Abstract
The matricellular SPARC family member hevin (SPARC-like 1/SPARCL-1/SC1/Mast9) contributes to neural development and alters tumor progression in a range of mammalian models. The distribution of hevin in mouse tissues was reexamined with a novel monoclonal antibody that discriminates between hevin and its ortholog SPARC. We now report proteolysis of hevin in many tissues, with the most extensive processing in the brain. We demonstrate a cleavage site within the hevin sequence for the neural tissue proteinase ADAMTS4. Digestion of hevin by ADAMTS4 in vitro produced fragments similar to those present in brain lysates. Monoclonal antibodies revealed a SPARC-like fragment generated from hevin that was co-localized with ADAMTS4 in vivo. We show that proteolysis of hevin by ADAMTS4 in the mouse cerebellum is important for the normal development of this tissue. In conclusion, we have identified the fragmentation of hevin by ADAMTS4 in the mouse brain and propose that this specific proteolysis is integral to cell morphology and extracellular matrix deposition in the developing brain.
Keywords: Antibodies/Monoclonal, Cell/Secretion, Enzymes/Proteolytic, Extracellular Matrix, Neurobiology/Neuroscience, Organisms/Mouse, Tissue/Organ Systems/Brain
Introduction
The matricellular group of secreted proteins has been assigned myriad roles in organismal development and pathology (for review, see Ref. 1). SPARC2 (secreted protein, acidic and rich in cysteine), a prototypic matricellular protein, functions broadly in the assembly of extracellular matrix (ECM) and modulates cell cycle and cellular differentiation (for review, see Ref. 2). However, less is known about its family member hevin, discovered in tonsillar tissue of adult humans (3). Initial work in murine models showed significant transcription of hevin mRNA in a diverse group of tissues, with brain consistently exhibiting the highest levels among hevin-positive tissues in unchallenged mice. Subsequent studies revealed hevin protein within the central nervous system, most notably the Purkinje cell-associated Bergmann glial cells, as well as astrocytes within the white matter of the cerebellum and throughout the murine brain (4, 5). Recent work has shown increased expression of hevin in activated astrocytes during central nervous system stress and/or injury and in the excitatory synapses in induced-seizure models in rats (6–8).
The shared homology and distribution of SPARC and hevin have lent credence to the concept of compensatory functions for these two matricellular proteins. Although the proteins are significantly different in size, the functional domains of SPARC are highly conserved within the C-terminal region of hevin. SPARC has been shown to be the target of several proteases that liberate specific domains, such as the follistatin and EF-hand motifs, or peptides within these domains, with functions different from those of the parent protein. Because these peptides are present in vivo (9–11), it is not unexpected that proteolytic cleavage could act as a mechanism regulating the functions of SPARC and many other secreted, ECM-associated proteins (12). It is, therefore, likely that proteolysis of hevin would affect both its function and tissue distribution.
ADAMTS4 (a disintegrin and metalloproteinase with thrombospondin motifs) cleaves the proteoglycans aggrecan and brevican (13). ADAMTS4 activity has been correlated with arthritis and the remodeling of cartilage, of which aggrecan is a major component, and with glioma in the brain, a tissue in which cleavage of brevican has been demonstrated (14–17). The deposition and breakdown of proteoglycans is important not only during neural development but also in central nervous system injury and neurodegenerative disease (for review, see Ref. 18). Recent studies have revealed additional substrates for ADAMTS4 and have thereby broadened the potential role of this protease in the brain.
Herein we demonstrate the cleavage of hevin by ADAMTS4 in vivo and in vitro. This proteolysis liberates a hevin peptide, termed SPARC-like fragment (SLF), with significant homology to SPARC. Moreover, the cleavage is tissue-dependent, with predominance in the mouse brain. A monoclonal antibody was developed that selectively detects the ADAMTS4-derived peptide and differentiates it from full-length hevin as well as SPARC and its related peptides. The lack of hevin expression and hevin cleavage, as seen in hevin-null and ADAMTS4-null mice, respectively, results in altered Purkinje-cell morphology and a reactive gliosis phenotype in the brain of these adult animals.
EXPERIMENTAL PROCEDURES
Antibodies
For immunoblotting and immunostaining procedures, the following antibodies (IgG) were used: polyclonal goat anti-hevin (specific for murine hevin) (R&D Systems, Minneapolis, MN), polyclonal rabbit anti-calbindin (Cell Signaling Technology, Danvers, MA), polyclonal goat anti-ADAMTS4 (Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal rabbit anti-glial fibrillary acidic protein (GFAP) (Lab Vision, Fremont, CA), monoclonal mouse anti-FLAG M2 (Sigma), normal IgG isotype controls (goat, rabbit, and mouse) (R&D Systems), monoclonal rat antibodies 12-155 and 12-54 against human and murine hevin, and a polyclonal rabbit antibody from our laboratory that was produced against murine hevin, affinity-purified against its immunogen, and cleared of SPARC-reactive species by a pass through SPARC-conjugated resin.
Cell Culture and Transfection
The expression vector for murine hevin with a C-terminal His6 tag was kindly provided by Dr. Cagla Eroglu (Duke University, Durham, NC). The expression vector for human ADAMTS4 with a C-terminal FLAG epitope (19) was the generous gift of Dr. Duanqing Pei (University of Minnesota, Minneapolis, MN). HEK 293 cells were transfected with Lipofectamine 2000 (Invitrogen) to express hevin, ADAMTS4, or both. The morning after transfection, the cells were rinsed with Dulbecco's modified Eagle's medium (DMEM) and subsequently grown for 3 days in DMEM with 1 mm sodium pyruvate. Conditioned media were clarified, treated with protease inhibitor mixture (Thermo Scientific, Rockford, IL), and analyzed by immunoblot. Hevin and its breakdown products were detected with polyclonal goat anti-hevin IgG followed by horseradish peroxidase-conjugated donkey anti-goat IgG (Jackson ImmunoResearch, West Grove, PA) and Immobilon substrate (Millipore Corp., Billerica, MA). The presence of ADAMTS4 was confirmed by an immunoblot of cell lysates. Detection with monoclonal antibody M2 anti-FLAG was followed by horseradish peroxidase-conjugated goat anti-mouse IgG (Thermo Scientific) and Immobilon substrate.
Identification of ADAMTS4 as a Potential Interacting Partner with Hevin
A random library of phage-displayed CX7C peptides (where C is cysteine, and X is any amino acid) was screened for binding to recombinant murine hevin, and its homolog SPARC was coated on 96-well plates. Negative controls were bovine serum albumin and collagen I. Phage that initially bound to hevin were selected, amplified, and subjected to two further rounds of binding, selection, and amplification. Phage clone #65, displaying the sequence CAGTVSLRC, bound to hevin and to SPARC but not to the controls (supplemental Fig. 1A). A search by NCBI BLAST identified a number of proteins containing sequences of significant similarity to CAGTVSLRC, including the ADAMTS4 protein, which contains the sequence GAVSLR within its spacer region (supplemental Fig. 1B).
