Abstract
The role of azadirachtin, an active component of a medicinal plant Neem (Azadirachta indica), on TNF-induced cell signaling in human cell lines was investigated. Azadirachtin blocks TNF-induced activation of nuclear factor κB (NF-κB) and also expression of NF-κB-dependent genes such as adhesion molecules and cyclooxygenase 2. Azadirachtin inhibits the inhibitory subunit of NF-κB (IκBα) phosphorylation and thereby its degradation and RelA (p65) nuclear translocation. It blocks IκBα kinase (IKK) activity ex vivo, but not in vitro. Surprisingly, azadirachtin blocks NF-κB DNA binding activity in transfected cells with TNF receptor-associated factor (TRAF)2, TNF receptor-associated death domain (TRADD), IKK, or p65, but not with TNFR, suggesting its effect is at the TNFR level. Azadirachtin blocks binding of TNF, but not IL-1, IL-4, IL-8, or TNF-related apoptosis-inducing ligand (TRAIL) with its respective receptors. Anti-TNFR antibody or TNF protects azadirachtin-mediated down-regulation of TNFRs. Further, in silico data suggest that azadirachtin strongly binds in the TNF binding site of TNFR. Overall, our data suggest that azadirachtin modulates cell surface TNFRs thereby decreasing TNF-induced biological responses. Thus, azadirachtin exerts an anti-inflammatory response by a novel pathway, which may be beneficial for anti-inflammatory therapy.
Keywords: Cytokines, Cytokines/Tumor Necrosis Factor, Membrane/Biophysics, Protein/Protein-protein interactions, Receptors/Membrane, Receptors/Regulation, Signal Transduction/Protein Kinases, Transcription/Regulation
Introduction
Activated NF-κB (transcription factor) up-regulates the expression of pro-inflammatory cytokines, chemokines, and adhesion molecules that enhance inflammation, tumorigenesis, and angiogenesis (1). Tumor necrosis factor (TNF)2 is one of the multifunctional pro-inflammatory Th1 cytokines known to cause apoptosis, cellular proliferation, differentiation, inflammation, tumorigenesis, etc. The primary role of TNF is in the regulation of immune cells. Dysregulation and, in particular, overproduction of TNF have been implicated in a variety of human diseases including cancer (2). TNF induces signaling through two distinct receptors, p60 (TNF receptor 1) and p80 (TNF receptor 2), which have similar extracellular domains but distinct cytoplasmic domains. Both TNFR1 and -2 induce cell signaling targeting activation of NF-κB, whereas TNFR1-mediated signaling also follows cell apoptosis. TNFα is the most potent inducer of NF-κB and is in-turn regulated by NF-κB. TNFα activates NF-κB by degradation of IκBα and expresses almost 400 genes that have the NF-κB responsive element within their promoter (3). IKKs differentially regulate cells for their tumorigenic or inflammatory responses (4). Thus, regulation of TNF-induced NF-κB activation is useful in the study of inflammatory diseases.
Azadirachtin, a secondary metabolite derived from the Neem tree, is a highly oxidized tetranortriterpenoid, comprising an enol ether, acetal, hemiacetal, and tetra-substituted oxirane as well as carboxylic ester. Azadirachtin shows strong antifeedant and anti-malarial activity (5). Azadirachtin masks the endocrine action of insects and molluscs. The bark and leaf extracts have been therapeutically used as folk medicine (6). The molecular mechanism of action of azadirachtin is not known. Neem leaf extract has been reported to be non-toxic, non-mutagenic, and found to possess immunomodulatory, anti-inflammatory, and anticarcinogenic properties (7, 8). Our data indicate that azadirachtin suppresses TNF-induced activation of NF-κB and expression of genes dependent on NF-κB. Azadirachtin suppresses TNF binding in the cell-free plasma membrane, and it affinity-purified TNFR levels. In silico studies involving docking of azadirachtin with TNFR1 revealed that it binds to TNFR1 at the TNF binding site, thereby preventing binding of TNF. Similar binding of azadirachtin is also expected with TNFR2 as the two receptors share some similarity and are structurally similar. Our studies with azadirachtin treatment suggest that Neem is an important herbal cure against diseases involving TNF-mediated inflammation.
EXPERIMENTAL PROCEDURES
Materials
Tissue culture chemicals were obtained from Invitrogen (Grand Island, NY). Azadirachtin and most of the general chemicals were obtained from Sigma Aldrich Chemicals. Recombinant cytokines were obtained from Peprotech Inc. (Rocky Hill, NJ). Most of the antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit polyclonal anti-TNFR1 (sc-7895) and -TNFR2 (sc-7862) antibodies were obtained from Santa Cruz Biotechnology Inc. and used to detect the amounts of TNFR1 and -2 proteins in U-937 and HeLa cells, respectively. Cell lines used for this study were obtained from American Type Culture Collection (Manassas, VA). TNFR2 stably transfected HeLa (TNFR2+/+-HeLa) cells were from Prof. Bharat B. Aggarwal, MD Anderson Cancer Center, Houston, Texas.
Assay of NF-κB
To determine TNF-induced NF-κB activation, a gel shift assay (EMSA) was conducted essentially as described previously (9) using 32P end-labeled double-stranded NF-κB oligonucleotide from HIV-LTR, 5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG-3′ (bold indicates the NF-κB binding site).
