Abstract
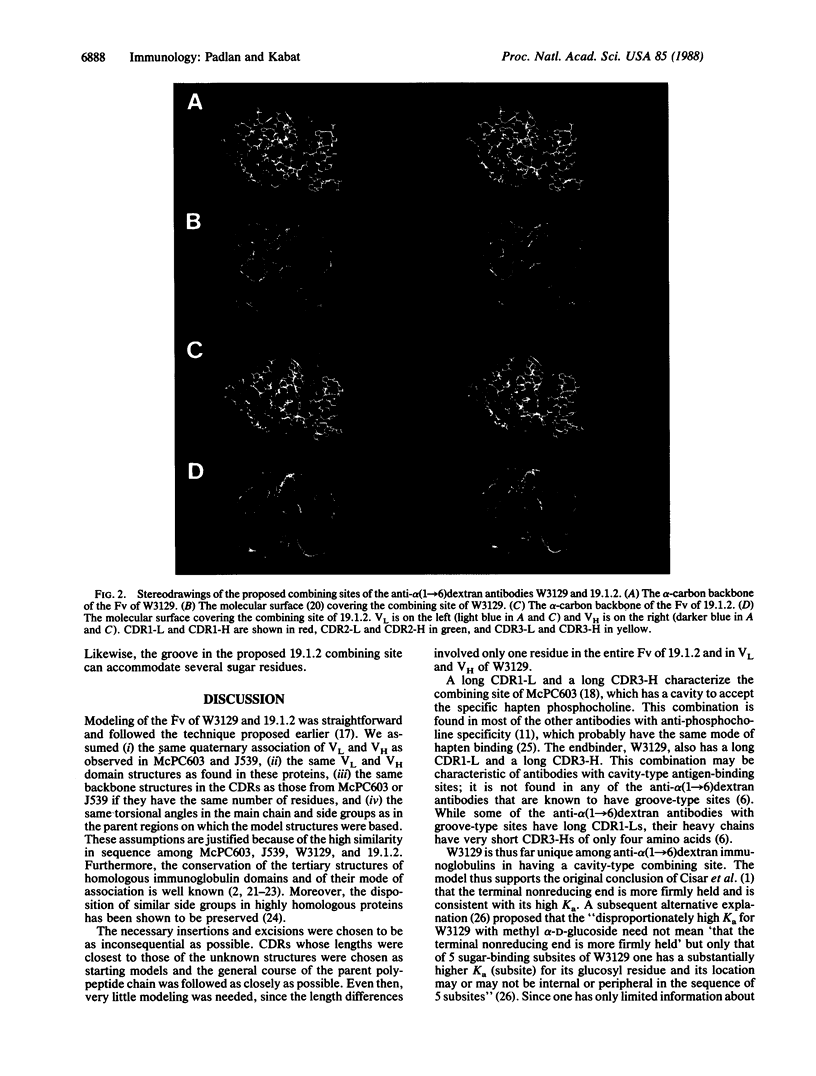
Models of the Fv portion (containing the variable regions of the heavy and light chains) of two monoclonal anti-alpha (1----6)dextran antibodies, W3129 and 19.1.2, were constructed from amino acid sequences and the known three-dimensional structures of the Fv portions of McPC603 and J539. The modeled combining site of W3129 has a protrusion on one side, formed by the long complementarity-determining region 1 of the light chain and the long complementarity-determining region 3 of the heavy chain, and has a cavity accommodating a glucose moiety. The model of the 19.1.2 site is basically flat with a shallow groove that can accommodate several internal glucose units. These results support the earlier conclusions, from ligand binding data, that W3129 has a cavity-type site, involving the terminal nonreducing glucose residue (endbinder), whereas 19.1.2 has a groove-type site.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akolkar P. N., Sikder S. K., Bhattacharya S. B., Liao J., Gruezo F., Morrison S. L., Kabat E. A. Different VL and VH germ-line genes are used to produce similar combining sites with specificity for alpha(1----6)dextrans. J Immunol. 1987 Jun 15;138(12):4472–4479. [PubMed] [Google Scholar]
- Amzel L. M., Poljak R. J. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Bennett L. G., Glaudemans C. P. The affinity of a linear, alpha-D-(1 leads to 6)-linked D-glucopyranan (dextran) for homogeneous immunoglobulin A W3129. Carbohydr Res. 1979 Jul;72:315–319. doi: 10.1016/s0008-6215(00)83958-0. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Borden P., Kabat E. A. Nucleotide sequence of the cDNAs encoding the variable region heavy and light chains of a myeloma protein specific for the terminal nonreducing end of alpha(1----6)dextran. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2440–2443. doi: 10.1073/pnas.84.8.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Chen H. T., Kabat E. A. Immunochemical studies on blood groups. The combining site specificities of mouse monoclonal hybridoma anti-A and anti-B. J Biol Chem. 1985 Oct 25;260(24):13208–13217. [PubMed] [Google Scholar]
- Chen H. T., Kabat E. A., Lundblad A., Ratcliffe R. M. Nucleotide and translated amino acid sequences of cDNA coding for the variable regions of the light and heavy chains of mouse hybridoma antibodies to blood group A and B substances. J Biol Chem. 1987 Oct 5;262(28):13579–13583. [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Levitt M., Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. The predicted structure of immunoglobulin D1.3 and its comparison with the crystal structure. Science. 1986 Aug 15;233(4765):755–758. doi: 10.1126/science.3090684. [DOI] [PubMed] [Google Scholar]
- Chothia C., Novotný J., Bruccoleri R., Karplus M. Domain association in immunoglobulin molecules. The packing of variable domains. J Mol Biol. 1985 Dec 5;186(3):651–663. doi: 10.1016/0022-2836(85)90137-8. [DOI] [PubMed] [Google Scholar]
- Cisar J., Kabat E. A., Dorner M. M., Liao J. Binding properties of immunoglobulin combining sites specific for terminal or nonterminal antigenic determinants in dextran. J Exp Med. 1975 Aug 1;142(2):435–459. doi: 10.1084/jem.142.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. R., Metzger H. Structural basis of antibody function. Annu Rev Immunol. 1983;1:87–117. doi: 10.1146/annurev.iy.01.040183.000511. [DOI] [PubMed] [Google Scholar]
