Abstract
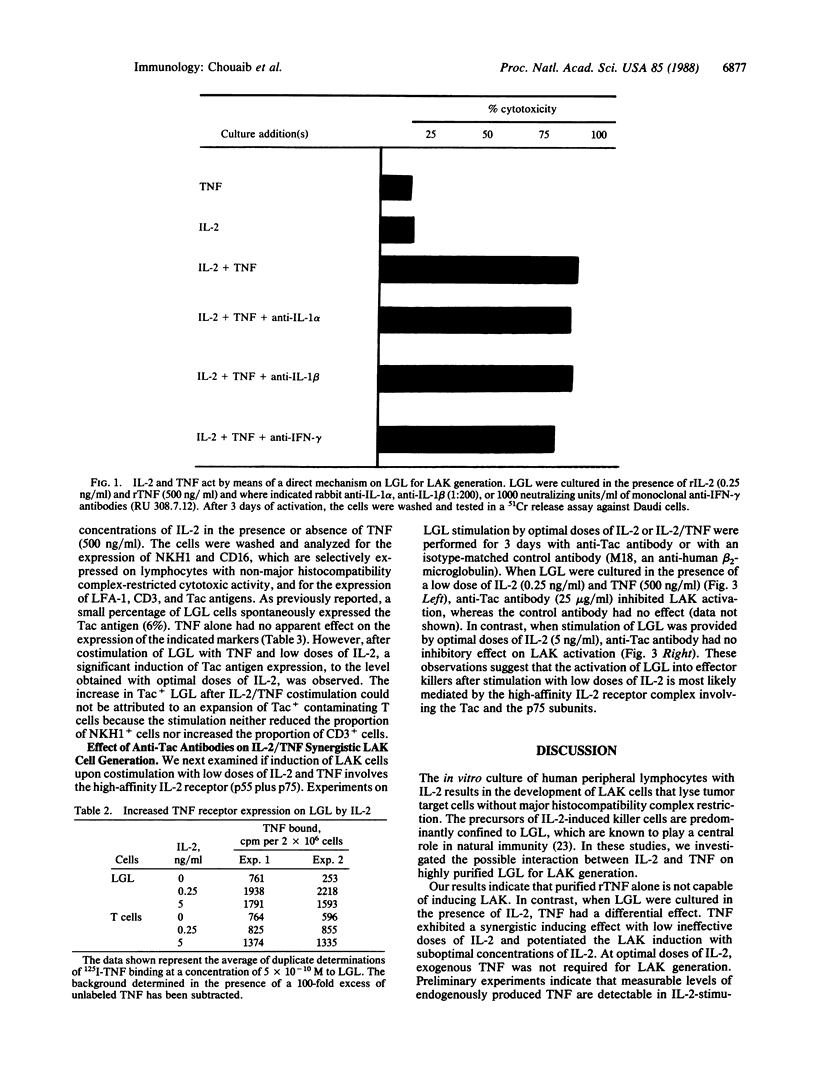
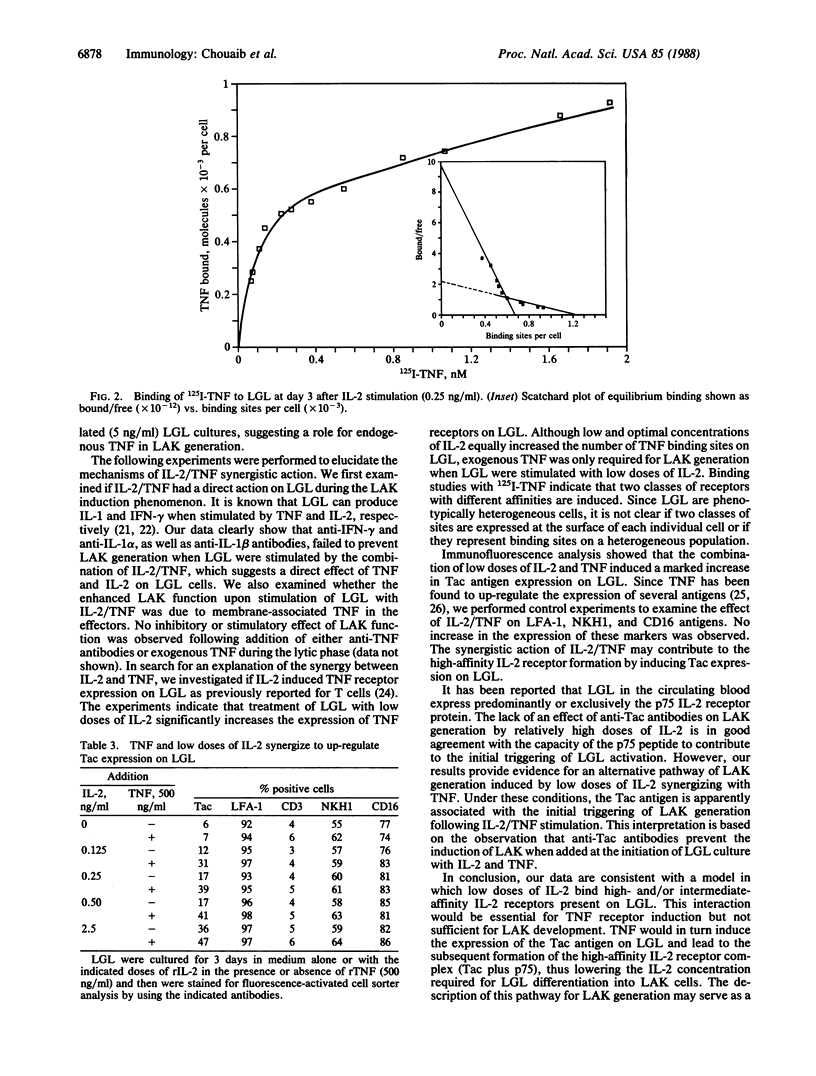
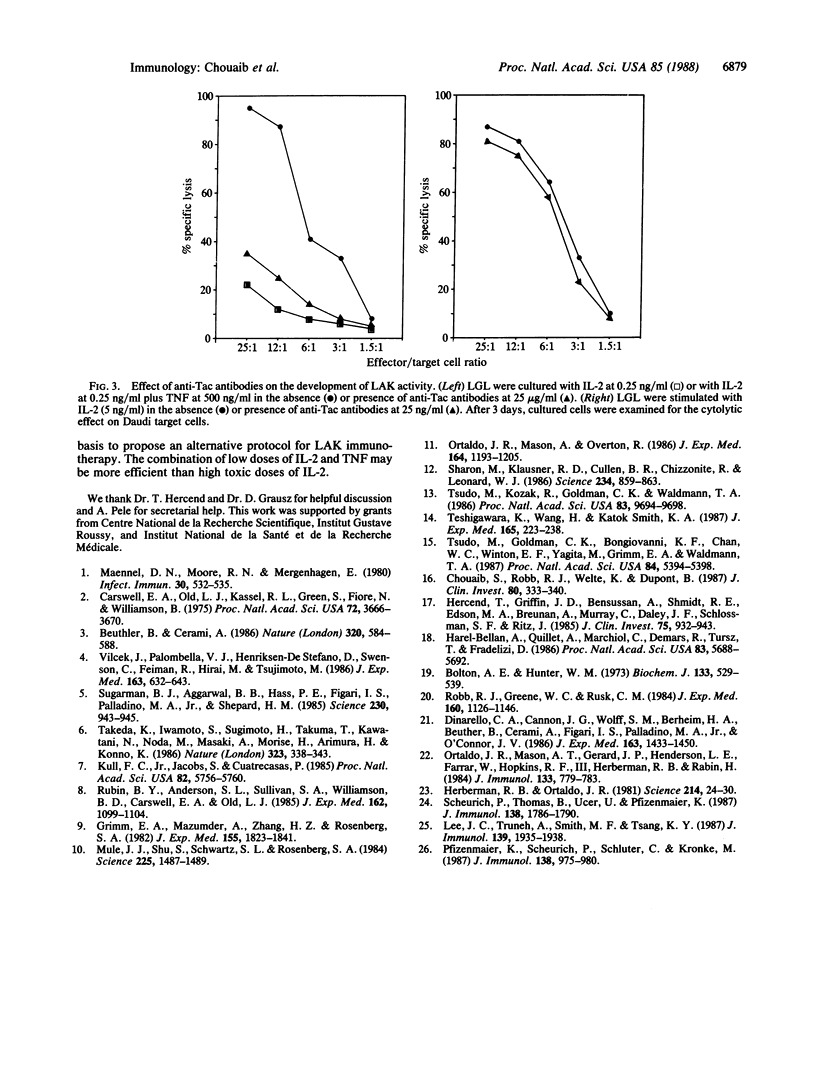
Large granular lymphocytes (LGL) can be activated by interleukin 2 (IL-2) to lymphokine-activated killers (LAK). The effect of tumor necrosis factor (TNF) on LAK generation was investigated. TNF was found to act synergistically with low concentrations of IL-2 (0.10-0.25 ng/ml), which were ineffective by themselves in inducing LAK activity, to promote the differentiation of LGL into non-major histocompatibility complex-restricted killers. When IL-2 was used at concentrations optimal for LAK generation, TNF did not further enhance this phenomenon. Specific binding of 125I-labeled TNF to LGL was increased by IL-2 stimulation. Scatchard analysis of TNF binding revealed the existence of two classes of binding sites with markedly different affinities (Kd values of 57 and 600 pM). We also demonstrated that the IL-2/TNF synergistic induction of LAK activity did not involve either IL-1 or interferon-gamma. This IL-2/TNF synergistic effect was blocked by anti-Tac antibodies. Immunofluorescence analysis revealed that IL-2/TNF selectively up-regulated Tac antigen expression on LAK precursors. Our results suggest a functional interaction between IL-2 and TNF on LAK precursors, which results in a reduction of the IL-2 concentration required for differentiation of LGL into LAK killers.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaib S., Robb R. J., Welte K., Dupont B. Analysis of prostaglandin E2 effect on T lymphocyte activation. Abrogation of prostaglandin E2 inhibitory effect by the tumor promotor 12.0 tetradecanoyl phorbol-13 acetate. J Clin Invest. 