Abstract
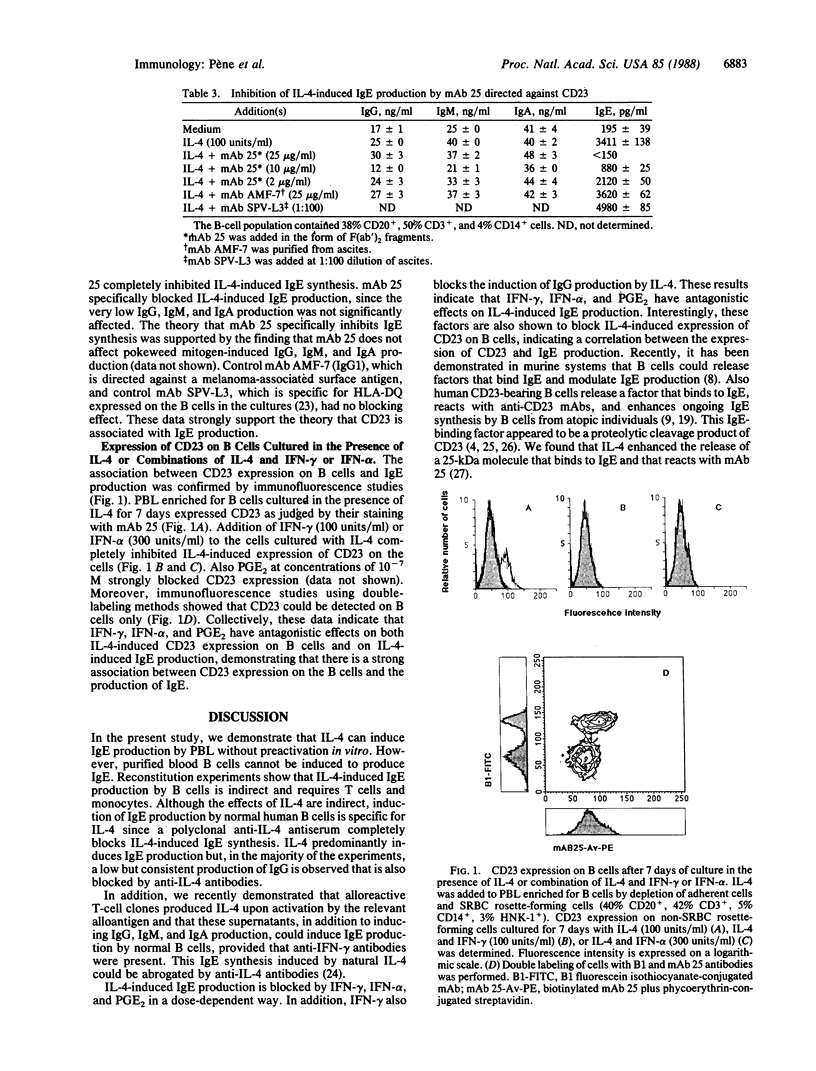
The effect of human recombinant interleukin 4 (IL-4) on antibody production by normal peripheral blood mononuclear cells enriched for B cells was investigated. IL-4 preferentially induced IgE synthesis in vitro. In addition, a low induction of IgG production was observed, whereas IL-4 had no effect on IgA and IgM synthesis. The IL-4-induced IgE production by B cells required T cells and monocytes but was specifically inhibited by an anti-IL-4 antiserum indicating that, although IL-4 acts indirectly, it is responsible for the induction of IgE synthesis. IL-4-induced IgE production was blocked in a dose-dependent way by interferon gamma (IFN-gamma), interferon alpha (IFN-alpha), and prostaglandin E2. IFN-gamma also inhibited IL-4-induced IgG production. These inhibitory effects of IFN-gamma and IFN-alpha on IgE production cannot be attributed to toxic effects since IFN-alpha induced IgM production in the presence of IL-4, whereas IFN-gamma was ineffective in inhibiting IgG production induced by IL-2. IFN-gamma, IFN-alpha, and prostaglandin E2 also inhibited IL-4-induced expression of the low-affinity receptor for the Fc portion of IgE (CD23) on B cells, indicating that there is an association between CD23 expression and IL-4-induced IgE production. This theory was supported by the finding that IL-4-induced IgE production was inhibited by F(ab')2 fragments of an anti-CD23 monoclonal antibody.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnefoy J. Y., Aubry J. P., Peronne C., Wijdenes J., Banchereau J. Production and characterization of a monoclonal antibody specific for the human lymphocyte low affinity receptor for IgE: CD 23 is a low affinity receptor for IgE. J Immunol. 1987 May 1;138(9):2970–2978. [PubMed] [Google Scholar]
- Bonnefoy J. Y., Defrance T., Peronne C., Menetrier C., Rousset F., Pène J., De Vries J. E., Banchereau J. Human recombinant interleukin 4 induces normal B cells to produce soluble CD23/IgE-binding factor analogous to that spontaneously released by lymphoblastoid B cell lines. Eur J Immunol. 1988 Jan;18(1):117–122. doi: 10.1002/eji.1830180118. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Rousset F., Vanbervliet B., Bonnefoy J. Y., Arai N., Takebe Y., Yokota T., Lee F., Arai K. Human recombinant interleukin 4 induces Fc epsilon receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987 Jun 1;165(6):1459–1467. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Aubry J. P., Takebe Y., Arai N., Miyajima A., Yokota T., Lee F., Arai K., de Vries J. E. B cell growth-promoting activity of recombinant human interleukin 4. J Immunol. 1987 Aug 15;139(4):1135–1141. [PubMed] [Google Scholar]