- Fine R. M., Wang H., Shenkin P. S., Yarmush D. L., Levinthal C. Predicting antibody hypervariable loop conformations. II: Minimization and molecular dynamics studies of MCPC603 from many randomly generated loop conformations. Proteins. 1986 Dec;1(4):342–362. doi: 10.1002/prot.340010408. [DOI] [PubMed] [Google Scholar]
- Glaudemans C. P. Immunodominance of terminal sugars revisited. Mol Immunol. 1986 Aug;23(8):917–918. doi: 10.1016/0161-5890(86)90078-7. [DOI] [PubMed] [Google Scholar]
- Glaudemans C. P. Seven structurally different murine monoclonal galactan-specific antibodies show identity in their galactosyl-binding subsite arrangements. Mol Immunol. 1987 Apr;24(4):371–377. doi: 10.1016/0161-5890(87)90179-9. [DOI] [PubMed] [Google Scholar]
- Gooi H. C., Hounsell E. F., Picard J. K., Lowe A. D., Voak D., Lennox E. S., Feizi T. Differing reactions of monoclonal anti-A antibodies with oligosaccharides related to blood group A. J Biol Chem. 1985 Oct 25;260(24):13218–13224. [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Kimura H., Cook R., Meek K., Umeda M., Ball E., Capra J. D., Marcus D. M. Sequences of the VH and VL regions of murine monoclonal antibodies against 3-fucosyllactosamine. J Immunol. 1988 Feb 15;140(4):1212–1217. [PubMed] [Google Scholar]
- Lemieux R. U., Wong T. C., Liao J., Kabat E. A. The combining site of anti-I Ma (group 1). Mol Immunol. 1984 Sep;21(9):751–759. doi: 10.1016/0161-5890(84)90161-5. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Cohen G. H., Davies D. R. On the specificity of antibody/antigen interactions: phosphocholine binding to McPC603 and the correlation of three-dimensional structure and sequence data. Ann Inst Pasteur Immunol. 1985 Mar-Apr;136C(2):271–276. doi: 10.1016/s0769-2625(85)80058-1. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Davies D. R., Pecht I., Givol D., Wright C. Model-building studies of antigen-binding sites: the hapten-binding site of mopc-315. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):627–637. doi: 10.1101/sqb.1977.041.01.072. [DOI] [PubMed] [Google Scholar]
- Padlan E. A. Structural basis for the specificity of antibody-antigen reactions and structural mechanisms for the diversification of antigen-binding specificities. Q Rev Biophys. 1977 Feb;10(1):35–65. doi: 10.1017/s0033583500000135. [DOI] [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Sikder S. K., Akolkar P. N., Kaladas P. M., Morrison S. L., Kabat E. A. Sequences of variable regions of hybridoma antibodies to alpha (1----6) dextran in BALB/c and C57BL/6 mice. J Immunol. 1985 Dec;135(6):4215–4221. [PubMed] [Google Scholar]
- Suh S. W., Bhat T. N., Navia M. A., Cohen G. H., Rao D. N., Rudikoff S., Davies D. R. The galactan-binding immunoglobulin Fab J539: an X-ray diffraction study at 2.6-A resolution. Proteins. 1986 Sep;1(1):74–80. doi: 10.1002/prot.340010112. [DOI] [PubMed] [Google Scholar]
- Summers N. L., Carlson W. D., Karplus M. Analysis of side-chain orientations in homologous proteins. J Mol Biol. 1987 Jul 5;196(1):175–198. doi: 10.1016/0022-2836(87)90520-1. [DOI] [PubMed] [Google Scholar]