Prediction of Proteolytic Targets of ADAMTS4
The ScanProsite motif program (20), utilizing a previously described ADAMTS4 cleavage motif, E(A/F/V/L/M/Y)(X)(2/3)(S/T)(V/Y/ I/F/W/M/L/A) (21), identified conserved patterns in both the mouse (Swiss-Prot sequence P70663) and human (Swiss-Prot sequence Q14515) sequences of hevin.
Immunoblot
Immunoblot procedures were followed as described previously (22) with some minor modifications. Briefly, mouse tissues were lysed with M-PER lysis reagent (Pierce) containing Halt protease inhibitor mixture (Pierce). Lysates and recombinant proteins were resolved by SDS-PAGE on 10% acrylamide gels. Proteins were transferred to polyvinylidene difluoride membranes (Millipore). Nonspecific binding was blocked by incubation for 30–60 min at room temperature in AquaBlock (EastCoast Bio, North Berwick, ME) diluted 1:1 with PBS containing 0.2% Tween 20. Membranes were incubated for 20 h at 4 °C in polyclonal rabbit anti-hevin IgG (1 μg/ml), rat monoclonal antibody 12-155 (1 μg/ml), or rat monoclonal antibody 12-54 (1 μg/ml). Blots were washed 3 × 5 min in PBS containing 0.2% Tween 20 and incubated for 1 h in horseradish peroxidase-conjugated secondary antibody (donkey anti-rabbit IgG (Jackson ImmunoResearch) or goat anti-rat IgG (Pierce). Immobilon substrate was used for detection of antibody-antigen complexes.
Protein Production
The vectors for full-length murine hevin and for the C-terminal peptide (with CTNFQ at the N terminus), both with His6 at the C terminus, were generously provided by Dr. Cagla Eroglu. We modified the full-length hevin vector with the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) to express the SLF, beginning with YLKYG at the N terminus. PCR was performed with the following primers: 5′-CCACTGGTGACGCGTACCTCAAATATGGC-3′ and 5′-GCCATATTTGAGGTACGCGTCACCAGTGG-3′. This and subsequent steps of the mutagenesis were completed according to the manufacturer's protocol. The SLF insert was verified by DNA sequencing (DNA Sequencing Facility, University of Washington, Seattle, WA) and by restriction digestion of sequences that were expected to exist only in the full-length template and not in the modified vector. HEK 293 cells grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum were transfected with Lipofectamine 2000 (Invitrogen) to express hevin, the C-terminal peptide, or the SLF. Cells were rinsed the next day with PBS and were grown for two more days in serum-free media. Conditioned media were purified on a nickel-nitrilotriacetic acid-agarose (Invitrogen) affinity column.
Protease Digestion and Edman Sequencing of the SLF
Recombinant human ADAMTS4 (R&D Systems) (250 ng) and murine hevin (5 μg) were combined in PBS and incubated for 15 min, 1 h, 4 h, or overnight at 37 °C. Reactions were stopped by the addition of SDS-PAGE sample buffer containing 0.1 m dithiothreitol. Proteins were analyzed by SDS-PAGE followed by staining with Coomassie Blue.
Murine hevin produced in Sf9 cells (50 μg) was digested by ADAMTS4 (2.5 μg) according to the Cleavage Assay Protocol by R&D Systems. The SLF was isolated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and submitted to the Stanford Protein and Nucleic Acid Facility for Edman sequencing.
ELISA
We selected conditioned media from partially cloned hybridomas developed by Brekken et al. (23) that had previously been rejected for further cloning because of their reactivity with protein bands of a lower apparent Mr than that of hevin. We screened the selected conditioned media for binding to the SLF as follows; hevin and the SLF were coated onto a 96-well ELISA plate at 20 ng per well. After the wells were blocked, conditioned media were added at a dilution of 1:10 in PBS and were incubated for 1–2 h followed by washes, incubation with horseradish peroxidase-conjugated goat anti-rat IgG, additional washes, and detection by tetramethyl benzidine substrate (Thermo Scientific). Color development was stopped with 10% H3PO4. Monoclonal rat antibody 12-155 served as the negative control antibody because of its reaction with hevin but not with the SLF. The 12-54 conditioned medium was of special interest because of its capacity to detect hevin and possibly hevin fragments by immunohistochemistry. The conditioned medium was assayed by ELISA, as above, to demonstrate lack of binding to the C-terminal peptide of hevin.
The 12-54 clone was expanded for conditioned media production, and the monoclonal antibody (isotype IgG2a) was purified from the conditioned media by affinity chromatography on Protein G resin (EastCoast Bio). ELISAs similar to those described above were repeated with purified monoclonal antibodies at 1 μg/ml.
Immunohistochemistry
Brains from C57Bl6/J x 129/SVJ (24) 1-month-old mice and ADAMTS4-null (provided by Dr. Duanqing Pei) mice of the same genetic background and age were fixed in fresh methyl Carnoy's solution (60% methanol, 30% chloroform, and 10% glacial acetic acid) for 4 h at 4 °C and subsequently washed and stored in 70% EtOH at 4 °C. Brains were dehydrated in a series of ethanol solutions and embedded in paraffin. Sections were next deparaffinized and rehydrated. Antigen unmasking was performed by heating of the slides for 10 min in 10 mm citrate buffer (pH 6.0). Nonspecific binding sites of all sections were blocked with 20% Aquablock in PBS, Tween (0.2%) for 1 h. Sections were incubated with rabbit anti-calbindin IgG, goat anti-ADAMTS4 IgG, rat monoclonal antibody 12-155 (10 μg/ml), or rat monoclonal antibody 12-54 (10 μg/ml) for 15–18 h at 4 °C, washed in PBS, Tween, and exposed to goat anti-rabbit IgG conjugated to fluorescein isothiocyanate (FITC) or TRITC and/or goat anti-rat IgG conjugated to FITC or TRITC (Invitrogen) for 1 h. All co-staining antibodies described above were used at concentrations according to the manufacturers' protocols. Cell nuclei were stained with Hoechst 33258 fluorochrome (4 μg/ml; Invitrogen). Negative controls included substitution of primary antibody by non-immune rabbit and/or rat IgG. Routine sample analysis and identification of astrocytes/microglial cells were performed on serial sections of brain tissue with hematoxylin and eosin (H+E) stain. All images were compiled into figures in Adobe Illustrator (San Jose, CA).
