component of ori α
We wished to determine whether the G site was an essential component of ori α and whether one could, with appropriate mutations, separate the ability of the site to promote SS to DS replication of the recombinant M13R6Kα (Fig. 3, A and B) from its possible function in R6K plasmid replication in vivo initiated at ori α. The assay was based on the observation that single-stranded (SS) M13 DNA containing the origin of replication of the phage G4 can be replicated to the DS form in the presence of DnaG primase, but in the absence of RNA polymerase. This is because M13 ori requires priming by RNA polymerase whereas that of G4 requires DnaG primase (32). Therefore, the hybrid SS DNA of M13 Gori1 is able to replicate in vitro when supplied with purified DnaG primase, SSB, DNA polymerase III*, and the processivity factor β (29, 33).
FIGURE 3.
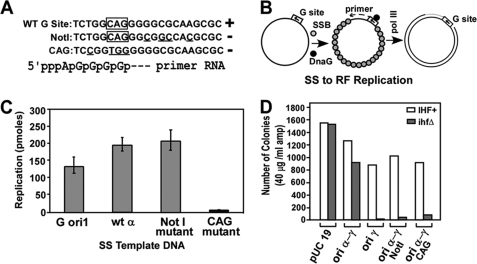
SS to DS replication in vitro of DNA templates carrying the wt R6K G site, the origin of G4 (M13 G ori1), and the two mutant forms of the plasmid G site. A, sequences showing the wt and the Not1 and the CAG mutant forms; the bottom line shows the start point of the presumptive primer RNA. B, diagram showing the purified proteins used and their action in replicating SS to DS DNA. C, quantification of the reaction product from each template from three separate experiments, presented with S.E. bars. D, in vivo measurements of maintenance of amp-resistant IHF+ and ihfΔ hosts carrying the wt and the various G site mutants along with a pUC19 control plated on LB media containing 40 μg/ml ampicillin.
We cloned the mini α segment (Fig. 1B) into an M13mp18 DS vector DNA and generated SS DNA containing the putative R6K G site in the correct orientation. We then performed site-directed mutagenesis of the site and isolated two mutants namely the Not1 and the CAG (Fig. 3A). The rationale for selecting these two mutants was as follows. E. coli DNA primase recognizes the sequence CTG on the template strand and synthesizes the primer starting with pppApGp .., etc. The mutation from 5′-TGGCAG to 5′-CGGTGG was made in the hope of inactivating the consensus initiation sequence of the primer CAG (34). The Not1 mutation was made in attempt to alter the sequence of the G site located downstream of the initiation site in the hope of reducing its affinity for the primase (Fig. 3A). The in vitro SS to DS replication was carried out in a reaction mixture containing purified DnaG, SSB, and pol III holoenzyme to determine whether one or both of the G site mutants were defective in priming activity in comparison with that of the wt G site template located in the region of ori α and the Gori1 sequences (Fig. 3B). The data (average of three independent experiments presented with S.E. bars) showed that both the M13 Gori1, and the R6K wt templates, as expected, were vigorously replicated in vitro, generating up to 200 pmol of reaction products under the specified reaction conditions. The Not1 mutant was at least as active as the wt α sequence in promoting SS to DS replication. In contrast, the CAG mutation severely reduced the replication in vitro (Fig. 3C).
We incorporated the mutant G sites into the α-γ plasmid and measured the ability of the wt and the mutant forms to replicate in vivo in the IHF+ and the isogenic ihfΔ hosts. The data revealed that whereas the wt pAM738αγ replicated with nearly equal efficiency in both of the host strains, the mutant forms of the plasmid namely pMAα-Not1, pMAα-CAG, while maintaining normal self-propagation in vivo in IHF+ cells, were significantly deficient in doing so in the ihfΔ strain (Fig. 3D). Control experiments showed that pUC19 DNA, as expected, replicated equally well in both the hosts. The control plasmid pAM738γ, which included a functional γ but was deleted for α, as expected, could only be maintained in the IHF+ but not in the ihfΔ host (Fig. 3D). The in vitro and in vivo data, taken together, support the conclusion that the primase-loading G site was an essential component of ori α.