Assay of NF-κB-dependent SEAP Reporter Gene
U-937 cells were transiently transfected with Qiagen SuperFect transfection reagent (Hilden, Germany) with 0.5 μg of reporter plasmid containing NF-κB binding site cloned upstream of heat-stable secretory alkaline phosphatase (SEAP) designated as NF-κB-SEAP; 0.5 μg of plasmid DNA of TNFR1, TRAF2, TRADD, IKK, p65, or dominant negative IκBα (IκBα-DN); and 0.5 μg of β-galactosidase or green fluorescence peptide (GFP) constructs. After different treatments, cell culture-conditioned medium (25 μl) was analyzed for SEAP activity essentially per the Clontech protocol (Palo Alto, CA) and reported as fold activation with respect to vector-transfected cells as described previously (10).
Assay of Cox-2-dependent Luciferase Gene Transcription
Cells were transiently transfected with 0.5 μg of each reporter plasmid containing the Cox-2 binding site cloned upstream of luciferase (designated as Cox-2-luciferase) and GFP constructs. After different treatments, the cell pellets were extracted with lysis buffer, and the extracts were incubated with firefly luciferin (substrate, Promega).
Radiolabeling of lL-8, TNF, TRAIL, IL-4, and IL-1 and Assay of Receptor Binding
Human IL-8, IL-4, IL-1, TNF, and TRAIL were iodinated with [125I]Na by the IODO-GEN method. Radiolabeled ligands were purified by G25-Sepharose column. The specific activities of radiolabeled ligands were 0.5 × 107 to 1 × 107 cpm/μg protein. Cell surface receptors for different ligands were detected following the method described previously (11).
Assay of IKK
The IKK assay was performed by a method described previously (10). Briefly, IKK complex from whole cell extract (300 μg) was precipitated with anti-IKKα and anti-IKKβ antibodies (1 μg each), incubated with protein A/G-Sepharose beads (Pierce), and assayed for IKK activity using 2 μg of GST-IκBα (amino acids 1–54) substrate protein.
Chemical Cross-linking
For chemical cross-linking, U-937 (1 × 107 cells/2 ml) after 125I-TNF binding at 4 °C for 2 h were pelleted, washed, and suspended in 200 μl of phosphate-buffered saline. 20 μl of disuccinimydyl suberate (DSS) (from 10 mg/ml DMSO) was added slowly in 200 μl of cell suspension and incubated for 1 h at 4 °C. Then cells were washed, extracted, and analyzed in 10% SDS-PAGE under reducing conditions. The gel was dried, exposed, and scanned in a PhosphorImager (Fuji, Japan).
Membrane Preparation
U-937 cell membranes were isolated from cells (1 × 107) with hypotonic lysis buffer followed by sucrose gradient centrifugation as described earlier (12).
Study of Molecular Docking
The x-ray structure of the extracellular domain of TNFR1 (PDB code: 1TNR) (13) in complex with TNF is available. However, such a complex or isolated structure is not yet available for the extracellular domain of TNFR2. We, therefore, carried out docking studies on the extracellular domain of TNFR1. The x-ray structure of the TNFR1-TNF complex (1TNR) (13) was downloaded from the PDB data base. From this complex, the TNFR1 structure was extracted and used for docking with azadirachtin. Before docking, missing hydrogen atoms were added to the TNFR1 structure, and the resultant structure was subjected to energy minimization.
The x-ray structure of azadirachtin was extracted from the Cambridge Structural data base (CSD) available at the Bioinformatics facility, Indian Institute of Science, Bangalore, India. The structure was subjected to energy minimization after fixing the missing hydrogen atoms. All energy minimizations were carried out using the conjugate gradient method (convergence criteria: energy gradient ∼0.01; force field: MMFF94 with the implicit solvation energy term as implemented in MOE software (CCG Inc., Canada)).
Docking studies were carried out using Genetic Optimization for Ligand Docking (GOLD) software (version 2.1). Docking searches were made within a sphere of 50 Å from the centroid of the TNF binding residues of TNFR1. The number of runs was set to 100. The annealing parameters for van der Waals and hydrogen bonding were set to 4.0 and 2.5 Å, respectively. The parameters used for the genetic algorithm were: population size 100, selection pressure 1.1, number of operations 1,00,000, number of islands 5, niche size 2, migrate 10, mutate 95, and crossover 95 (all default values). The docking solution (TNFR-azadirachtin complex) with the best GOLD score was considered for further analysis.
RESULTS
In this study, we examined the effect of azadirachtin on TNF-induced biological responses in different cell types. U-937 cells were used in most of our experiments. We did not find any cytolysis effect as detected by the lactate dehydrogenase (LDH) assay. Only 6.7% of LDH was released at 200 μm azadirachtin at 72 h of incubation, considering LDH release in 9 mm CHAPS-treated cells as 100%. Azadirachtin is highly oxygenated C-secomeliacins, and its structure is indicated in Fig. 1A.