Statistical Analysis
All densitometry readings are expressed as the means ± S.E. from a minimum of three independent experiments. Unpaired t tests were used to assess statistical significance between groups. p < 0.05 was considered statistically significant.
RESULTS
Processing of Hevin Protein Is Tissue-specific
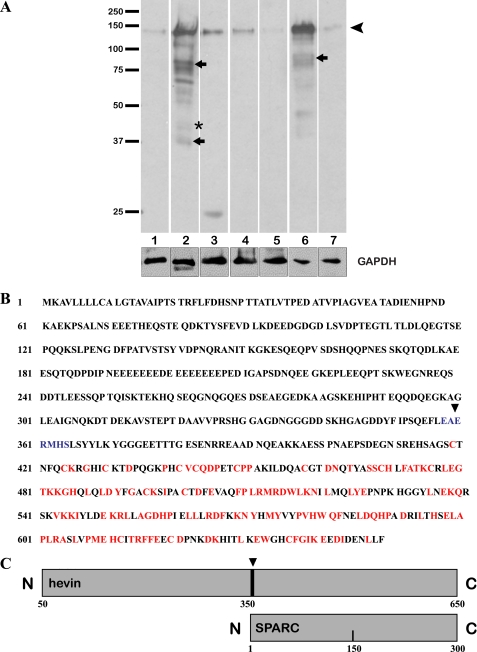
Whereas the distribution of hevin mRNA in mouse tissues has been reported, less is known regarding expression of the protein in these same tissues. Accordingly, immunoblots were performed against a range of mouse tissue lysates from the organs previously reported to express hevin mRNA. A rabbit polyclonal antibody against murine hevin minus the N-terminal signal sequence was used for immunodetection. The multiple epitopes identified from the use of a polyclonal antibody against the entire sequence allowed the detection of the full-length protein as well as hevin-derived peptides. As expected, these proteins were evident in all tissue lysates (Fig. 1A). In addition to the band representing full-length hevin (∼130 kDa) (Fig. 1A, arrowhead), there were several additional bands, most notably in lung and brain (Fig. 1A, arrows). Of particular interest were the smaller proteins/peptides of similar apparent Mr as that of the hevin homolog SPARC (∼43 kDa, asterisk). Our subsequent experiments were focused on brain tissue due to the abundance of hevin and its breakdown products in this organ (Fig. 1A, lane 2).
FIGURE 1.
Proteolysis of hevin in mouse tissues and predicted cleavage by ADAMTS4. A, protein from tissue lysates was resolved by SDS-PAGE, immunoblotted, and probed with anti-hevin antibodies. Equal amounts of total protein were added to each lane. Arrowhead, intact hevin; small arrows and asterisk, major cleavage products of hevin. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Lanes: 1, adrenal gland; 2, brain; 3, eye; 4, heart; 5, lacrimal gland; 6, lung; 7, pancreas. Results shown are from the same gel (equal exposure times) and are representative of at least three independent experiments. Molecular masses (in kDa) are indicated on the left. B, a single predicted cleavage site was identified in the sequence for ADAMTS4 (indicated by the arrowhead and blue coloring). Red indicates regions of homology between hevin and SPARC. C, diagram indicates the position of the cleavage site in hevin (arrowhead) relative to SPARC.
The Aggrecanase ADAMTS4 Cleaves Hevin
Previous work has shown that the hevin family member SPARC is cleaved by a number of proteases that release peptides exhibiting functions in some cases unique from those of the parent protein (9–11). From this observation it was hypothesized that hevin is also susceptible to proteolytic cleavage and harbors biologically active peptides. To determine binding partners and interacting proteins, we subjected both SPARC and hevin to phage display analysis (see Refs. 25 and 26 and the SPARC analysis detailed in Ref. 27). As shown in supplemental Fig. 1, the aggrecanase ADAMTS4 was identified as a potential protease targeting hevin as a substrate. The murine hevin sequence was scanned with an algorithm shown to identify potential cleavage sites for ADAMTS4 (21). The scan revealed a cut-site (EAERMHS) just N-terminal to the region of highest SPARC homology within hevin (Fig. 1B). Cleavage at this site would result in a C-terminal peptide (Fig. 1C) predicted to migrate on SDS-PAGE comparably to the SPARC-like band observed in the brain lysate (Fig. 1A, lane 2).
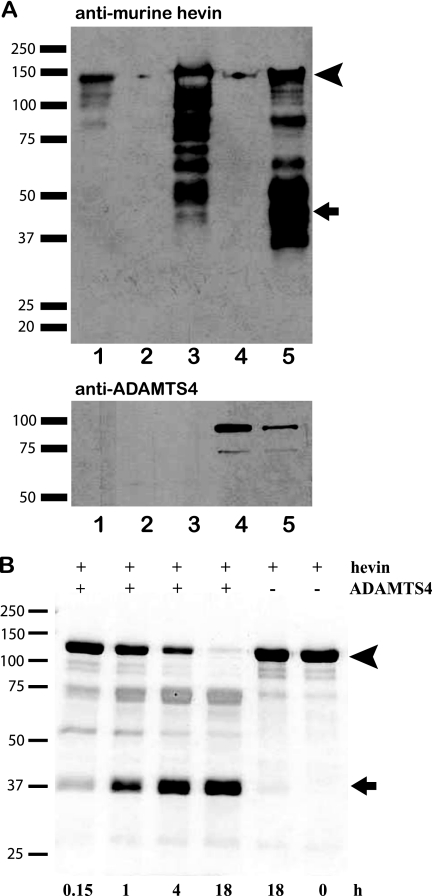
We used two approaches to ask whether hevin was a substrate for ADAMTS4. First, HEK 293 cells were transfected with a murine hevin expression vector with or without an ADAMTS4 expression vector. The resulting conditioned media were collected, and proteins were resolved by SDS-PAGE. The subsequent immunoblot revealed a significant increase in hevin processing coincident with the expression of ADAMTS4 (Fig. 2A). Second, murine hevin was incubated with purified ADAMTS4 at 37 °C to establish a time course for proteolysis (Fig. 2B). By 18 h, all of the full-length hevin (Fig. 2B, arrowhead) had been cleaved to an ∼Mr 40,000 fragment (Fig. 2B, arrow). Subsequent N-terminal sequencing of this smaller band revealed the cleavage site to be as predicted by the ADAMTS4 algorithm (Fig. 1B, arrowhead).
FIGURE 2.