Two-dimensional Gel Electrophoresis Showed Initiation From Within the α Region in the Plasmid Derivatives
Previous work using electron microscopy had localized the site of initiation to the middle of the α region (Fig. 1B) (7, 35, 36). In fact, the bubble seems to be initiated ∼400 bp away from the genetically indispensable sequence namely the iteron and the G site, although the site where the bubble formed seemed to be dispensable. The preceding observation suggested that initiation of replication, while being absolutely dependent on the ori α sequences, did not necessarily start at a unique location. This phenomenon has also been observed in the plasmid P1 (37). We have also reported that in a reconstituted system, ori γ replication did not start from just one location but at multiple sites within ∼200 bp (38).
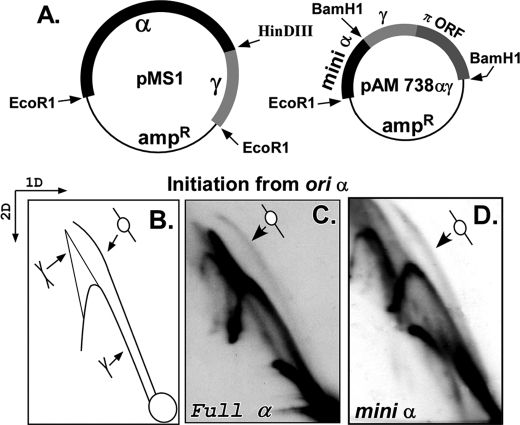
The genetic evidence presented above suggested that α was active in maintaining the α-γ plasmid in ihfΔ cells but did not necessarily reveal whether initiation in the plasmid derivatives started at or near the genetically defined α sequences. The plasmid maintenance data (Fig. 2) also did not reveal whether DNA looping to α was helping ori γ to be activated in an ihfΔ host or the converse. We therefore wished to seek more direct evidence to determine whether replication bubbles were actually forming in the restriction fragment that contained the genetic determinants of ori α. We approached this question by performing two-dimensional neutral-neutral agarose gel electrophoresis of plasmid replication intermediates (24) of α-γ (pMS1) or mini α-γ replicon (pAM738αγ) (from IHF+ cells). The full α-γ plasmid was cut with EcoR1 and HindIII and that of the mini α-γ with BamH1 to physically separate the fragment containing α sequences from that containing γ (Figs. 4A and 1, A and B). Furthermore, by selecting these restriction sites, the γ fragment was made longer than the α fragment, the result of which the replication intermediates from α and γ were clearly resolved in two-dimensional gels. The gel slabs were blotted onto nitrocellulose membranes and hybridized with a labeled α-specific probe (or a γ-specific probe that was used to look separately at initiation from γ). The expected pattern of the intermediates is shown schematically in Fig. 4B. If initiation had occurred exclusively within the α or mini α sequences, one would have expected to observe a bubble, Y, and double Y arcs in blots probed with the α-specific probe. If all initiations, on the other hand had occurred within ori γ and none at α, then forks would have entered the α fragment from outside, thereby passively replicating it to generate only Y and double Y but no bubble arcs. The gel patterns were unequivocal and showed that both the complete α-γ (Fig. 4C) and the mini α-γ replicons generated bubble arcs in addition to more intense Y arcs and some double Y arcs (Fig. 4D). The results were consistent with the early EM data that had localized an initiation bubble within approximately the same restriction fragment (7, 35, 39). Similar experiments were performed with plasmid replication intermediates isolated from the ihfΔ strain with almost identical results (not shown). The data are consistent with the interpretation that although an ori located outside the α region, probably γ, could have accounted for some of the Y arc, it was unlikely to account for all of it. Because of the relatively small size of the restriction fragment containing α (660 bp), many of the replication bubbles must have replicated beyond the end of the linear DNA fragment, thereby getting rapidly converted into Y-shaped intermediates. This interpretation was not inconsistent with data generated by separately studying replication of the fragment containing ori γ using a labeled γ-specific probe. The gel patterns of γ were similar to that of α and also revealed a fainter bubble arc and a stronger Y arc (not shown). In contrast, our earlier work in which fork movement initiated from γ was arrested by using replication termini placed close to and on either side of the γ origin had revealed robust bubble arcs and very faint Y arcs (38). Taken together, the data support the interpretation that many if not all of the Y arc in the ori α samples were generated by forks crossing the restriction sites used to linearize the DNA and separate the two origins.