- Del Prete G., Maggi E., Macchia D., Tiri A., Parronchi P., Ricci M., Romagnani S. Human T cell clones can induce in vitro IgE synthesis in normal B cells regardless of alloantigen recognition or specificity for peculiar antigens. Eur J Immunol. 1986 Dec;16(12):1509–1514. doi: 10.1002/eji.1830161207. [DOI] [PubMed] [Google Scholar]
- Delespesse G., Sarfati M., Rubio-Trujillo M. In vitro production of IgE-binding factors by human mononuclear cells. Immunology. 1987 Jan;60(1):103–110. [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Vogel S. N. Interferons with special emphasis on the immune system. Adv Immunol. 1983;34:97–140. doi: 10.1016/s0065-2776(08)60378-8. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Takami M., Kim C. W., Honjo T., Miyoshi T., Tagaya Y., Kawabe T., Yodoi J. Human lymphocyte Fc receptor for IgE: sequence homology of its cloned cDNA with animal lectins. Proc Natl Acad Sci U S A. 1987 Feb;84(3):819–823. doi: 10.1073/pnas.84.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K. Regulation of IgE synthesis. Annu Rev Immunol. 1984;2:159–182. doi: 10.1146/annurev.iy.02.040184.001111. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Sandberg K. Formation of IgE binding factors by human T lymphocytes. J Immunol. 1981 May;126(5):1692–1696. [PubMed] [Google Scholar]
- Katz D. H. The IgE antibody system is coordinately regulated by FcR epsilon-positive lymphoid cells and IgE-selective soluble factors. Int Arch Allergy Appl Immunol. 1985;77(1-2):21–25. doi: 10.1159/000233747. [DOI] [PubMed] [Google Scholar]
- Kikutani H., Inui S., Sato R., Barsumian E. L., Owaki H., Yamasaki K., Kaisho T., Uchibayashi N., Hardy R. R., Hirano T. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986 Dec 5;47(5):657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. B-cell stimulatory factors (BSFs): molecular structure, biological function, and regulation of expression. J Clin Immunol. 1987 Sep;7(5):343–355. doi: 10.1007/BF00917012. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. One out of five peripheral blood B lymphocytes is activated to high-rate Ig production by human alloreactive T cell clones. Eur J Immunol. 1983 Oct;13(10):820–824. doi: 10.1002/eji.1830131008. [DOI] [PubMed] [Google Scholar]
- Leung D. Y., Young M. C., Wood N., Geha R. S. Induction of IgE synthesis in normal human B cells. Sequential requirements for activation by an alloreactive T cell clone and IgE-potentiating factors. J Exp Med. 1986 Mar 1;163(3):713–723. doi: 10.1084/jem.163.