Cleavage of hevin in vitro by ADAMTS4. Hevin and ADAMTS4 were co-expressed by transfection of HEK 293 cells (A), or the recombinant proteins were incubated together for the time intervals indicated (B). A, shown is an immunoblot of conditioned media from single- or co-transfected HEK 293 cells probed with polyclonal rabbit anti-hevin or anti-ADAMTS4 IgG on two separate gels run in parallel. Lanes: 1, recombinant hevin; 2, mock-transfected conditioned media; 3, hevin-transfected conditioned media; 4, ADAMTS4-transfected conditioned media; 5, hevin/ADAMTS4 co-transfected conditioned media. B, a 10% SDS-polyacrylamide gel stained with Coomassie Blue shows proteolysis of hevin by ADAMTS4 as a function of time. Arrowheads (A and B) indicate intact hevin. Arrows (A and B) indicate major peptides corresponding in size to the C-terminal peptide predicted by the ADAMTS4 cleavage site in Fig. 1. Molecular masses (in kDa) are indicated on the left.
Monoclonal Antibodies Identify Hevin Fragments
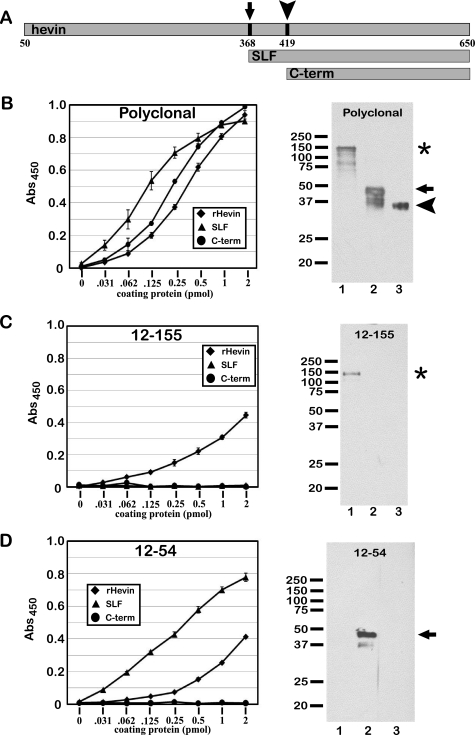
The identification of a SLF produced from hevin necessitated the generation of specific reagents that could differentiate between full-length hevin and hevin peptides. Our laboratory had produced several monoclonal anti-hevin antibodies that have been used extensively for detection of full-length hevin (but not SPARC) in a variety of experimental models (24, 28). This collection of monoclonal hybridomas was used in a series of screening experiments to identify individual clones reactive with the hevin peptides described above. We developed an expression vector to produce a recombinant SLF in vitro and performed ELISAs to screen for monoclonal antibodies with which to identify the fragment. We identified one, 12-54, which reacts with the SLF (and less so with full-length hevin) but not with the C-terminal peptide, a fragment lacking the 51 residues at the N terminus of the SLF (Fig. 3A). We demonstrated that the 12-54 monoclonal antibody detects the SLF (but not the C-terminal peptide) by immunoblot, whereas 12-155 detects only the full-length hevin (Fig. 3, panels B–D). These results indicate that 1) the epitope for 12-155 resides in the region of hevin N-terminal to the SLF, and 2) the 51 residues at the N terminus of the SLF (residues 368–419 of full-length hevin) contain the epitope for 12-54. As a positive control, hevin, the SLF, and the C-terminal peptide were detected in each assay by polyclonal rabbit anti-hevin IgG. These data are shown in Fig. 3.
FIGURE 3.
Monoclonal antibodies identify hevin fragments. Stocks of hybridoma-conditioned media were used to identify clones that react with full-length hevin and hevin-derived peptides. ELISA and immunoblotting were performed with full-length hevin, a recombinant SLF mimicking a major fragment from proteolysis, and a recombinant C-terminal peptide (C-term). Polyclonal goat anti-hevin IgG and the previously described rat monoclonal antibody 12-155 were used as positive and negative controls, respectively. A, shown are sizes and N termini of the SLF (arrow) and C-terminal peptide (arrowhead) relative to full-length hevin. B, shown are ELISA and an immunoblot comparing reactivity of polyclonal goat anti-hevin IgG with recombinant hevin (rHevin) and hevin peptides. C, shown are ELISA and immunoblot comparing the reactivity of monoclonal rat antibody 12-155 with recombinant hevin and hevin peptides. D, shown are ELISA and an immunoblot comparing the reactivity of monoclonal rat antibody 12-54 with recombinant hevin and hevin peptides. Equivalent molar concentrations of proteins were added to each lane for SDS-PAGE and immunoblotting. Lanes 1 and the asterisk, full-length hevin; lanes 2 and arrows, SLF; lane 3 and arrowhead, C-terminal peptide. Abs, absorbance.
ADAMTS4 and Hevin Co-localize within the Mouse Brain
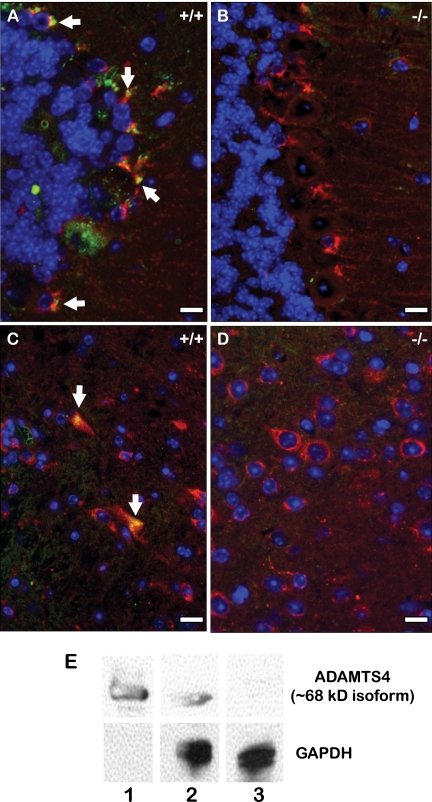
To determine whether ADAMTS4 and hevin proteins interact in vivo, we co-stained sections of cerebellum from wild-type (WT) and ADAMTS4-null mice with polyclonal anti-murine hevin (red) and anti-ADAMTS4 antibodies (green) (Fig. 4). The polyclonal anti-hevin antibody was used in this analysis to attain the most broad spectrum of reactivity with both full-length protein and hevin peptides. In WT tissue there was an apparent co-localization of hevin and ADAMTS4 in the Purkinje cell-associated Bergmann glial cells and in astrocytes located within the white matter (Fig. 4, panels A and C, yellow stain). The lack of ADAMTS4 expression in ADAMTS4-null tissue was verified by immunoblot with recombinant ADAMTS4 as a positive control (Fig. 4, panel E). For visual clarity, the individual and merged fluorescent images are shown in supplemental Fig. 2. This result was not unexpected because both of these cell types have been shown to express hevin (4–6); however, this is the first report showing that hevin is co-localized with ADAMTS4. In the ADAMTS4-null tissue, where lack of ADAMTS4 reactivity was expected, the specificity of the ADAMTS4 antibody within the WT Purkinje cell layer and white matter was verified (Fig. 4, panels B and D).