FIGURE 4.
Replication of α-γ and mini α-γ plasmids analyzed by Brewer-Fangman two-dimensional gel electrophoresis. A, schematic diagrams of the α-γ and the mini α-γ templates plasmids used. B, schematic representation of the expected two-dimensional gel pattern after probing with an α-specific labeled DNA probe of gels blotted onto nitrocellulose membranes showing the bubble, the Y, and the double Y arcs. C, replication intermediates of full-length α-γ plasmid showing a fainter bubble arc and strong Y and double Y arcs. D, replication intermediates of the mini α-γ plasmid showing a pattern very similar to that in C.
Mutations at γ that did not affect π binding to the 7 iterons still abolished initiation from α. Previous work has shown that only mutations in the γ iterons or in the DNA-binding domains of π protein, abolished π-iteron interaction (40). The following experiments were performed to distinguish between two alternative possibilities. First, π mediated α-γ looping is a necessary and sufficient contribution by γ for induction of initiation at α. An alternative model proposes that delivery of π by DNA looping to α was necessary but not a sufficient contribution and that the latter also delivered a protein-DNA complex that probably included DnaA and a functional ori γ sequence. Ori α and γ but not β are known to be strictly DnaA-dependent (12, 41). If the first model were correct, only mutations in the γ iterons, which abolished π binding or otherwise interfered with DNA looping, would have abolished α activation. The second model predicts that other mutations in γ such as those that abolished DnaA binding to γ or altered the spacing between the dnaA site(s) and the iterons or altered the AT-rich region could also abolish α activation, even if π-mediated γ-α looping was left unperturbed. Besides π, two other proteins are known to interact with γ, namely DnaA (41) and IHF (15, 30). But IHF is not required for initiation from α, as previously noted. We proceeded to mutate the γ ori and study its effect on α utilization in vivo as described below.
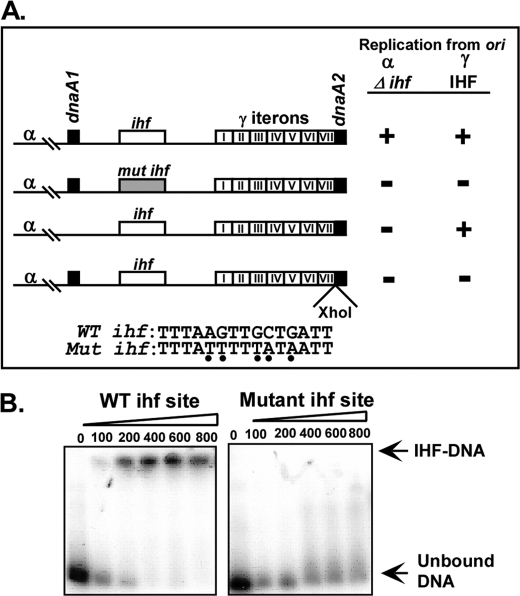
The wt γ sequence is shown in Fig. 5A, row 1. We introduced multiple mutations in and around the ihf site that converted several G/C sequences to A/T; this mutated form abolished both α- and γ-mediated replication (Fig. 5A, row 2). The mutated site no longer bound specifically to IHF protein as contrasted with the wt site (Fig. 5B). Because, ori α can support plasmid propagation in the absence of IHF protein, we interpret the results to mean that multiple mutations in the AT-rich region of γ were detrimental to α function because of structural alterations in the ori γ sequence rather than failure of IHF to bind to the mutated site.
FIGURE 5.
Mutants of ori γ sequences that impact ori α-mediated plasmid maintenance. A, diagram showing the normal and the mutated γ sequences. (+) means at least 500 colonies of IHF+ and/or ihfΔ cells carrying the plasmid derivatives plated on 40 μg/ml amp plates were recorded. (−) means that the ihfΔ host carrying the various plasmid derivatives formed less than 1% of the colonies formed by the IHF+ cells carrying the same plasmid derivative under identical conditions. Data are averages of three independent sets of experiments. The wt and the mutant form of the AT-rich region with the embedded ihf site are shown; the Xho1 insertion precisely separated the adjacent 7th iteron and the DnaA2 box. B, gel mobility shift experiments performed with 20 fmol of labeled ori γ probe/reaction and containing the wt or the mutated ihf site and incubated with the indicated amounts (fmol) of purified IHF. The mutant form of the ihf site failed to bind to the protein as contrasted with the normal ihf site.
