Abstract
Neisseria meningitidis is a leading cause of bacterial meningitis and other serious infections worldwide. The epidemiology of N. meningitidis is highly changeable, with major changes in disease incidence and serogroup distribution. Six serogroups are responsible for most meningococcal disease worldwide, namely serogroups, A, B, C, W-135, X, and Y; the epidemiology of disease caused by each serogroup is unique. No vaccine is available for endemic disease caused by serogroup B strains. Two tetravalent (A/C/Y/W-135) meningococcal vaccines are licensed in the United States, a purified polysaccharide product and a polysaccharide–protein conjugate vaccine. The conjugate vaccine is recommended for all adolescents, although vaccine coverage remains low, and other high risk groups. A comprehensive program to prevent invasive meningococcal disease in the US will require immunization of infants; several conjugate vaccines for infants may become available in the near future. Broadly protective vaccines for endemic serogroup B disease are also needed.
Keywords: Neisseria meningitidis, meningococcal, United States, vaccine
Introduction
Neisseria meningitidis remains a major cause of bacterial meningitis and other invasive bacterial infections worldwide [1-4]. A remarkable characteristic of meningococcal epidemiology is that it is highly fluid, with major fluctuations in the incidence of endemic disease and the occurrence of outbreaks and epidemics. In addition, meningococcal serogroup distribution is also highly regional and cyclical.
The purpose of this review is to discuss the current epidemiology of meningococcal disease and the vaccines that are presently available in the United States. Recent experiences with meningococcal vaccines in the United Kingdom and New Zealand will also be reviewed because they illustrate the potential public health impact of meningococcal conjugate and outer-membrane protein–based serogroup B vaccines, respectively.
Meningococcal Serogroups
Virulent N. meningitidis strains have a polysaccharide capsule, which allows the organism to cause invasive diseases such as bacteremia and meningitis. Unencapsulated strains, which are frequently found in the pharynx of asymptomatic carriers, have only rarely been determined to cause invasive infections [5, 6]. Of the 13 different polysaccharide capsular types, only 6 commonly cause disease globally: A, B, C, W-135, X, and Y, although substantial rates of serogroup X disease are restricted to parts of sub-Saharan Africa [1].
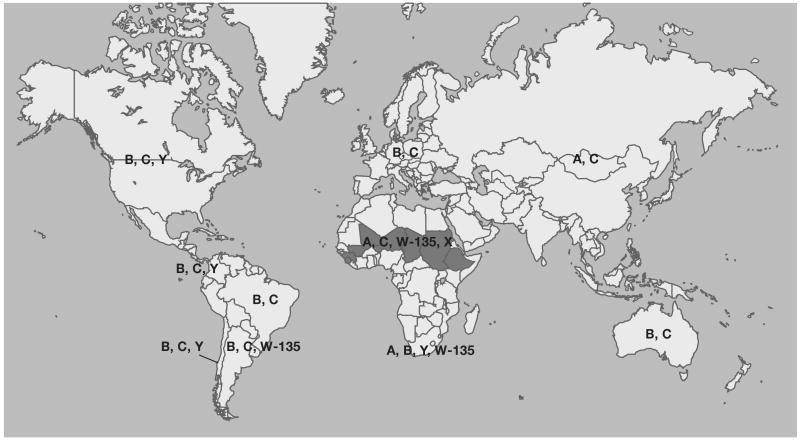
Serogroup A N. meningitidis occurs primarily in the meningitis belt of sub-Saharan Africa and is responsible for the largest and most devastating meningococcal epidemics [7, 8]. Serogroup A meningococcal disease also occurs in other parts of the world, such as China and Russia, but is currently extremely rare in the United States. The lack of serogroup A disease in the U.S. is despite the documented introduction of virulent serogroup A strains into this country [9]. Serogroup B strains are a cause of a substantial proportion of endemic meningococcal disease in many parts of the world, including the United States, as well as prolonged epidemics [10, 11]. Serogroup C, also a prominent serogroup in many regions of the world, has occasionally caused epidemics and frequently causes outbreaks [12]. Serogroup Y strains cause a high proportion of cases in the US and other countries in the Americas [13, 14]. Although not generally considered to be one of the major meningococcal serogroups, serogroup X strains have been reported to cause a substantial amount of meningococcal disease in some countries in Africa, such as Niger, Togo, and Western Kenya [15-17]. The reasons for the distinct serogroup distribution in different parts of the world are unknown but possible mechanisms include differences in population immunity and environmental factors. A summary of the global distribution of meningococcal serogroups is shown in figure 1.
