Abstract
Interleukin-17 (IL-17A) is a pro-inflammatory cytokine that has recently been implicated in pathogenesis of Langerhans Cell Histiocytosis (LCH), a potentially fatal disease characterized by lesions including CD207+ (langerin +) histiocytes. However, in this study we were unable to identify IL-17A gene expression in Langerhans cell lesions, and plasma levels of IL-17A did not correlate with disease activity. Therefore, this study does not support a central role for IL-17A in LCH pathogenesis.
Langerhans Cell Histiocytosis (LCH) is a potentially fatal disease characterized by lesions including CD207+ (langerin +) histiocytes. A current model hypothesizes that LCH lesions arise due to proliferation of Langerhans cells, bone-marrow derived dendritic cells normally restricted to skin and lymphatics1, 2. However, the etiology of LCH remains speculative. In a recent publication, Coury et al. (2008) reported expression of the proinflammatory cytokine interleukin 17A (IL-17A) by Langerhans cells (LCs) derived from LCH lesions3. IL-17A is a pro-inflammatory cytokine that initially was thought to be restricted to CD4+ T cells (Th17 cells) and has been described as an important mediator of inflammation, granulopoiesis, and immune responses including granuloma formation. Pathologic IL-17A expression has been implicated in autoimmune diseases including rheumatoid arthritis, psoriasis, multiple sclerosis and inflammatory bowel disease 4–7. LCH lesions are comprised of multiple cell types including LCs (36–58%), T cells (13–18%), macrophages (2–30%), eosinophils (1–10%) and rare B cells (1–3%) 8. Several studies have evaluated expression of select cytokines within the LCH lesions using immunohistochemistry, leading to the consensus that the microenvironment of the LCH lesion pro-inflammatory “cytokine storm” 9–11. If the inflammation in LCH were mediated by IL-17A, it would be an attractive target for therapy.
In order to further define IL-17A expression in LCH lesions, we first analyzed cell-specific IL-17A gene expression. T cells (CD3+) and LCs (CD207+) were isolated from 14 fresh LCH biopsy samples by flow cytometry, including lesions from patients with multisystem disease and relapsed disease (Table 1, Figure 1). Single-cell suspensions of unsorted cells were also processed from two of the biopsied lesions to determine if cells within the LCH lesion other than CD3+ or CD207+ expressed IL-17A. LCs isolated from foreskin from healthy donors and T cells isolated from tonsils from healthy donors were used as controls. RNA was extracted, quality was verified, and then cDNA was amplified. Polymerase chain reaction (PCR) was then performed to determine qualitative gene expression. (Methods are detailed in “Supplemental Methods”).
Table 1. Clinical details of LCH patients.
Disease categories (Group 1–3) correspond to Histiocyte Society groups used in the LCH-III treatment protocol. Group 4 includes patients with single non-risk lesions.
Chemotherapy abbreviations:
LCHIII-1A: vinblastine, prednisone, mercaptopurine.
LCHIII-1B: vinblastine, prednisone, mercaptopurine, methotrexate.
VP-16: etoposide
LCH-A1: vinblastine, prednisone, mercaptopurine (Histiocyte Society adult LCH protocol).