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdin C., Hofstetter H., Sarfati M., Levy C. A., Suter U., Alaimo D., Kilchherr E., Frost H., Delespesse G. Cloning and expression of the cDNA coding for a human lymphocyte IgE receptor. EMBO J. 1987 Jan;6(1):109–114. doi: 10.1002/j.1460-2075.1987.tb04726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman T. B., Volkman D. J., Hussain R., Fauci A. S., Ottesen E. A. Filarial parasite-specific T cell lines: induction of IgE synthesis. J Immunol. 1985 Feb;134(2):1178–1184. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Wideman J., Bonnefoy J. Y., De Vries J. E. Interleukin 5 enhances interleukin 4-induced IgE production by normal human B cells. The role of soluble CD23 antigen. Eur J Immunol. 1988 Jun;18(6):929–935. doi: 10.1002/eji.1830180615. [DOI] [PubMed] [Google Scholar]
- Rousset F., Malefijt R. W., Slierendregt B., Aubry J. P., Bonnefoy J. Y., Defrance T., Banchereau J., de Vries J. E. Regulation of Fc receptor for IgE (CD23) and class II MHC antigen expression on Burkitt's lymphoma cell lines by human IL-4 and IFN-gamma. J Immunol. 1988 Apr 15;140(8):2625–2632. [PubMed] [Google Scholar]
- Sarfati M., Rector E., Rubio-Trujillo M., Wong K., Sehon A. H., Delespesse G. In vitro synthesis of IgE by human lymphocytes. III. IgE-potentiating activity of culture supernatants from Epstein-Barr virus (EBV) transformed B cells. Immunology. 1984 Oct;53(2):207–214. [PMC free article] [PubMed] [Google Scholar]
- Spits H., Borst J., Giphart M., Coligan J., Terhorst C., De Vries J. E. HLA-DC antigens can serve as recognition elements for human cytotoxic T lymphocytes. Eur J Immunol. 1984 Apr;14(4):299–304. doi: 10.1002/eji.1830140404. [DOI] [PubMed] [Google Scholar]
- Spits H., Yssel H., Takebe Y., Arai N., Yokota T., Lee F., Arai K., Banchereau J., de Vries J. E. Recombinant interleukin 4 promotes the growth of human T cells. J Immunol. 1987 Aug 15;139(4):1142–1147. [PubMed] [Google Scholar]
- Umetsu D. T., Leung D. Y., Siraganian R., Jabara H. H., Geha R. S. Differential requirements of B cells from normal and allergic subjects for the induction of IgE synthesis by an alloreactive T cell clone. J Exp Med. 1985 Jul 1;162(1):202–214. doi: 10.1084/jem.162.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Otsuka T., Mosmann T., Banchereau J., DeFrance T., Blanchard D., De Vries J. E., Lee F., Arai K. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5894–5898. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. C., Leung D. Y., Geha R. S. Production of IgE-potentiating factor in man by T cell lines bearing Fc receptors for IgE. Eur J Immunol. 1984 Oct;14(10):871–878. doi: 10.1002/eji.1830141003. [DOI] [PubMed] [Google Scholar]
- te Velde A. A., Klomp J. P., Yard B. A., de Vries J. E., Figdor C. G. Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. J Immunol. 1988 Mar 1;140(5):1548–1554. [PubMed] [Google Scholar]