FIGURE 4.
ADAMTS4 appears coincident with hevin in the mouse brain. Immunohistochemical staining was performed on the Purkinje cell layer (A and B) and white matter (C and D) of serially sectioned WT (+/+) (A and C) and ADAMTS4-null (−/−) (B and D) paraffin-embedded mouse brain. Sections were co-stained with polyclonal rabbit anti-ADAMTS4 (green) and polyclonal goat anti-hevin antibodies (red). Yellow indicates coincidence of ADAMTS4 and hevin. E, immunoblotting of active ADAMTS4 (∼68 kDa) in lysates from WT and ADAMTS4-null cerebellum. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Lane 1, recombinant ADAMTS4; lane 2, WT brain lysate; lane 3, ADAMTS4-null brain lysate. Results shown are from the same gel (equal exposure times) and are representative of at least three independent experiments. Scale bar, 1 μm.
Brain-specific Proteolysis of Hevin Is ADAMTS4-dependent
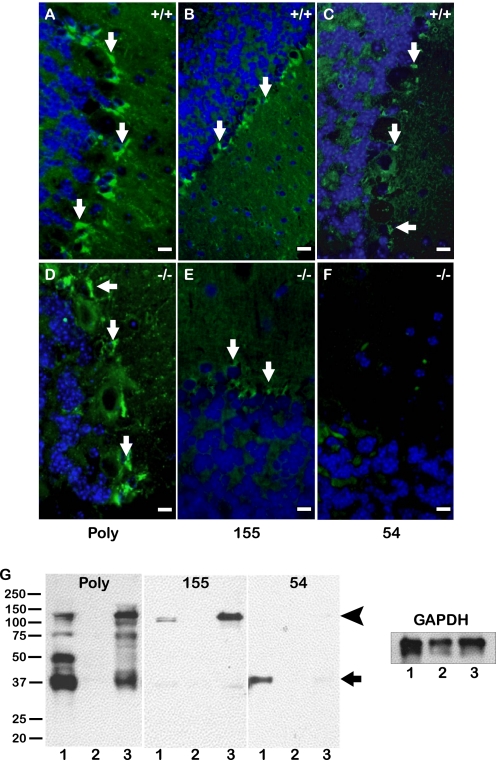
To determine whether the fragmentation of hevin in the mouse brain was due to the activity of ADAMTS4, we used the selected monoclonal antibodies to assess the presence of hevin peptides in ADAMTS4-null brain. A monoclonal IgG specific for full-length hevin (12-155) showed localization in the glia of both WT and ADAMTS4-null tissue (Fig. 5, panels B and E). In contrast, the monoclonal antibody specific for the SLF (12-54) revealed hevin peptides in WT tissue but not in ADAMTS4-null tissue, with some minimal background staining equivalent to that of the control IgG antibody (Fig. 5, panels C and F). Fig. 5G shows an immunoblot of WT, hevin-null, and ADAMTS4-null brain lysates probed with the polyclonal and monoclonal anti-hevin antibodies. As demonstrated by immunohistochemistry, hevin fragmentation is reduced in the ADAMTS4-null brain. This change is accompanied by a relative increase in full-length hevin found in these tissues. Hevin-null tissues were used as a negative control to demonstrate antibody specificity.
FIGURE 5.
Proteolysis of hevin in the brain is dependent on ADAMTS4. Immunohistochemical staining was performed on the Purkinje cell layer of serially sectioned WT (A–C) and ADAMTS4-null (D–F) mouse brain. Sections were stained with polyclonal goat anti-hevin IgG (Poly) (A and D), monoclonal rat antibody 12-155 (155) (B and E), or monoclonal rat antibody 12-54 (54) (C and F). Peptide-specific staining observed in WT brain (green) (C) is absent in ADAMTS4-null brain (F). Images are representative of staining patterns seen in three independent experiments. Scale bar, 10 μm. G, immunoblotting was performed on cerebellar lysates from WT, hevin-null, and ADAMTS4-null animals. Blots were probed with polyclonal goat anti-hevin IgG (Poly), monoclonal rat antibody 12-155 (155), or monoclonal rat antibody 12-54 (54). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Lanes: 1, WT brain; 2, hevin-null brain; 3, ADAMTS4-null brain. The arrowhead indicates intact hevin. The arrow indicates major peptides corresponding in size to the C-terminal peptide predicted by the ADAMTS4 cleavage site in Fig. 2. Molecular masses (in kDa) are indicated on the left.
Mice Lacking Expression or ADAMTS4-mediated Cleavage of Hevin Share Altered Purkinje Cell Phenotypes
Whereas no behavioral studies have been performed on hevin-null animals, ADAMTS4-null animals have been reported to exhibit reduced muscle memory and coordination. Additionally, these mice have an increased susceptibility to metrazol-induced seizures (Mouse Genomic Informatics accession ID: 3604450). These phenomena have been associated with a range of functions including astrocyte activation, synaptogenesis, and basic cerebellar function (29–31). Because astrocyte activation has been connected to the expression of hevin in the brain (4–6), it was of interest to compare the basic cellular morphology and astrocyte activation within the cerebellum and white matter of adult WT, hevin-null, and ADAMTS4-null mice. In addition, sections of the cerebellum of each genotype were stained with anti-calbindin IgG to examine changes in Purkinje cell-dendrite morphology in the presence or absence of hevin and hevin peptides (Fig. 6).
FIGURE 6.
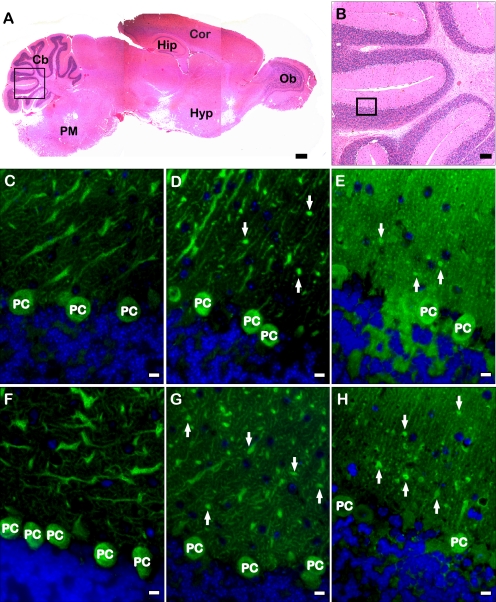
Absence of hevin fragments affects Purkinje cell morphology in the mouse cerebellum. A, H+E-stained mouse brain showing a representative section that was stained in experiments detailed below. The box highlights the image magnified in panel B. Ob, olfactory bulb; Hyp, hypothalamus; Cor, cortex; Hip, hippocampus; Cb, cerebellum; PM, pons-medulla. Scale bar, 240 μm. B, shown is a magnified image of a H+E section of mouse cerebellum. The box indicates a representative area of cerebellum stained for all subsequent analyses of all genotypes. Scale bar, 60 μm. Immunohistochemical staining was performed on the Purkinje cell layer of serially sectioned WT (C and F), hevin-null (D and G), and ADAMTS4-null (E and H) mouse brains. Sections were stained with anti-calbindin (green). Altered morphology of Purkinje cell dendritic processes (arrows) is apparent in hevin-null and ADAMTS4-null tissue. Images are representative of staining patterns seen in three independent experiments. PC, Purkinje cell. Scale bar, 1 μm.