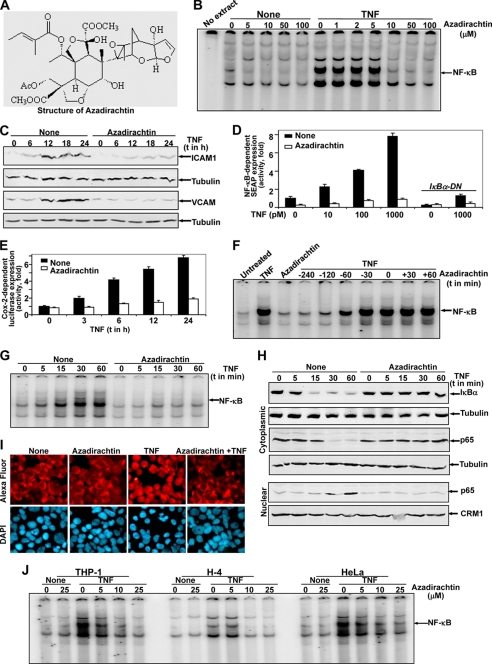
FIGURE 1.
Azadirachtin inhibits TNF-induced NF-κB activation. Chemical structure of azadirachtin (A). U-937 cells, pretreated with varying concentrations of azadirachtin for 6 h were stimulated with 100 pm TNF for 1 h. Nuclear extracts were prepared, and 8 μg of NE proteins were assayed for NF-κB (B). Cells, pretreated with 10 μm azadirachtin for 6 h were stimulated with TNF for different times. ICAM1 and VCAM were measured from cell extracts (100 μg of proteins) by Western blot (C). Cells were transfected with Qiagen Superfect transfection reagent for 6 h with plasmids for NF-κB promoter DNA that had been linked to SEAP (NF-κB-SEAP) and GFP. After washing, cells were cultured for 12 h. The GFP-positive cells were counted, and transfection efficiency was calculated. Cells treated with 10 μm azadirachtin for 6 h were stimulated with varying concentrations of TNF for 12 h. The culture supernatant was assayed for SEAP activity. Results are represented as fold of activation over the nontransfected control (D). U-937 cells were transfected with Cox-luciferase construct, treated with azadirachtin (10 μm for 6 h), stimulated with TNF (100 pm) for different times, and luciferase activity was measured from cell extracts. Luciferase activity was indicated as fold of activation over nonstimulated cells (E). U-937 cells, either pre-, co-, or postincubated with 10 μm azadirachtin at 37 °C for the indicated times were stimulated with 100 pm TNF at 37 °C for 1 h. After these treatments, nuclear extracts were prepared and then assayed for NF-κB (F). U-937 cells were treated with 10 μm azadirachtin for 6 h and then stimulated with TNF (100 pm) for different times. Nuclear (NE) and cytoplasmic (CE) extracts were prepared. NF-κB was assayed from 8 μg of NE by gel shift assay (G), p65 was measured from 30 μg of NE by Western blot, amounts of IκBα and p65 were measured from 50 μg of CE as detected by Western blot (H). The blot for NE was reprobed for CRM1, and blots for CE were reprobed for tubulin. U-937 cells, treated without or with azadirachtin (10 μm) were stimulated with TNF (100 pm) for 1 h. Cells were subjected to immunocytochemistry to detect p65 as described under “Experimental Procedures” (I). Human monocytic macrophage (THP-1), neuronal (H4), and epithelial (HeLa) cells were incubated at 37 °C with varying concentrations of azadirachtin for 6 h and then stimulated with 100 pm TNF for 1 h. After these treatments, nuclear extracts were prepared and then assayed for NF-κB (J).
Azadirachtin Inhibits TNF-induced Cell Signaling
TNF, a potent inducer of cell signaling that leads to NF-κB activation, can induce cell death either by inducing expression of proapoptotic genes and/or recruitment of death domain-containing proteins. Thus, regulation of TNF-mediated signaling especially the NF-κB pathway, would be important for therapeutic intervention. TNF-induced NF-κB DNA binding as measured from the nuclear extract (NE) by gel shift assay was inhibited by pretreatment of azadirachtin in a dose-dependent manner (Fig. 1B). TNF-induced increases in the amounts of adhesion molecules (Fig. 1C), and activities of SEAP (secretary alkaline phosphatase, an NF-κB-dependent reporter gene) (Fig. 1D) and luciferase (Cox-2-dependent) (Fig. 1E) were inhibited by pretreatment of azadirachtin. Complete inhibition of TNF-induced NF-κB activation was observed by azadirachtin pretreated for 4 h (Fig. 1F). TNF-induced increase in NF-κB DNA binding (Fig. 1G), which proceeded through cytoplasmic IκBα degradation (Fig. 1H, upper panel) and p65 nuclear translocation (Fig. 1, H, lower panel and I), was completely prevented by pretreatment of azadirachtin. The azadirachtin-mediated inhibition of NF-κB DNA binding was not only restricted in U-937 cells, but also shown in other cell types like THP-1, H-4, and HeLa cells (Fig. 1J). These data suggest that the TNF-induced NF-κB activation pathway is completely inhibited by azadirachtin.