We deleted the dnaA1 box from γ and monitored α-specific plasmid propagation in an ihfΔ host on by plating the cells on LB plates containing 50 or 100 μg/ml ampicillin. The data showed that dnaA1 was absolutely necessary for α-directed plasmid maintenance. In contrast, deletion of dnaA1 did not abolish γ replication (Fig. 5A, row 3). Insertion of an XhoI site between the 7th iteron and dnaA2 box that interrupted the contiguity of the two sites also abolished plasmid maintenance driven both a γ and an α origin (Fig. 5A, row 4). In Fig. 5A, a plus sign means that the colony counts of IHF+ cells were greater than 500 and minus means that less than 1–5% of the colonies were formed by the corresponding ihfΔ cells containing the same plasmid under identical conditions of growth.
In summary, the present work for the first time has unambiguously identified sequence elements that were necessary for promoting ori α activity at a distance and under conditions that reduced or eliminated plasmid maintenance by γ. The critical α elements were identified to be the βHR region, the single iteron, the G site and the sequences inbetween the G site and the α iteron. This latter sequence could be a part of the extended G site (31). The sequences located 125 bp immediately downstream of the G site also reduced the efficiency of α-directed plasmid maintenance. A similar analysis of the γ region, that was necessary for activating α, revealed that both dnaA boxes, the AT-rich region, and the 7 iterons were critical elements.
DISCUSSION
The data presented in this report suggest that a complex of plasmid-encoded π and host-encoded DnaA proteins formed at the ori γ sequence and probably was delivered to α by a transient π-mediated α-γ iteron contact. This interaction was necessary to impart biological activity to ori α at a distance. The R6K system provides an interesting variation on the theme of origin recognition. Past work on replication SS phages revealed that origin recognition in SS to DS replication in 3 phage systems required different mechanisms such as recognition of a hairpin sequence of M13 by RNA polymerase to generate a primer that was extended by DNA polymerase III holoenzyme in the presence of SSB; an origin-specific “nickase” nicked the viral DNA strand to induce DS to DS rolling circle replication. In the phage G4, the origin recognition is promoted by a G site where as in the ϕX174 system it was a more complex multiprotein mobile primosome that carried out SS to DS replication. The DS to DS replication was initiated by an origin-specific nickase (32). It is interesting that in the region of ori α, origin-specific initiation appears to require a specific primase recognition site and primase, the iteron and its cognate initiator protein π, and the host-encoded initiator protein DnaA to initiate replication.
Does π play any role in the recruitment of the primase to the G site? Our previous work has shown that DnaG primase physically interacts with π (42). However, the biological implication of this interaction has not yet been elucidated. It would be interesting to isolate point mutants of π and possibly of DnaG that would abolish this binary interaction and to study its impact on plasmid replication in vivo and in vitro. It is tempting to postulate that ori α-specific initiation of replication might be compromised in such mutants, while these might retain the ability to support replication from γ. Such mutations that specifically disrupt binary protein-protein interactions could be selected by employing a reverse 2-hybrid technique (43, 44).
Why did the Not1 mutant of the G site promote SS to DS phage replication but not DS plasmid maintenance? We suspect that the mutation might have reduced the affinity of the G site for DnaG, which is known to be present in limiting amounts in vivo. This could account for its inability to support DS plasmid replication in vivo where both the bacterial chromosome (synthesis of Okazaki pieces) and the plasmids are competing for a limited pool of DnaG. In contrast, the in vitro SS to DS replication was carried out with an excess of DnaG where it was not likely to have been a limiting factor. Alternatively, the Not1 mutation could have interfered with protein-protein interaction between the primase-G site complex and some other replication protein such as π, DnaA, or DnaB.