Figure 1.
Worldwide distribution of major meningococcal serogroups.
Molecular Mechanisms that Play a Role in the Dynamic Epidemiology of Meningococal Disease
The meningococcus uses several mechanisms to change its characteristics, such as antigenic structure or resistance to antibiotics. Many of these changes occur through horizontal gene transfer, which permits the organism to obtain large DNA sequences from other meningococcal strains or other species [18]. This organism also utilizes multiple other mechanisms to achieve antigenic variation [19-26]. One such mechanism is gene conversion, which involves autologous recombination. As an example, PilE is a prominent component of the N. meningitidis pilus that is encoded by pilE. Contiguous to pilE are eight truncated pseudogenes that are able to undergo recombination with pilE, which allows for generation of major antigenic variability without the acquisition of foreign DNA.
Capsular switching is a genetic mechanism that allows N. meningitidis to change its capsular phenotype. N. meningitidis outbreaks can be started or sustained using this mechanism, which permits immunologic escape from immunity to the original serogroup [27-30]. Capsular switching occurs through horizontal gene transfer and is generally defined as strains that belong to the same genetic lineage—as determined, for example, by multilocus sequence typing (MLST)—but have a different polysaccharide capsule. Capsular switching presumably occurs when a person is co-colonized in the pharynx with at least 2 or more meningococcal strains [31, 32]. For example, the boyfriend of a young girl who died of serogroup B meningococcal disease had pharyngeal colonization with serogroup C strain that was otherwise genetically indistinguishable [29]. A significant percentage of meningococcal strains that cause disease in the US apparently have arisen through the mechanism of capsular switching [33].
Capsular switching appears to have been responsible for an outbreak of serogroup W-135 disease during the 2000 Hajj in Mecca, Saudi Arabia. Subsequent to this outbreak, the epidemic strain spread globally and, in one example, caused an epidemic in Burkina Faso [34, 35]. Capsular switching was also observed in the 1990s during Oregon's serogroup B outbreak, with some serogroup C strains found to be otherwise genetically indistinguishable from the serogroup B outbreak strain [10, 27].
A key concern is that, with mass immunization using vaccines that do not include protection against all of the major meningococcal serogroups, there could be an increase in meningococcal disease caused by strains not included in the vaccine. This could occur through the mechanisms of capsular switching or capsular replacement. Serotype replacement has been observed since the routine use of pediatric pneumococcal conjugate vaccine began in the US in 2000 [36, 37]. However, meningococcal serogroup replacement has not been observed in the UK since the introduction of routine immunization with serogroup C conjugate vaccines [38]. Serogroup C carriage is relatively uncommon, even in the face of a substantial incidence of serogroup C meningococcal disease; it remains to be seen if serogroup replacement will occur with the use of meningococcal vaccines that cover a higher proportion of carriage strains.
Increases in the incidence of meningococcal disease have also been associated with changes in noncapsular outer-membrane proteins. This observation has implications for outer-membrane protein–based vaccines for the prevention of serogroup B meningococcal disease. In Maryland, an increase in the incidence of serogroup C and serogroup Y meningococcal disease occurred in association with substantial antigenic shift in several outer-membrane proteins [14]. For serogroup C, horizontal gene transfer led to major antigenic changes in FetA, and the porA gene was entirely deleted from some strains. For serogroup Y, major antigenic changes were caused by horizontal gene transfer involving three outer-membrane protein genes.