Site of Action of Azadirachtin in TNF-induced Signal Transduction Pathway
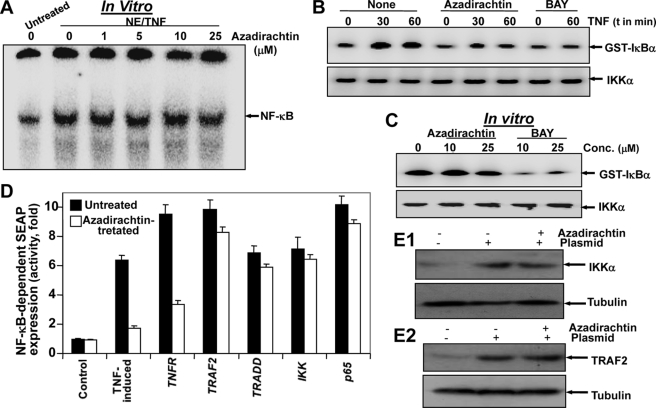
Azadirachtin did not decrease the DNA binding to NF-κB in vitro (Fig. 2A). TNF-induced IKK activity was inhibited in azadirachtin-pretreated cells (Fig. 2B), but not in TNF-induced IKK activity under cell-free conditions (Fig. 2C). These data suggest that azadirachtin-mediated inhibition of NF-κB activation is not at the DNA binding level or not in the IKK complex but may be more upstream of the IKK complex. TNF-induced NF-κB activation is mediated through sequential interaction of the TNF receptor (TNFR) with associated factor (TRAF2), associated death domain (TRADD), NIK followed by phosphorylation of IKK complex and subsequent phosphorylation and degradation of IκBα (14). As shown in Fig. 2D, cells transfected with TNFR1, TRAF2, TRADD, IKK, and p65 increased SEAP activity, and azadirachtin treatment suppressed activity of SEAP in TNFR1-transfected cells but had little effect on TRAF2, TRADD, IKK, or p65-transfected cells. The cell viability was decreased 8–15% upon transfection of different constructs. A significant amount of IKK and TRAF2 was expressed in the transfected cells with IKK and TRAF2 plasmids (Fig. 2, E1 and E2). Thus, azadirachtin must act at a step upstream from TRAF2, possibly at the TNFR level.
FIGURE 2.
Azadirachtin inhibits TNF-induced signaling by inhibiting TNFRs, but not NF-κB DNA binding or IKK activity. Nuclear extracts (NE) were prepared from 100 pm TNF-treated U-937 cells; 8 μg/sample NE proteins were treated with indicated concentrations of azadirachtin for 6 h at 37 °C and then assayed for NF-κB DNA binding (A). U-937 cells, treated with azadirachtin (10 μm) or BAY (25 μm) for 6 h were stimulated with TNF for 30 or 60 min. Whole cell extracts were prepared, and 250 μg of extract proteins were immunoprecipitated with anti-IKKα and β antibodies. Thereafter, immunocomplex kinase assay was performed (B). TNF-induced whole cell extracts (250 μg of protein) were incubated with varying concentrations of azadirachtin or BAY for 6 h and then immunoprecipitated with anti-IKKα and β antibodies. Thereafter, IKK activity was assayed (C). U-937 cells, transiently transfected with the indicated plasmids along with an NF-κB-SEAP constructs either untreated or treated with azadirachtin (10 μm) for 6 h and cultured for 12 h. Cell supernatants were assayed for SEAP activity as described under “Experimental Procedures.” Results are expressed as fold activity over the vector-transfected control (D). For expression control, extracts from IKK and TRAF-2 plasmid-transfected cells were used to detect IKK and TRAF-2 by Western blot, respectively (E).
Azadirachtin Inhibits TNF Binding
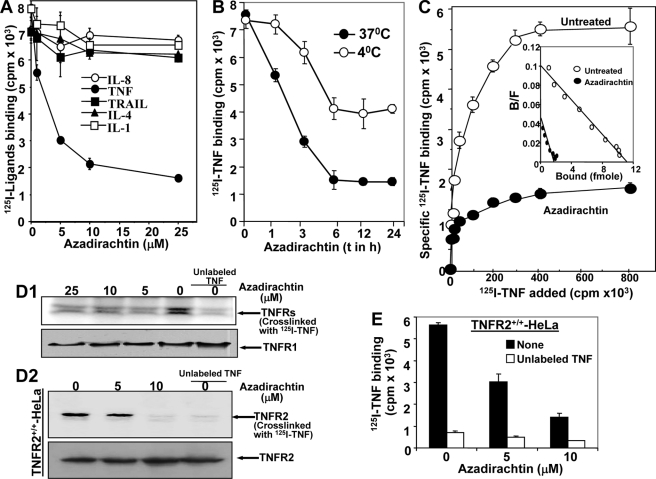
As azadirachtin inhibited TNF-mediated cell signaling that might be at the TNF receptor level, TNF binding was measured. The 125I-TNF binding decreased with increasing concentrations of azadirachtin, but not the binding of 125I-labeled IL-1, IL-4, IL-8, or TRAIL (Fig. 3A). For various times of pre-incubation, U-937 cells were treated with azadirachtin (10 μm) at 37 and 4 °C, and 125I-TNF binding was assayed at 4 °C. TNF binding gradually decreased up to 6 h at 37 °C and partially at 4 °C with increasing times of azadirachtin treatment (Fig. 3B). From these results, it is clear that the optimal time required for maximal inhibition of TNF binding by azadirachtin is 6 h. From the data at 4 °C treatment, it is clear that azadirachtin might be interfering in TNF-TNFR binding. The number of TNFRs available for TNF in the surface of azadirachtin-treated U-937 was counted by Scatchard analysis (11, 15). U-937 cells, either untreated or treated with azadirachtin for 6 h at 37 °C, were incubated with different concentrations of 125I-TNF at 4 °C for 2 h. In another set, the same binding was assayed in the presence of 50-fold unlabeled TNF. The specific binding of TNF was shown in Fig. 3C as the mean count of duplicate samples. From specific counts, bound/free values were calculated and plotted against bound values (Fig. 3C, inset). From this Scatchard plot data, the total number of TNFRs per U-937 cell was calculated as 5546 (Kd, 0.42 nm) and 986 (Kd, 0.48 nm) before and after azadirachtin treatment, respectively without changing affinity toward TNF. Azadirachtin decreases TNFRs by 79%, and this correlates with TNF binding data. The azadirachtin-mediated inhibition of TNF binding was further supported by the amount of cross-linked 125I-TNF with cell surface TNFRs (Fig. 3D1, upper panel) without changing the amounts of TNFR1 as detected by Western blot (Fig. 3D1, lower panel). The cross-linking data with TNFR2+/+-HeLa cells further suggest that azadirachtin inhibited TNF binding to TNFR2 (Fig. 3D2, upper panel) without altering the amounts of TNFR2 as detected by Western blot (Fig. 3D2, lower panel). Labeled TNF binding to these TNFR2+/+-HeLa cells upon azadirachtin treatment decreased TNF binding (Fig. 3E). These data suggest that azadirachtin inhibited TNF binding with both types of receptors only by inhibiting protein-protein interaction, without affecting the amount of cell surface TNFR protein.