WT cerebellum displayed normal Purkinje cell morphology, with an extensive branching of dendrites into the molecular layer of the cerebellum. In contrast, the hevin-null and ADAMTS4-null cerebella exhibited altered Purkinje cell morphology (Fig. 6, panels D and E and panels G and H). The dendritic processes extending from the Purkinje cell bodies into the molecular layer appeared stunted and displayed significantly less branching. These stunted dendritic processes, most evident after staining for calbindin, appeared to terminate in blunt formations, in sharp contrast to the finer dendritic branching seen in the WT tissue (Fig. 6, panels D and E and panels G and H, arrows). The null genotypes also showed an apparent decrease in synaptophysin staining along the Purkinje cell dendritic processes (data not shown).3
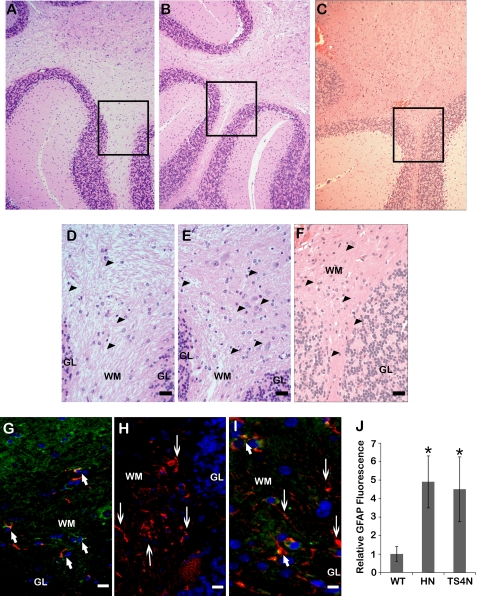
The glial cell population in the white matter of the three genotypes was also examined by H+E stain, which revealed an increased presence of glial cells, characterized by their smaller, dense nuclei within the white matter of both null tissues (Fig. 7, panels d–F). These cells were further defined by their staining with GFAP, with increased numbers of GFAP-positive cells in the tissue and potentially increased levels of GFAP in individual cells of the null tissues (Fig. 7, panels G–I). Quantification of total GFAP staining was also performed on these sections; hevin-null and ADAMTS4-null tissues showed a significant increase in total staining for this marker (Fig. 7, panel J).
FIGURE 7.
Decreased expression or cleavage of hevin promotes gliosis in the mouse cerebellum. Immunohistochemical and H+E staining were performed on serially sectioned WT (A, D, and G), hevin-null (B, E, and H), or ADAMTS4-null (C, F, and I) mouse cerebellum. Areas of higher magnification stained with H+E (D–F) are indicated by boxes in A–C. In D–F, astrocyte/microglial cells are indicated by arrowheads. For immunohistochemistry (G–I), sections were co-stained with polyclonal goat anti-hevin (green) and polyclonal rabbit anti-GFAP antibodies (red). Arrows indicate GFAP+/hevin+ cells (short arrows) and areas of increased GFAP without hevin expression (long arrows). Images are representative of staining patterns seen in three independent experiments. WM, white matter; GL, granular layer. Scale bar, 40 μm (A–C) and 10 μm (D–I). J, quantification of total GFAP staining from a minimum of 6 fields per genotype is shown. Increased GFAP signal is evident in the white matter of hevin-null (HN) and ADAMTS4-null (TS4N) tissue, relative to WT brain (*, p < 0.05).
DISCUSSION
The matricellular proteins hevin and SPARC share similar functions that include inhibition of adhesion, stimulation of cell migration, and increased expression after tissue injury or stress in vitro (1). Both proteins are expressed during development and tissue remodeling in adult animals (1). Although hevin and SPARC exhibit significant protein similarity, tissue distribution, and apparent function, considerably less is known about the mechanisms controlling the distribution of hevin and its role in vivo. The degradation of SPARC by proteases has been shown as a valid mechanism for the liberation of biologically active peptides from the parent sequence (for review, see Ref. 32). The data presented here indicate that a similar control mechanism exists for hevin that generates peptides which can act in a SPARC-like manner; these findings link the two proteins both structurally and functionally.
Previous work had shown widespread distribution of hevin mRNA in a range of fetal and adult tissues (33). After reagents that distinguished hevin from SPARC became available, it was evident that hevin protein was also present in most of these tissues (Fig. 1A). In addition to expression of the full-length protein, there was also apparent fragmentation of hevin in some tissues, particularly in mouse brain and lung, as indicated by immunoblots of lysates of these tissues. From a previous phage display analysis of hevin binding partners, ADAMTS4 was selected as a brain-specific protease with potential activity toward hevin. A cleavage site search revealed a site within hevin from which a peptide of apparent Mr ∼ 40,000 would be released; indeed, this peptide was observed upon immunoblotting. Glycosylation of SPARC and hevin proteins results in an increase in Mr by SDS-PAGE over the Mr predicted by amino acid composition; the same is likely to be the case for the SLF. Analysis of ADAMTS4 activity on hevin in vitro by co-transfection of HEK 293 cells or co-incubation of the purified proteins revealed a SLF produced from full-length hevin, with an almost complete loss of the parent protein within 18 h of incubation (Fig. 2). The identity of the resulting peptide of approximately the same Mr as SPARC was verified by N-terminal sequencing; moreover, the N-terminal residue of the peptide matched the cleavage location initially predicted by the site search of the hevin sequence.
Until now there has been no reagent with which to discriminate between hevin and hevin fragments by immunohistochemistry. A library of rat hybridomas produced against hevin, previously screened for clones recognizing full-length hevin, was rescreened to identify possible peptide-specific reagents. Several clones with reactivity to the SLF were identified and validated by ELISA and immunoblot analysis (data not shown). Of these clones, one (12-54) was chosen for its selective reactivity to the SLF over full-length hevin in ELISA and immunoblotting assays (Fig. 3). 12-54 was affinity-purified and used to identify the SLF in all subsequent immunohistochemistry and immunoblot experiments.