| Table 1A. Clinical Details of LCH Patients – Biopsy Samples | ||||||
|---|---|---|---|---|---|---|
|
Patient Number |
Age (years) | Gender | Disease Category |
Sites of LCH Lesions | Biopsy Site | Chemotherapy |
| 1 | 2.3 | M | 2 | single skull (occipital), recurrent tibia |
tibia | N |
| 2 | 0.95 | M | 3 | multifocal skull, orbit | orbit | N |
| 3 | 0.9 | F | 1 | skin, lungs, multiple skull, mastoid, gingiva |
mastoid | LCHIII-IA |
| 4 | 6.2 | F | 4 | single skull (parietal) | parietal skull | N |
| 5 | 2.8 | M | 4 | single skull (parietal) | parietal skull | N |
| 6 | 5.5 | F | 2 | pelvis | pelvis | N |
| 7 | 0.8 | M | 2 | orbit, skin | skin | N |
| 8 | 1.8 | M | 4 | mandible | mandible | N |
| 9 | 4.9 | F | 2 | skin, bone | scalp | N |
| 10 | 14 | M | 4 | single skull (parietal) | parietal skull | N |
| 11 | 6 | M | 3 | single skull (frontal) | frontal skull | N |
| 12 | 0.9 | F | 3 | mastoid | mastoid | N |
| 13 | 2.1 | F | 1 | mandible, skull, vertebrae, recurrent orbit |
orbit | LCHIII-IB |
| 14 | 5.9 | M | 4 | single skull | skull | N |
| Table 1B. Clinical Details of LCH Patients – Plasma Samples | ||||||
|---|---|---|---|---|---|---|
|
Patient Number |
Age (years) | Gender | Disease Category |
Sites of LCH Lesions | Chemotherapy | Therapy Details |
| 3 | 0.7 | F | 1 | skin, lungs, multiple skull, mastoid, gingival |
N | |
| 3 | 0.9 | F | 1 | skin, lungs, multiple skull, mastoid, gingival |
Y | LCHIII - IA |
| 15 | 0.1 | F | 1 | skin, liver, bone marrow | N | |
| 15 | 0.4 | F | 1 | skin, liver, bone marrow | Y | LCHIII - IA |
| 16 | 0.5 | F | 1 | skin, multiple skull, multifocal bone, liver, bone marrow |
N | |
| 16 | 0.6 | F | 1 | skin, multiple skull, multifocal bone, liver, bone marrow |
Y | VP-16, LCHIII-IA |
| 17 | 56 | F | 1 | lung, lymph node | N | |
| 18 | 0.4 | F | 1 | skin, lung, multiple skull | N | |
| 19 | 1.9 | M | 1 | skin, multiple skull, mastoid - refractory |
Y | LCHIII - IB |
| 20 | 57 | M | 1 | lung, skin, jaw | Y | prednisone |
| 21 | 38 | F | 1 | skin, lung | N | |
| 22 | 43 | F | 1 | lung, multifocal bone | Y | LCH-A1 |
| 23 | 50 | F | 1 | skin, mouth, lungs | N | |
| 24 | 38 | F | 1 | LN, malig histiocytosis | N | |
| 25 | 11 | F | 1 | lung, LN, multifocal bone bone | Y | VCR, Ara-C |
| 26 | 34 | F | 1 | mastoid, lung | N | |
| 38 | 31 | F | 1 | lung | Y | prednisone |
| 27 | 7.8 | F | 3 | skull (frontal), femur | N | surgery |
| 28 | 1.4 | F | 3 | multifocal bone | N | |
| 29 | 5.9 | M | 3 | pituitary (relapse), history of liver, multiple skull |
N | |
| 30 | 38 | F | 3 | multifocal bone, pituitary | N | |
| 31 | 3.6 | M | 3 | pituitary (relapse) | N | |
| 32 | 44 | F | 3 | thymus, pituitary | N | |