FIGURE 3.
Azadirachtin inhibits TNF binding. U-937 (1 × 106/ml) cells were incubated in triplicate with varying concentrations of azadirachtin for 6 h at 37 °C. Labeled TNF, TRAIL, IL-4, IL-1, and IL-8 binding was assayed at 4 °C and indicated as mean cpm ± S.D. of triplicate samples (A). Cells, treated with azadirachtin for different times at 37. Then 125I-TNF binding was carried out at 4 °C for 2 h (B). Azadirachtin-treated and untreated U-937 cells (1 × 106) were incubated without or with 200 ng of TNF in duplicate. Then TNF binding was assayed using different amounts of 125I-TNF at 4 °C for 2 h. To determine the specific binding, nonspecific binding (obtained from a 50-fold excess of cold TNF used in binding) was subtracted. The result shown is representative of three independent experiments. From specific binding, ligand bound versus bound/free ratio was indicated in the inset (C). U-937 or TNFR2+/+-HeLa) cells were treated with different concentrations of azadirachtin for 6 h, and then 125I-TNF binding was carried out. Then cells were used to cross-link for ligand receptor. Cells were extracted, 250 μg of proteins were analyzed in 10% SDS-PAGE, and the dried gel was scanned in PhosphorImager. 100 μg of protein were probed for TNFR1 using rabbit polyclonal anti-TNFR1 antibody for U-937 (D1) and TNFR2 using rabbit polyclonal anti-TNFR2 antibody for TNFR2+/+-HeLa cells by Western blot for loading control (D2). TNFR2+/+-HeLa cells were treated with varying concentrations of azadirachtin in triplicate for 6 h at 37 °C. Then 125I-TNF binding was carried out at 4 °C for 2 h and indicated as mean cpm ± S.D. of triplicate samples (E).
Azadirachtin Blocks the TNF Binding Domain of TNFRs
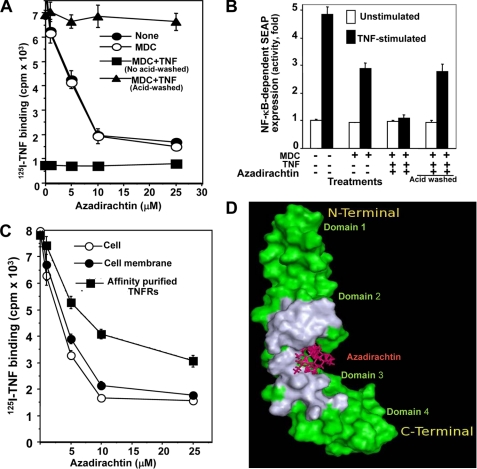
To determine whether azadirachtin interferes with the TNF binding domain of TNFRs, ligand protection experiments were performed. Because TNFRs are rapidly internalized after binding with TNF at 37 °C and monodansyl cadaverine (MDC) can protect endocytosis at 37 °C (16), cells were incubated with MDC for 15 min at 37 °C and then incubated with unlabeled TNF for 2 h at 4 °C. The cells were washed, treated with different concentrations of azadirachtin for 6 h, and then acid washed (with glycine-HCl: 50 mm glycine, pH 3.0) for 2 min to remove surface-bound unlabeled TNF. The binding of 125I-TNF was then assayed at 4 °C immediately after washing the cells. The binding of TNF decreased in MDC-treated or untreated U-937 cells by azadirachtin in a dose-dependent manner (Fig. 4A). Azadirachtin was unable to decrease TNF binding in TNF-preincubated and acid-washed U-937 cells. MDC-treated and/or acid-washed cells did not suppress TNF binding. TNF alone or in the presence of MDC suppressed TNF binding. TNF incubated in the presence or absence of MDC but in acid-washed cells showed almost complete protection of TNF binding (data not shown). In NF-κB-SEAP-transfected cells (Fig. 4B), the activity of SEAP was increased by almost 5-fold by TNF stimulation, with an almost 35% decrease in SEAP activity in MDC-pretreated and TNF-stimulated cells. MDC-treated, TNF-incubated, azadirachtin-treated, but TNF-stimulated cells showed complete inhibition of SEAP activity, and MDC-treated, TNF-incubated, azadirachtin-treated, acid-washed followed by TNF-stimulated cells showed almost 35% inhibition of SEAP activity. These results suggest that TNF protects cells from azadirachtin effect of decreases in TNF binding and of TNF-induced functions in U-937 cells.