1987 Aug;80(2):333–340. doi: 10.1172/JCI113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel-Bellan A., Quillet A., Marchiol C., DeMars R., Tursz T., Fradelizi D. Natural killer susceptibility of human cells may be regulated by genes in the HLA region on chromosome 6. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5688–5692. doi: 10.1073/pnas.83.15.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Hercend T., Griffin J. D., Bensussan A., Schmidt R. E., Edson M. A., Brennan A., Murray C., Daley J. F., Schlossman S. F., Ritz J. Generation of monoclonal antibodies to a human natural killer clone. Characterization of two natural killer-associated antigens, NKH1A and NKH2, expressed on subsets of large granular lymphocytes. J Clin Invest. 1985 Mar;75(3):932–943. doi: 10.1172/JCI111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Cuatrecasas P. Cellular receptor for 125I-labeled tumor necrosis factor: specific binding, affinity labeling, and relationship to sensitivity. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5756–5760. doi: 10.1073/pnas.82.17.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Truneh A., Smith M. F., Jr, Tsang K. Y. Induction of interleukin 2 receptor (TAC) by tumor necrosis factor in YT cells. J Immunol. 1987 Sep 15;139(6):1935–1938. [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Schwarz S. L., Rosenberg S. A. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984 Sep 28;225(4669):1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A. T., Gerard J. P., Henderson L. E., Farrar W., Hopkins R. F., 3rd, Herberman R. B., Rabin H. Effects of natural and recombinant IL 2 on regulation of IFN gamma production and natural killer activity: lack of involvement of the Tac antigen for these immunoregulatory effects. J Immunol. 