To verify an interaction between hevin and ADAMTS4 in the mouse brain, we performed immunohistochemistry and immunoblots on paraffin-embedded sections and on lysates of WT, hevin-null, and ADAMTS4-null brains, respectively (Figs. 4 and 5). The ADAMTS4-null tissue exhibited a significant decrease in the fragmentation of hevin within the brain relative to that observed in WT brain (Fig. 5, panels F and G). When they were used in combination on WT tissue, one monoclonal antibody specific for full-length hevin and another specific for the ADAMTS4-generated hevin fragment showed an increase in the SLF associated with a decrease in full-length hevin. In contrast, the full-length version composed the majority of hevin in ADAMTS4-null tissue. The use of hevin-null tissue confirmed the specificity of antibody reactivity in this immunoblot series.
With the apparent loss of hevin processing, ADAMTS4-null brain was compared with age-matched hevin-null and WT mouse brains to identify possible roles for hevin fragmentation in brain development and maintenance. Calbindin, commonly used as a marker for healthy Purkinje cells (34), is an intracellular calcium-binding protein involved in calcium regulation and calcium-dependent signaling in neurons. Diminished levels of calbindin are found in models of neurological disorders such as epilepsy and in neurodegenerative diseases (35, 36). By immunohistochemistry, it was apparent that Purkinje cell morphology was altered in the absence of hevin as well as in the absence of ADAMTS4-dependent hevin processing (Fig. 6).
Additionally, increased numbers of glial cells, indicated by their enhanced GFAP reactivity, were observed in the white matter of both null (hevin and ADAMTS4) brain tissues (Fig. 7). Increased expression of GFAP has been associated with an activated state of astrocytes and glial lineage cells. Termed “reactive gliosis” (for review, see Ref. 37), this condition develops upon central nervous system injury, during tumor progression, after seizure, and in a number of neurodegenerative pathologies (e.g. Alzheimer and Parkinson diseases) (for review, see Ref. 38). Examination of ADAMTS4-null animals revealed poor coordination and muscle memory, indicated by reduced aptitude on the rotorod test and an increased susceptibility to chemical-induced seizures (Mouse Genomic Informatics accession ID: 3604450). Hevin has been shown to play a role during ECM deposition, cell-ECM interaction, and wound healing (39, 40). Although the observations in hevin-null and ADAMTS4-null tissues closely resemble a condition of reactive gliosis, the contribution of various cells of the glial lineage (microglia and astrocytes) to the GFAP reactivity in these specific tissues remains unknown. It is possible that altered Purkinje cell morphology and the increased GFAP reactivity, resembling reactive gliosis, in hevin- and ADAMTS4-null tissue are responses to altered ECM deposition, initiated by the absence of hevin or hevin processing. Future experiments using established reactive gliosis models (e.g. seizure induction, central nervous system wounding, and neural lesions) in hevin-null and ADAMTS4-null animals should define a role for hevin and hevin peptides in this important neurological process.
In conclusion, we have shown that the matricellular protein hevin undergoes proteolytic cleavage in vivo. Within the brain this activity appears to be effected by the aggrecanase ADAMTS4. ADAMTS4-mediated cleavage results in a hevin peptide that is highly similar to SPARC in both size and sequence. The data presented here reveal this cleavage as a mechanism regulating the function of hevin in the mouse brain. ADAMTS4-null animals, which have full-length hevin, as indicated by staining and immunoblotting, present with altered Purkinje cell morphology and reactive gliosis that have also been described in the hevin-null animals. In addition to a functional mechanism, generation of a SLF in tissues could account for some of the overlapping biological roles hypothesized for hevin and SPARC. This functional integration has been most recently observed in the foreign body response (24) and during tumor cell growth/angiogenesis in hepatocellular carcinoma (41). In contrast, there is an inverse relationship in the expression of SPARC and hevin in the progression of pancreatic cancer (28). These seemingly conflicting results highlight the complex interrelationship between hevin and SPARC. Whereas the full-length proteins fulfill unique biological roles, their primary structures provide substrates for proteolytic generation of peptides that have similar, if not identical, functions in vivo. This wide range of functions attributed to hevin and SPARC, achieved in part by proteolytic processing, allows not only for separate roles but also for compensatory activity as required by specific tissue environments.
Acknowledgments
We thank the laboratory of Dr. Daunqing Pei for the generous contribution of the FLAG-tagged ADAMTS4 expression vector and ADAMTS4-null mouse tissue. We thank Dr. Cagla Eroglu for contributing the expression vectors for full-length hevin and the C-terminal peptide. We also thank Dr. Jing Nie for input regarding ADAMTS4 function and hevin proteolysis. Drs. Ben Barres and Cagla Eroglu provided valuable input and discussion.
This work was supported, in whole or in part, by National Institutes of Health Grants GM40711 and CA109559.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
H. Kucukdereli, N. J. Allen, A. Lee, C. Chakraborty, G. Workman, M. S. Weaver, E. H. Sage, B. A. Barres, and C. Eroglu, manuscript in preparation.