FIGURE 4.
Azadirachtin interacts with TNFRs and inhibits TNF-induced NF-κB activation. U-937 cells (2 × 106/200 μl), preincubated with MDC (900 μm) for 15 min at 37 °C were incubated with TNF (250 ng/ml) for 2 h at 4 °C. After washing, cells were treated with varying concentrations of azadirachtin for 6 h at 37 °C. Cells were washed with 0.05 m glycine-HCl, pH 3 and then assayed for 125I-TNF binding (A). U-937 cells (2 × 106), transfected with NF-κB-SEAP constructs, pretreated with MDC for 15 min at 37 °C, incubated with TNF (250 ng) at 4 °C for 2 h, washed with medium, and treated with azadirachtin for 6 h at 37 °C. Cells were washed without or with glycine-HCl and then stimulated with TNF for 12 h. Culture supernatant was used to measure SEAP activity and indicated as fold of activation (B). Purified membrane and affinity-purified CXCRs, taken on nitrocellulose discs and U-937 cells were treated with different concentrations of azadirachtin in triplicate samples. The nitrocellulose discs and cells were washed and then assayed for 125I-TNF binding (C). The TNFR (green)-azadirachtin (maroon) complex structure is shown and the TNF binding region on the receptor is in gray (D).
Labeled 125I-TNF binding was assayed in intact U-937 cells, membranes, and purified TNFRs in the presence of different concentrations of azadirachtin for 6 h at 37 °C. The results shown in Fig. 4C indicated that 125I-TNF binding decreased in all these cases. These data suggest that down-regulation of TNFRs by azadirachtin is mediated by its interaction with the cell membrane at the TNFR level.
As mentioned under “Experimental Procedures,” we carried out molecular docking studies on TNFR1 with azadirachtin. Docking studies showed that azadirachtin binds at the junction of the two TNF binding regions (Fig. 4D). It is to be noted that the TNF binding region on TNFR1 distinctly comprises two regions belonging to the two central domains 2 and 3 (13). Most of the residues of TNFR1 that are in contact with azadirachtin are also found at the interface of the TNFR1-TNF complex (Table 1, top). Taking a cue from this observation, it can be argued that binding of azadirachtin to the receptor blocks further binding of TNF at its binding site on the receptor. It is also interesting to note that azadirachtin makes two hydrogen-bonding interactions with the Lys-75 and Arg-77 residues of the receptor. Of these residues, only Lys-75 is involved in hydrogen-bonding interaction with TNF in the TNF-TNFR1 complex, which is also stabilized by five other hydrogen-bonding interactions (Table 1, bottom). This indicates that azadirachtin and TNF have different binding specificities with TNFR.
TABLE 1.
Residues (top) and hydrogen-bonding interactions (bottom) found at the interfaces of TNFR1-TNF and TNFR1-azadirachtin complexes
The common residues found at the interfaces of TNFR1-TNF and TNFR1-Azadirachtin complexes are shown in bold. The interfacial residues of the complexes involving TNFR2 are shown within parentheses. (−) indicates the deletion of the residue in TNFR2.
| TNFR1(TNFR2)-TNF complex | TNFR1(TNFR2)-Azadirachtin |
|---|---|
| P16(R), Q17(L), G18(R), Q24(Q), R31(S), N41(V), P46(T), Q48(-), E56(E), G58(D), S59(S), L67(V), H69(E), C70(C), L71(L), S72(S), C73(C), K75(-), R77(R), K78(C), E79(S), M80(S), G81(D), I85(T), V90(R), V95(I), W107(S), L111(C), Q113(L), R124(F), V136(V), R138(K), G142(G), L145(S), V151(T) | L71(L), S72(S), C73(C), S74(G), K75(-), R77(R), D93(N), T94(R), C96(C), S108(K), N110(E), L111(G), F112(C) |
| TNFR1(TNFR2) | TNF | Azadirachtin (AZA) | |
|---|---|---|---|
| K75.O (-) | – | AZA.O9 | |
| R77.NH1 (R) | – | AZA.O6 | |
| 1 | K75.NZ (-) | P37.O | – |
| 2 | S72.OG (S) | R46.NH2 | – |
| 3 | S72.OG (S) | T49.OG1 | – |
| 4 | H69.ND1 (E) | D50.O | – |
| 5 | S72.OG (S) | D50.OD2 | – |
| 6 | K78.NZ (S) | P155.O | – |
Although we could not carry out detailed docking studies on TNFR2, it is reasonable to assume that the binding of azadirachtin with TNFR2 is very similar to TNFR1. The extracellular domains of both the receptors share good sequence similarity (sequence similarity: 54%) marked by conserved Cys residues, indicating a structural similarity between the two domains (13). Inspection of pairwise sequence alignment of the two receptors further revealed that most of the TNF binding residues of TNFR1 are also conserved in TNFR2 (Table 1, top). Furthermore, 5 of 13 residues found in the azadirachtin binding region of TNFR1 are conserved in TNFR2 (Table 1, top), which includes the Arg that makes a hydrogen bond with azadirachtin. All these aforementioned results suggest that the mechanism of inhibition of TNF binding as expounded in the case of TNFR1 also holds true for TNFR2.