| 33 | 24 | F | 3 | degenerative CNS | N | |
| 34 | 2.8 | M | 3 | pituitary | N | |
| 35 | 22 | M | 3 | brain, skull (base) | N | surgery |
| 36 | 3.7 | M | 3 | single bone (orbit) | N | surgery |
| 37 | 2.9 | M | 4 | skull (parietal) | N | surgery |
| 39 | 2.2 | M | 4 | single skull (occiput) | N | surgery |
| 40 | 0.3 | M | 4 | skin only | N | |
| 41 | 3.5 | F | 4 | skin only | N | |
| 42 | 0.1 | F | 4 | skin only | N | |
| 43 | 6.1 | F | 4 | single skull (parietal) | N | surgery |
| 44 | 5 | F | 4 | single bone (femur) | N | surgery |
| 45 | 7.9 | M | 4 | single bone (femur) | N | surgery |
| 46 | 8.7 | M | 4 | single bone (femur) | N | surgery |
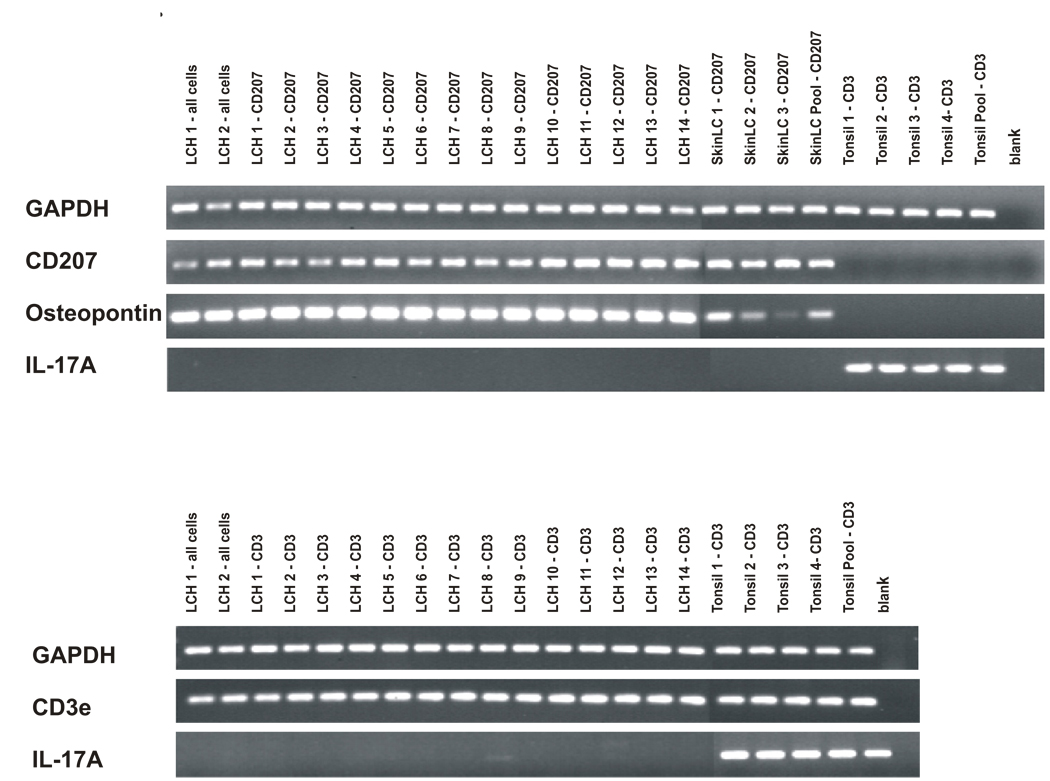
Figure 1. Cell-specific IL-17A expression.
Upper Panel: Cells were isolated from LCH lesions and normal skin and tonsil control samples. After RNA extraction and cDNA amplification, PCR was performed using primers for GAPDH, CD207, osteopontin-1, and IL-17A. Samples were run on agarose gels stained with ethidium bromide, and PCR products were photographed. PCR primers are indicated along the left border. Sample description is detailed along the top of the figure. “All cells” indicates that all cells from that lesion were included in the sample. “CD207” indicates cells were sorted with antibody specific for LCs. “CD3” indicates cells were sorted with antibody specific for T cells. The control skin “LC Pool” contains RNA extracted from 20 different normal skin samples. The control “Tonsil Pool” contains RNA extracted from 20 different normal tonsil samples.
Lower Panel: Cells were isolated from LCH lesions and normal tonsil control samples. PCR primers specific for GAPDH, CD3e and IL-17A were used.
For the LCH lesion and control LCs, 20 ng of amplified template cDNA was used in PCR reactions with primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), osteopontin (OPN), and IL-17A. Uniform expression of GAPDH validated overall quality of the cDNA template and equal loading. CD207 expression was restricted to normal and LCH lesion LCs. As a positive control for LCH-specific LCs, osteopontin expression was increased in LCH LCs compared to control LCs12. IL-17A expression was undetectable in all of the experimental and control LCs, but was identified in the control tonsil CD3 cells. For the LCH lesion and control tonsil T cells, primers for GAPDH, CD3e and IL-17A were used. In the LCH lesion T cells, IL-17A expression was absent or present at extremely low levels, though it was readily detected in control tonsil T cell samples. Therefore, this study is unable to support the observation by Coury et al that IL-17A is expressed at significant levels by LCs or T cells in LCH lesions 3.