1984 Aug;133(2):779–783. [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A., Overton R. Lymphokine-activated killer cells. Analysis of progenitors and effectors. J Exp Med. 1986 Oct 1;164(4):1193–1205. doi: 10.1084/jem.164.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizenmaier K., Scheurich P., Schlüter C., Krönke M. Tumor necrosis factor enhances HLA-A,B,C and HLA-DR gene expression in human tumor cells. J Immunol. 1987 Feb 1;138(3):975–980. [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Sullivan S. A., Williamson B. D., Carswell E. A., Old L. J. High affinity binding of 125I-labeled human tumor necrosis factor (LuKII) to specific cell surface receptors. J Exp Med. 1985 Sep 1;162(3):1099–1104. doi: 10.1084/jem.162.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurich P., Thoma B., Ucer U., Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987 Mar 15;138(6):1786–1790. [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Takeda K., Iwamoto S., Sugimoto H., Takuma T., Kawatani N., Noda M., Masaki A., Morise H., Arimura H., Konno K. Identity of differentiation inducing factor and tumour necrosis factor. 1986 Sep 25-Oct 1Nature. 323(6086):338–340. doi: 10.1038/323338a0. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Goldman C. K., Bongiovanni K. F., Chan W. C., Winton E. F., Yagita M., Grimm E. A., Waldmann T. A. The p75 peptide is the receptor for interleukin 2 expressed on large granular lymphocytes and is responsible for the interleukin 2 activation of these cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5394–5398. doi: 10.1073/pnas.84.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]