DISCUSSION
In this report, we demonstrate a novel action of azadirachtin against TNF-mediated biological responses. TNF is a potent inducer of inflammation. Inflammatory response is always mediated by activation of nuclear transcription factor κB (NF-κB). NF-κB plays a very important role in the regulation of genes involved in inflammation and tumorigenesis. Our data show that TNF-induced NF-κB is inhibited by pretreatment of azadirachtin. Azadirachtin was able to inhibit TNF-induced ICAM1 and VCAM expression. Adhesion molecules are known to play a central role in the regulation of cellular inflammatory responses (17). Thus, azadirachtin-mediated down-regulation of adhesion molecules might have an important role in anti-inflammatory responses. Cyclooxygenase (Cox) 2 is not normally expressed by inflammatory stimuli such as cytokines or bacterial endotoxin (18). Enhanced Cox 2 induced synthesis of prostaglandins, and these proliferate tumor cells, promote angiogenesis, inhibit apoptosis, and increase metastatic potential (19, 20). Our data indicated just this as azadirachtin was able to inhibit the TNF-induced Cox-2 activation. Thus, azadirachtin may turn out to be an important anti-tumor agent particularly because the concentrations used in this study are non-toxic.
There are various ways by which azadirachtin might decrease TNF-induced NF-κB activation. TNF-induced NF-κB activation involves the sequential interaction of TNF receptor with TRAF2, TRADD, and NIK, which then activates IκBα kinase (IKK) (4, 14). IKK in turn phosphorylates IκBα. NF-κB activation requires sequential phosphorylation, ubiquitination, and degradation of IκBα. Azadirachtin blocks IKK activation followed by IκBα degradation, indicating that the azadirachtin effect on NF-κB may be due to inhibition of proteolysis of IκBα. Surprisingly, our findings suggest that azadirachtin blocks NF-κB-dependent reporter gene expression induced by TNFR1 but not by TRAF2, TRADD, IKK, and p65. So, the azadirachtin effect lies more upstream of TRAF2 and possibly at the TNFR level. Ligand binding data suggest that azadirachtin specifically inhibits binding of TNF, but not IL-1, IL-4, IL-8, or TRAIL. Molecular docking studies further confirmed that azadirachtin binds at the junction of the two distinct parts of the TNF binding region on TNFR1, thereby, precluding TNF binding. Even azadirachtin treatment at 4 °C, where cells do not show any fluidity for its function showed the 30–40% inhibition of TNF binding. These findings further prove that azadirachtin interacts with both TNFR1 and -2 and modulates the TNF binding site. Inhibition of IKK activity mediated by azadirachtin ex vivo, but not in vitro is different from BAY, a known inhibitor of IKKs both ex vivo and in vitro. These data further support that the azadirachtin effect lies more upstream in the TNF signaling cascade.
In this report, we demonstrate a novel action of azadirachtin against TNF-mediated biological responses in mammalian cells through inhibition of NF-κB, thereby regulation of inflammation and tumorigenesis. Azadirachtin is known to be effective in insect cells (7, 21–24) and also to induce cell death in human cell lines at higher concentrations (7, 25). We are reporting for the first time that it inhibits inflammatory responses in diverse mammalian cell types. Azadirachtin interacts with TNFRs and prevents TNF binding through downstream signaling like IKK activation, IκBα degradation, p65 nuclear translocation, and NF-κB-dependent gene transcription. Even azadirachtin treatment at 4 °C also supports the effect of azadirachtin at the TNFR level. In silico data for the TNF binding site at TNFR and the interaction of azadirachtin on this binding site might help to address this issue further. The Th2 cytokine IL-4 sheds both types of TNFRs from the cell membrane (15), but azadirachtin does not do so. These data further suggest that azadirachtin-mediated interaction lies within the TNFRs.
As TNF is the most potent inducer of NF-κB, blocking TNF receptors is expected to control inflammation and tumorigenesis. Azadirachtin-mediated interactions with TNFR, thereby causing a decrease in TNF-induced biological activities in diverse cell types are novel findings. Azadirachtin-mediated inhibition of inflammatory responses might be the basis of the folklore use of Neem products against many diseases. Hence, understanding the azadirachtin (an active component of Neem products) -mediated inhibition of TNF-induced inflammatory responses might advance its use as a medicine against several inflammatory diseases.
Acknowledgments
We thank Raja Verma for help during the preparation of the manuscript and Drs. T. Ramasarma and A. Khar for critically and carefully reading the manuscript and valuable suggestions. Iodination work was carried out at MD Anderson Cancer Center, Houston, Texas. We acknowledge the Sun Center of Excellence facility of the Institute.
This work was supported in part by a grant from the Department of Science and Technology (DST), Government of India, a core grant from the Centre for DNA Fingerprinting and Diagnostics (CDFD), and fellowships (to M. T. and P. K.) from the Council for Scientific and Industrial Research (CSIR), Government of India.