Risk Factors
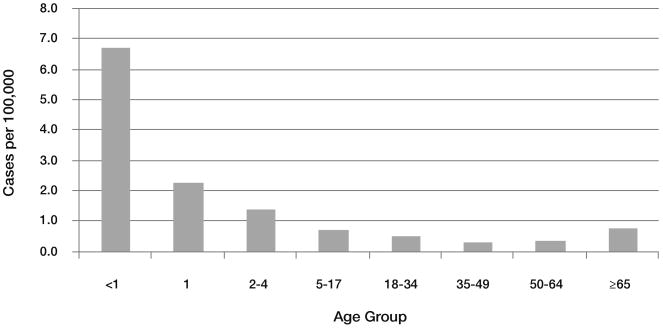
There are numerous known risk factors for meningococcal disease; incidence is influenced strongly by age, with infants having the highest risk [13] (figure 2). Low levels of serum bactericidal antibody (SBA) are among the most important host factors responsible for the risk of infection [39, 40]. Low socioeconomic status and minority ethnicity have also been found to be associated with increased risk [4, 13, 41, 42]. Conditions associated with immune compromise, such as functional or anatomic asplenia, HIV infection, and genetic polymorphisms and deficiencies in components of the innate immune system have all been associated with increased risk of meningococcal disease [43-51].
Figure 2.
Average annual incidence of invasive meningococcal disease, 1997–2007, by age group, US.
Environmental factors associated with the risk of both invasive disease and carriage include recent or concurrent upper respiratory infection, such as influenza [52-57]. In the meningitis belt of sub-Saharan Africa, epidemics commence during the dry season and end with the onset of the rainy season [58]. Population crowding has long been known to play a role in the risk of meningococcal disease [59]. More recently, behavioral risk factors, such as passive and active smoking, pub and bar patronage, kissing, and living in a university dormitory, have been shown to be associated with the risk of meningococcal carriage and disease in a variety of studies [60-70].
Epidemiology in the United States
The Active Bacterial Core surveillance (ABCs) network, a population-based surveillance system for invasive meningococcal disease and other serious bacterial pathogens, has provided invaluable information about the epidemiology of meningococcal disease in the United States [71]. Because active surveillance methods are used, ABCs is highly sensitive for culture-positive N. meningitidis disease and other bacterial pathogens. In 2009, approximately 40 million persons, or about 13% of the US population, lived in an area with ABCs meningococcal disease surveillance.
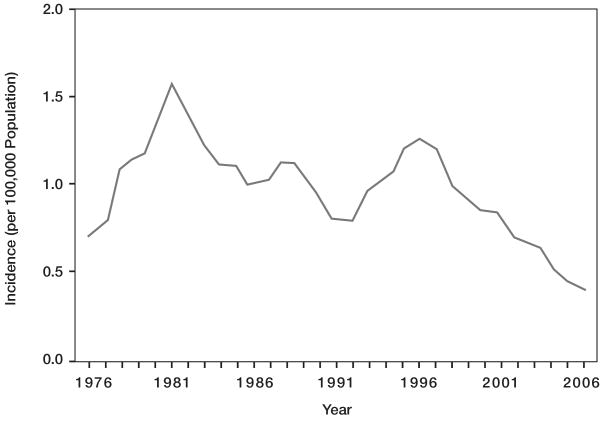
Since World War II, the annual incidence of meningococcal disease has varied between ∼0.5–1.5 cases per 100,000 population. During the past 3 decades, the incidence has waxed and waned in multiyear cycles [3, 72] (figure 3). The most recent peak in incidence occurred during the mid-1990s. Subsequently, there was a decline to ∼0.35 cases per 100,000 in 2007, caused by decreases in the 3 most common serogroups in the US: B, C, and Y. Interestingly, the current nadir in incidence began before the introduction of tetravalent meningococcal conjugate vaccine (MCV4) and has been more sustained than previously. In addition, MCV4 does not contain a serogroup B component and therefore would have had no influence on the reduction in serogroup B disease. The factors responsible for this substantial decline in the overall rate of meningococcal disease are unknown but could include population immunity to the strains currently circulating in the U.S., changes in the prevalence of behavioral risk factors, or unknown variables.