- SPARC
- secreted protein acidic and rich in cysteine
- SLF
- SPARC-like fragment of hevin
- ADAMTS4
- a disintegrin and metalloproteinase with thrombospondin motifs
- ECM
- extracellular matrix
- ELISA
- enzyme-linked immunosorbent assay
- GFAP
- glial fibrillary acidic protein
- H+E
- hematoxylin and eosin
- TRITC
- tetramethylrhodamine isothiocyanate
- WT
- wild type
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Bornstein P., Sage E. H. (2002) Curr. Opin. Cell Biol. 14, 608–616 [DOI] [PubMed] [Google Scholar]
- 2.Brekken R. A., Sage E. H. (2001) Matrix Biol. 19, 816–827 [DOI] [PubMed] [Google Scholar]
- 3.Girard J. P., Springer T. A. (1995) Immunity 2, 113–123 [DOI] [PubMed] [Google Scholar]
- 4.Lively S., Ringuette M. J., Brown I. R. (2007) Neurochem. Res. 32, 65–71 [DOI] [PubMed] [Google Scholar]
- 5.Lively S., Brown I. R. (2008) Neurochem. Res. 33, 1692–1700 [DOI] [PubMed] [Google Scholar]
- 6.McKinnon P. J., Margolskee R. F. (1996) Brain Res. 709, 27–36 [DOI] [PubMed] [Google Scholar]
- 7.Lively S., Brown I. R. (2008) J. Neurochem. 107, 1335–1346 [DOI] [PubMed] [Google Scholar]
- 8.Yin G. N., Lee H. W., Cho J. Y., Suk K. (2009) Brain Res. 1265, 158–170 [DOI] [PubMed] [Google Scholar]
- 9.Lane T. F., Iruela-Arispe M. L., Johnson R. S., Sage E. H. (1994) J. Cell Biol. 125, 929–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iruela-Arispe M. L., Lane T. F., Redmond D., Reilly M., Bolender R. P., Kavanagh T. J., Sage E. H. (1995) Mol. Biol. Cell 6, 327–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sage E. H., Reed M., Funk S. E., Truong T., Steadele M., Puolakkainen P., Maurice D. H., Bassuk J. A. (2003) J. Biol. Chem. 278, 37849–37857 [DOI] [PubMed] [Google Scholar]
- 12.Ehlen H. W., Sengle G., Klatt A. R., Talke A., Müller S., Paulsson M., Wagener R. (2009) J. Biol. Chem. 284, 21545–21556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao G., Westling J., Thompson V. P., Howell T. D., Gottschall P. E., Sandy J. D. (2002) J. Biol. Chem. 277, 11034–11041 [DOI] [PubMed] [Google Scholar]
- 14.Naito S., Shiomi T., Okada A., Kimura T., Chijiiwa M., Fujita Y., Yatabe T., Komiya K., Enomoto H., Fujikawa K., Okada Y. (2007) Pathol. Int. 57, 703–711 [DOI] [PubMed] [Google Scholar]
- 15.Held-Feindt J., Paredes E. B., Blömer U., Seidenbecher C., Stark A. M., Mehdorn H. M., Mentlein R. (2006) Int. J. Cancer 118, 55–61 [DOI] [PubMed] [Google Scholar]
- 16.Hu B., Kong L. L., Matthews R. T., Viapiano M. S. (2008) J. Biol. Chem. 283, 24848–24859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viapiano M. S., Hockfield S., Matthews R. T. (2008) J. Neurooncol. 88, 261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galtrey C. M., Fawcett J. W. (2007) Brain Res. Rev. 54, 1–18 [DOI] [PubMed] [Google Scholar]
- 19.Wang P., Tortorella M., England K., Malfait A. M., Thomas G., Arner E. C., Pei D. (2004) J. Biol. Chem. 279, 15434–15440 [DOI] [PubMed] [Google Scholar]
- 20.de Castro E., Sigrist C. J., Gattiker A., Bulliard V., Langendijk-Genevaux P. S., Gasteiger E., Bairoch A., Hulo N. (2006) Nucleic Acids Res. 34, W362–W365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hills R., Mazzarella R., Fok K., Liu M., Nemirovskiy O., Leone J., Zack M. D., Arner E. C., Viswanathan M., Abujoub A., Muruganandam A., Sexton D. J., Bassill G. J., Sato A. K., Malfait A. M., Tortorella M. D. (2007) J. Biol. Chem. 282, 11101–11109 [DOI] [PubMed] [Google Scholar]
- 22.Weaver M. S., Workman G., Sage E. H. (2008) J. Biol. Chem. 283, 22826–22837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brekken R. A., Sullivan M. M., Workman G., Bradshaw A. D., Carbon J., Siadak A., Murri C., Framson P. E., Sage E. H. (2004) J. Histochem. Cytochem. 52, 735–748 [DOI] [PubMed] [Google Scholar]
- 24.Barker T. H., Framson P., Puolakkainen P. A., Reed M., Funk S. E., Sage E. H. (2005) Am. J. Pathol. 166, 923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giordano R. J., Cardó-Vila M., Lahdenranta J., Pasqualini R., Arap W. (2001) Nat. Med. 7, 1249–1253 [DOI] [PubMed] [Google Scholar]
- 26.Kzhyshkowska J., Workman G., Cardó-Vila M., Arap W., Pasqualini R., Gratchev A., Krusell L., Goerdt S., Sage E. H. (2006) J. Immunol. 176, 5825–5832 [DOI] [PubMed] [Google Scholar]
- 27.Barker T. H., Baneyx G., Cardó-Vila M., Workman G. A., Weaver M., Menon P. M., Dedhar S., Rempel S. A., Arap W., Pasqualini R., Vogel V., Sage E. H. (2005) J. Biol. Chem. 280, 36483–36493 [DOI] [PubMed] [Google Scholar]
- 28.Esposito I., Kayed H., Keleg S., Giese T., Sage E. H., Schirmacher P., Friess H., Kleeff J. (2007) Neoplasia 9, 8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnewald U., Kondziella D. (2003) NMR Biomed. 16, 424–429 [DOI] [PubMed] [Google Scholar]
- 30.Hirai H., Launey T., Mikawa S., Torashima T., Yanagihara D., Kasaura T., Miyamoto A., Yuzaki M. (2003) Nat. Neurosci. 6, 869–876 [DOI] [PubMed] [Google Scholar]
- 31.Nguon K., Baxter M. G., Sajdel-Sulkowska E. M. (2005) Cerebellum 4, 112–122 [DOI] [PubMed] [Google Scholar]
- 32.Sage E. H. (1997) Trends Cell Biol. 7, 182–186 [DOI] [PubMed] [Google Scholar]
- 33.Soderling J. A, Reed M. J., Corsa A., Sage E. H. (1997) J. Histochem. Cytochem. 45, 823–835 [DOI] [PubMed] [Google Scholar]
- 34.Cheron G., Servais L., Dan B. (2008) Neuroscience 153, 1–19 [DOI] [PubMed] [Google Scholar]
- 35.Kang T. C., Park S. K., Hwang I. K., An S. J., Bahn J. H., Choi S. Y., Kim J. S., Won M. H. (2002) Neurochem. Int. 40, 115–122 [DOI] [PubMed] [Google Scholar]
- 36.Bastianelli E. (2003) Cerebellum 2, 242–262 [DOI] [PubMed] [Google Scholar]
- 37.Pekny M., Pekna M. (2004) J. Pathol. 204, 428–437 [DOI] [PubMed] [Google Scholar]
- 38.Pekny M., Wilhelmsson U., Bogestål Y. R., Pekna M. (2007) Int. Rev. Neurobiol. 82, 95–111 [DOI] [PubMed] [Google Scholar]
- 39.Sullivan M. M., Barker T. H., Funk S. E., Karchin A., Seo N. S., Höök M., Sanders J., Starcher B., Wight T. N., Puolakkainen P., Sage E. H. (2006) J. Biol. Chem. 281, 27621–27632 [DOI] [PubMed] [Google Scholar]
- 40.Sullivan M. M., Puolakkainen P. A., Barker T. H., Funk S. E., Sage E. H. (2008) Wound Repair Regen. 16, 310–319 [DOI] [PubMed] [Google Scholar]
- 41.Lau C. P., Poon R. T., Cheung S. T., Yu W. C., Fan S. T. (2006) J. Pathol. 210, 459–468 [DOI] [PubMed] [Google Scholar]