- TNF
- tumor necrosis factor
- BAY
- BAY 11-7082
- Cox-2
- cyclooxygenase
- ICAM
- intercellular cell adhesion molecule
- IκBα
- inhibitory subunit of κB
- IKK
- IκBα kinase
- MDC
- monodansyl cadavarine
- NF-κB
- nuclear transcription factor κB
- SEAP
- secretory alkaline phosphatase
- TNFR
- tumor necrosis factor (TNF) receptor
- TRAF
- TNF receptor-associated factor
- TRADD
- TNF receptor-associated death domain
- VCAM
- vascular cell adhesion molecule
- IL
- interleukin
- GFP
- green fluorescent protein
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- PDB
- Protein Data Bank.
REFERENCES
- 1.Hayden M. S., Ghosh S. (2008) Cell 132, 344–362 [DOI] [PubMed] [Google Scholar]
- 2.Locksley R. M., Killeen N., Lenardo M. J. (2001) Cell 104, 487–501 [DOI] [PubMed] [Google Scholar]
- 3.Ahn K. S., Aggarwal B. B. (2005) Ann. N.Y. Acad. Sci. 1056, 218–233 [DOI] [PubMed] [Google Scholar]
- 4.Karin M. (2008) Cell Res. 18, 334–342 [DOI] [PubMed] [Google Scholar]
- 5.Jones I. W., Denholm A. A., Ley S. V., Lovell H., Wood A., Sinden R. E. (1994) FEMS Microbiol. Lett. 120, 267–273 [DOI] [PubMed] [Google Scholar]
- 6.Biswas K., Chattopadhyay I., Banerjee R. K., Bandyopadhyay U. (2002) Curr. Sci. 82, 1336–1345 [Google Scholar]
- 7.Akudugu J., Gäde G., Böhm L. (2001) Life Sci. 68, 1153–1160 [DOI] [PubMed] [Google Scholar]
- 8.Subapriya R., Nagini S. (2005) Curr. Med. Chem. Anticancer Agents 5, 149–156 [DOI] [PubMed] [Google Scholar]
- 9.Manna S. K., Manna P., Sarkar A. (2007) Cell Death Differ. 14, 158–170 [DOI] [PubMed] [Google Scholar]
- 10.Manna S. K., Ramesh G. T. (2005) J. Biol. Chem. 280, 7010–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manna S. K., Sarkar A., Sreenivasan Y. (2006) Eur. J. Immunol. 36, 754–769 [DOI] [PubMed] [Google Scholar]
- 12.Matsushima K., Akahoshi T., Yamada M., Furutani Y., Oppenheim J. J. (1986) J. Immunol. 136, 4496–4502 [PubMed] [Google Scholar]
- 13.Banner D. W., D'Arcy A., Janes W., Gentz R., Schoenfeld H. J., Broger C., Loetscher H., Lesslauer W. (1993) Cell 73, 431–445 [DOI] [PubMed] [Google Scholar]
- 14.Nasuhara Y., Adcock I. M., Catley M., Barnes P. J., Newton R. (1999) J. Biol. Chem. 274, 19965–19972 [DOI] [PubMed] [Google Scholar]
- 15.Manna S. K., Aggarwal B. B. (1998) J. Biol. Chem. 273, 33333–33341 [DOI] [PubMed] [Google Scholar]
- 16.Ray E., Samanta A. K. (1997) Cytokine 9, 587–596 [DOI] [PubMed] [Google Scholar]
- 17.Dedrick R. L., Bodary S., Garovoy M. R. (2003) Expert Opin. Biol. Ther. 3, 85–95 [DOI] [PubMed] [Google Scholar]
- 18.O'Banion M. K., Winn V. D., Young. D. A. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 4888–4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheng H., Shao J., Morrow J. D., Beauchamp R. D., Dubois R. N. (1998) Cancer Res. 58, 362–366 [PubMed] [Google Scholar]
- 20.Kakiuchi Y., Tsuji S., Tsujii M., Murata H., Kawai N., Yasumaru M., Kimura A., Komori M., Irie T., Miyoshi E., Sasaki Y., Hayashi N., Kawano S., Hori M. (2002) Cancer Res. 62, 1567–1572 [PubMed] [Google Scholar]
- 21.Gonzalez M. S., Nogueira N. F., Mello C. B., De Souza W., Schaub G. A., Azambuja P., Garcia E. S. (1999) Exp. Parasitol. 92, 100–108 [DOI] [PubMed] [Google Scholar]
- 22.Sayah F. (2002) Tissue Cell 34, 53–62 [DOI] [PubMed] [Google Scholar]
- 23.Robertson S. L., Ni W., Dhadialla T. S., Nisbet A. J., McCusker C., Ley S. V., Mordue W., Mordue ‘Luntz’ A. J. (2007) Arch. Insect Biochem. Physiol. 64, 200–208 [DOI] [PubMed] [Google Scholar]
- 24.Anuradha A., Annadurai R. S., Shashidhara L. S. (2007) Insect Biochem. Mol. Biol. 37, 627–634 [DOI] [PubMed] [Google Scholar]
- 25.Di Ilio V., Pasquariello N., van der Esch A. S., Cristofaro M., Scarsella G., Risuleo G. (2006) Mol. Cell. Biochem. 287, 69–77 [DOI] [PubMed] [Google Scholar]