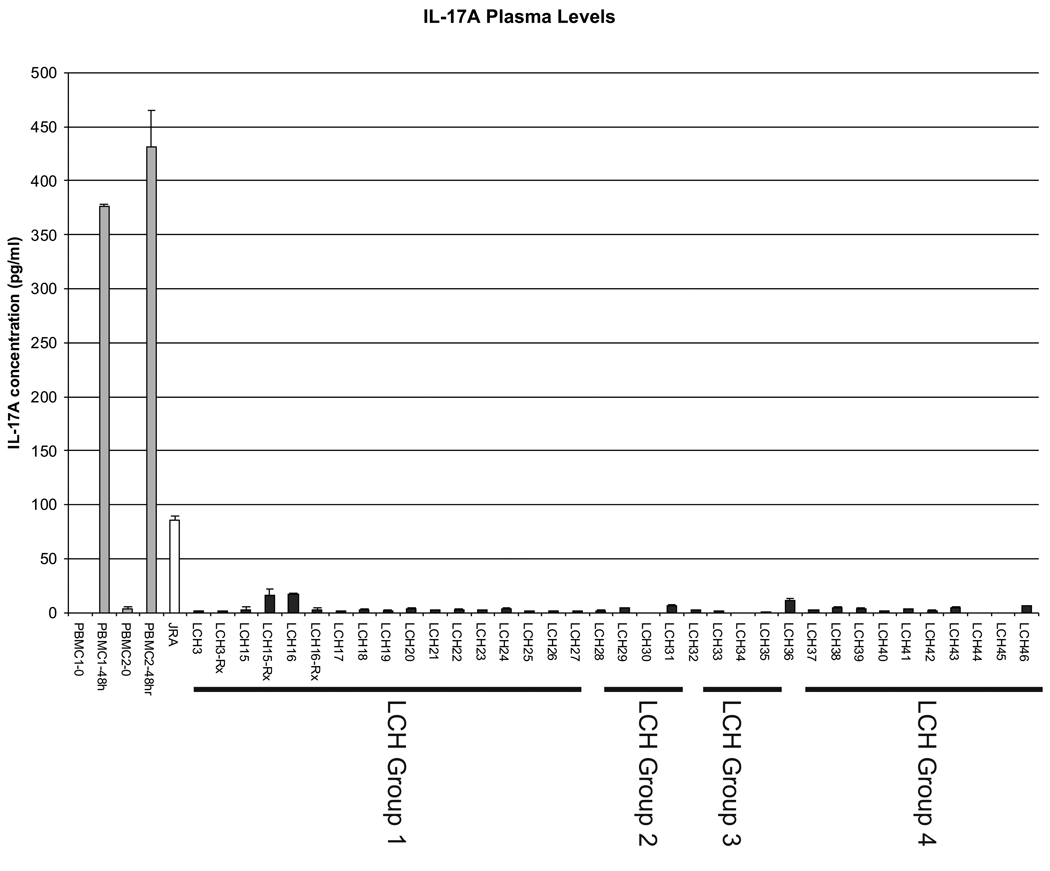
In order to test the possibility that IL-17A is produced in patients with LCH by cells outside the LCH lesions, we tested 36 plasma samples from 33 patients with active LCH ranging from single lesions in non-risk sites to multi-system disease (Table 1, Figure 2). Group 1 patients have multi-system disease in “high-risk” sites including bone marrow, liver, spleen, or lungs. Group 2 patients have multifocal lesions in “low-risk” sites. Group 3 patients have multifocal bone lesions or lesions in “CNS risk” sites13. We arbitrarily designate “Group 4” to describe patients with single lesions in all other “non-risk” sites. The ELISA experiments were performed with reagents from two commercially available kits (eBioscience, San Diego and R&D Systems, Minneapolis) with reproducible results. All tests were performed at least twice. For a positive control, peripheral blood monocytes (PBMCs) isolated from two healthy donors were incubated with phorbol myristate acetate (PMA) and ionomycin, and media was sampled at 0 hours and 48 hours with IL-17A detected at predictable levels (376–432 pg/ml at 48 hours) 14. A sample from a patient with active juvenile idiopathic arthritis (JIA) was also included as a positive plasma control (85 pg/ml). Coury et al. identified elevated IL-17A serum levels to up 1 ng/ml in some patients with active LCH. None of the samples from our patients with active disease, including patients with florid multisystem LCH, had abnormal IL-17A plasma levels (0–17 pg/ml). IL-17A has been detected in healing bone callus in a rat model 15, so it may be possible some patients may develop elevated IL-17A as a result of LCH-associated bone fracture or mechanical bone injury from surgery, although we did not observe this in our patients. In this study, plasma IL-17A does not correlate with extent or activity of LCH.