It is not known whether DNA looping leads to initiator-mediated DNA melting at α and whether the origin melting, as in the case of ori γ, also requires π-DnaA interaction (41, 45). It would be interesting to address this and other questions in the future by stepwise partial reconstitution experiments to resolve the preinitiation events and their requirements at this structurally atypical origin, from the subsequent steps of the reaction (38, 46, 47). Does DNA looping-dependent replication control also occur in eukaryotes? In multi-origin eukaryotic chromosomes, DNA replication appears to be occurring in discreet chromatin domains (48). There are groups of origins that initiate early and those that initiate late in the cell cycle, and the replicating chromatin regions are not randomly distributed in the nucleus but occur in localized replication zones (49). It has been suggested that multiple early replicating eukaryotic replication origins are likely to be located together in common replication factories (50). It would be surprising if the process of assembling multiple distantly located origins in the same chromosome or on different chromosomes at common replication centers did not involve long range looping interactions and/or interchromosomal site-site contacts that could perhaps be detected by chromosome conformation capture techniques (51).
Acknowledgments
We thank members of our laboratory, Drs. S. Zzaman, S. K. Singh, B. K. Mohanty for useful discussions and for help with the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1GM049264 from the NIAID (to D. B.).
- βHR
- β-homology region
- wt
- wild type
- ORF
- open reading frame
- IHF
- integration host factor
- SSB
- single-stranded DNA-binding protein
- SS
- single-stranded
- DS
- double-stranded.
REFERENCES
- 1.Ptashne M. (2004) Genetic Switch, 3rd Ed., Cold Spring Harbor Press, Cold Spring Harbor, NY [Google Scholar]
- 2.Ma J., Ptashne M. (1988) Cell 55, 443–446 [DOI] [PubMed] [Google Scholar]
- 3.Lobell R. B., Schleif R. F. (1991) J. Mol. Biol. 218, 45–54 [DOI] [PubMed] [Google Scholar]
- 4.Schleif R. (1992) Annu. Rev. Biochem. 61, 199–223 [DOI] [PubMed] [Google Scholar]
- 5.Tolhuis B., Palstra R. J., Splinter E., Grosveld F., de Laat W. (2002) Mol. Cell 20, 1453–1465 [DOI] [PubMed] [Google Scholar]
- 6.Jhunjhunwala S., van Zelm M. C., Peak M. M., Cutchin S., Riblet R., van Dongen J. J., Grosveld F. G., Knoch T. A., Murre C. (2008) Cell 133, 265–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crosa J. H., Luttropp L. K., Falkow S. (1978) J. Mol. Biol. 124, 443–468 [DOI] [PubMed] [Google Scholar]
- 8.Miron A., Mukherjee S., Bastia D. (1992) EMBO J. 11, 1205–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masai H., Nomura N., Kubota Y., Arai K. (1990) J. Biol. Chem. 265, 15124–15133 [PubMed] [Google Scholar]
- 10.Kubota Y., Arai K., Masai H. (1993) Gene 126, 9–16 [DOI] [PubMed] [Google Scholar]
- 11.Kelley W. L., Bastia D. (1992) New Biol. 4, 569–580 [PubMed] [Google Scholar]
- 12.Kelley W. L., Patel I., Bastia D. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 5078–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolter R., Helinski D. R. (1978) Plasmid 1, 571–580 [DOI] [PubMed] [Google Scholar]
- 14.Germino J., Bastia D. (1983) Cell 34, 125–134 [DOI] [PubMed] [Google Scholar]
- 15.Filutowicz M., Appelt K. (1988) Nucleic Acids Res. 16, 3829–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacAllister T. W., Kelley W. L., Miron A., Stenzel T. T., Bastia D. (1991) J. Biol. Chem. 266, 16056–16062 [PubMed] [Google Scholar]
- 17.Shafferman A., Flashner Y., Hertman I., Lion M. (1987) Mol. Gen. Genet. 208, 263–270 [DOI] [PubMed] [Google Scholar]