Figure 2. IL-17A plasma levels.
IL-17A plasma levels were determined using ELISA. As a control for the assay, media levels of IL-17A produced by peripheral blood mononuclear cells (PBMCs) from two healthy donors (PBMC1, PBMC2) after stimulation by PMA and ionomycin were used. A plasma sample from a patient with active JIA was also used as a positive control. The “LCH” number corresponds to the patient descriptions in Table 1. Group 1–3 correspond to clinical categories defined by the Histiocyte Society LCH-III protocol. Group 4 includes patients with single non-risk lesions.
In conclusion, IL-17A gene expression was undetectable in LCH LCs from 14 biopsy samples. Furthermore, IL-17A protein was not detected at abnormal levels in LCH patients, including those with severe, active disease. Therefore, IL-17A is unlikely to play a central role in pathogenesis of LCH.
Supplementary Material
Acknowledgements
We would like to thank Dr. Barry Myones for the JIA plasma sample and helpful discussions. We would also like to thank the Texas Children’s Cancer Center Flow Cytometry Core for their assistance in this project as well as Saida Ebrahim for data management. This project was supported by grants from the Histiocytosis Association of America and the NIH (R21 CA 114981-01A2). CEA received funding from the NIH Pediatric Oncology Research Training Grant (T32 CA115303-03).
Footnotes
Author Contributions
CEA and KLM conceived of the study, designed the experiments, and wrote the manuscript. CEA carried out the experiments.
Reference List
- 1.Arceci RJ. The histiocytoses: the fall of the Tower of Babel. Eur. J. Cancer. 1999;35:747–767. doi: 10.1016/s0959-8049(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 2.McClain KL, Natkunam Y, Swerdlow SH. Atypical cellular disorders. Hematology. Am. Soc. Hematol. Educ. Program. 2004:283–296. doi: 10.1182/asheducation-2004.1.283. [DOI] [PubMed] [Google Scholar]
- 3.Coury F, et al. Langerhans cell histiocytosis reveals a new IL-17Adependent pathway of dendritic cell fusion. Nat. Med. 2008;14:81–87. doi: 10.1038/nm1694. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 6.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin. Immunol. 2007;19:362–371. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favara BE, Steele A. Langerhans cell histiocytosis of lymph nodes: a morphological assessment of 43 biopsies. Pediatr. Pathol. Lab Med. 1997;17:769–787. [PubMed] [Google Scholar]
- 9.de Graaf JH, Tamminga RY, Dam-Meiring A, Kamps WA, Timens W. The presence of cytokines in Langerhans' cell histiocytosis. J. Pathol. 1996;180:400–406. doi: 10.1002/(SICI)1096-9896(199612)180:4<400::AID-PATH701>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Fadeel B, Henter JI. Langerhans-cell histiocytosis: neoplasia or unbridled inflammation? Trends Immunol. 2003;24:409–410. doi: 10.1016/s1471-4906(03)00171-6. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths CE, Dearman RJ, Cumberbatch M, Kimber I. Cytokines and Langerhans cell mobilisation in mouse and man. Cytokine. 2005;32:67–70. doi: 10.1016/j.cyto.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Allen C, et al. Langerhans Cells in LCH Lesions Over-Express Genes that Regulate Lymphocyte Trafficking. Pediatric Blood and Cancer [2008 ASPHO Abstracts] 2008 Ref Type: Generic. [Google Scholar]
- 13.Allen CE, McClain KL. Langerhans cell histiocytosis: a review of past, current and future therapies. Drugs Today (Barc. ) 2007;43:627–643. doi: 10.1358/dot.2007.43.9.1088823. [DOI] [PubMed] [Google Scholar]
- 14.Lenarczyk A, et al. Antigen-induced IL-17 response in the peripheral blood mononuclear cells (PBMC) of healthy controls. Clin. Exp. Immunol. 2000;122:41–48. doi: 10.1046/j.1365-2249.2000.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokubu T, Haudenschild DR, Moseley TA, Rose L, Reddi AH. Immunolocalization of IL-17A, IL-17B, and their receptors in chondrocytes during fracture healing. J. Histochem. Cytochem. 2008;56:89–95. doi: 10.1369/jhc.7A7223.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.