- 18.Flashner Y., Schlomai J., Shafferman A. (1996) Mol. Microbiol. 19, 985–996 [DOI] [PubMed] [Google Scholar]
- 19.Flashner Y., Shafferman A. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 9123–9127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Núñez B., Avila P., de la Cruz F. (1997) Mol. Microbiol. 24, 1157–1168 [DOI] [PubMed] [Google Scholar]
- 21.Avila P., Núñez B., de la Cruz F. (1996) J. Mol. Biol. 261, 135–143 [DOI] [PubMed] [Google Scholar]
- 22.Crosa J. H., Luttropp L. K., Falkow S. (1976) J. Bacteriol. 126, 454–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 24.Brewer B. J., Fangman W. L. (1987) Cell 51, 463–471 [DOI] [PubMed] [Google Scholar]
- 25.O'Donnell M., Studwell P. S. (1990) J. Biol. Chem. 265, 1179–1187 [PubMed] [Google Scholar]
- 26.Pritchard A. E., Dallmann H. G., McHenry C. S. (1996) J. Biol. Chem. 271, 10291–10298 [DOI] [PubMed] [Google Scholar]
- 27.Nash H. A., Robertson C. A., Flamm E., Weisberg R. A., Miller H. I. (1987) J. Bacteriol. 169, 4124–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loscha K., Oakley A. J., Bancia B., Schaeffer P. M., Prosselkov P., Otting G., Wilce M. C., Dixon N. E. (2004) Protein Exp. Purif. 33, 304–310 [DOI] [PubMed] [Google Scholar]
- 29.Stayton M. M., Kornberg A. (1983) J. Biol. Chem. 258, 13205–13212 [PubMed] [Google Scholar]
- 30.Kelley W. L., Bastia D. (1991) J. Biol. Chem. 266, 15924–15937 [PubMed] [Google Scholar]
- 31.Sims J., Benz E. W., Jr. (1980) Proc. Natl. Acad. Sci. U.S.A. 77, 900–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornberg A., Baker T. (1992) DNA Replication, W. H. Freeman and Co., San Francisco [Google Scholar]
- 33.Bouché J. P., Rowen L., Kornberg A. (1978) J. Biol. Chem. 253, 765–769 [PubMed] [Google Scholar]
- 34.Rowen L., Kornberg A. (1978) J. Biol. Chem. 253, 758–764 [PubMed] [Google Scholar]
- 35.Crosa J. H. (1980) J. Biol. Chem. 255, 11075–11077 [PubMed] [Google Scholar]
- 36.Lovett M. A., Sparks R. B., Helinski D. R. (1975) Proc. Natl. Acad. Sci. U.S.A. 72, 2905–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park K., Chattoraj D. K. (2001) J. Mol. Biol. 310, 69–81 [DOI] [PubMed] [Google Scholar]
- 38.Abhyankar M. M., Zzaman S., Bastia D. (2003) J. Biol. Chem. 278, 45476–45484 [DOI] [PubMed] [Google Scholar]
- 39.Inuzuka N., Inuzuka M., Helinski D. R. (1980) J. Biol. Chem. 255, 11071–11074 [PubMed] [Google Scholar]
- 40.Swan M. K., Bastia D., Davies C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 18481–18486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y. B., Datta H. J., Bastia D. (1998) EMBO J. 17, 5192–5200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abhyankar M. M., Reddy J. M., Sharma R., Büllesbach E., Bastia D. (2004) J. Biol. Chem. 279, 6711–6719 [DOI] [PubMed] [Google Scholar]
- 43.Mulugu S., Potnis A., Shamsuzzaman, Taylor J., Alexander K., Bastia D. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 9569–9574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma R., Kachroo A., Bastia D. (2001) EMBO J. 20, 4577–4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Datta H. J., Khatri G. S., Bastia D. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zzaman S., Abhyankar M. M., Bastia D. (2004) J. Biol. Chem. 279, 17404–17410 [DOI] [PubMed] [Google Scholar]
- 47.Kaguni J. M., Kornberg A. (1984) Cell 38, 183–190 [DOI] [PubMed] [Google Scholar]
- 48.de Laat W. (2007) Curr. Opin. Cell Biol. 19, 317–320 [DOI] [PubMed] [Google Scholar]
- 49.Vogelstein B., Pardoll D. M., Coffey D. S. (1980) Cell 22, 79–85 [DOI] [PubMed] [Google Scholar]
- 50.Göndör A., Ohlsson R. (2009) Nat. Rev. Genet. 10, 269–276 [DOI] [PubMed] [Google Scholar]
- 51.Simonis M., Kooren J., de Laat W. (2007) Nat. Methods 4, 895–901 [DOI] [PubMed] [Google Scholar]