Figure 3.
Invasive meningococcal disease incidence (per 100,000), 1976–2006, by year, US [72].
Meningococcal serogroup distribution varies over time. For example, serogroup Y disease accounted for only 2% of meningococcal infections during 1989–1991 [41]. By the mid-1990s, serogroup Y incidence increased and serogroup Y strains accounted for one third of meningococcal infections [13]. The increase in serogroup Y disease in Maryland occurred primarily in children < 15 years old and adults > 25 years old [73]. Serogroup C meningococcal disease also increased and subsequently decreased during the 1990s. Serogroup W-135 disease, which currently accounts for a small percentage of cases, was previously more common [13, 74, 75]. On the basis of ABCs data, the serogroup distribution in the US in 2007 was serogroup B, 25%; serogroup C, 30%; and serogroup Y, 37%, with 9% caused by serogroup W-135, other serogroups, and nongroupable strains (www.cdc.gov/ncidod/DBMD/abcs/survreports/mening07.pdf). The proportion of isolates that are serogroup B is higher in Oregon, as is its incidence, because of an ongoing outbreak involving a serogroup B sequence type (ST)-32 complex/enzyme type-5 complex clone [10].
As the incidence in meningococcal disease increased in the US during the 1990s, the number of N. meningitidis outbreaks also increased. Between mid-1994 and mid-2002, 76 outbreaks in the community, specifically in colleges, primary and secondary schools, and nursing homes, were identified throughout the US [12, 76-79]. The majority of outbreaks were caused by serogroup C strains.
Current Meningococcal Vaccines in the United States
Two tetravalent (A/C/Y/W-135) meningococcal vaccines are licensed in the US, one a purified polysaccharide product and the other a polysaccharide–protein conjugate vaccine (MCV4), with diphtheria toxoid as the protein carrier [80]. MCV4 was licensed in 2005 and is recommended for all US adolescents. In 2007, despite this universal recommendation, only 33% of 13-year-olds had received MCV4 as determined by the National Immunization Survey [81]. MCV4 is also recommended for college freshmen living in dormitories, travelers to areas with hyperendemic or epidemic meningococcal disease, microbiologists working with live N. meningitidis, military recruits, and persons with immunologic deficits, such as terminal complement deficiency and functional or anatomic asplenia [80].
The current focus on adolescents is a laudable step towards prevention of meningococcal disease in the US, although a comprehensive program will require immunization of infants, the group with the highest risk [82, 83]. Several meningococcal conjugate vaccines that are immunogenic in infants may soon be licensed in the US: a second tetravalent (A/C/Y/W-135) polysaccharide–protein conjugate vaccine that uses CRM197 as the protein carrier [84-86] and a combination Haemophilus influenzae serotype B and serogroup C/Y meningococcal conjugate vaccine, with each polysaccharide component conjugated to tetanus toxoid [87]. Outer-membrane protein–based vaccines for the prevention of endemic serogroup B disease are also in development for use in the US and globally [88, 89], although these vaccines will probably not be available for several more years. The addition of protection against serogroup B meningococcal disease is crucial because of the importance of disease caused by this serogroup in many countries [1].
United Kingdom Experience With Serogroup C Meningococcal Conjugate Vaccines
Because insufficient time has elapsed following the routine use of MCV4 and the low vaccine coverage rates among adolescents, it is not possible to draw conclusions about the eventual impact of routine meningococcal immunization in the US [81]. However, several recent experiences from abroad provide valuable information about this issue. In 1999, the UK was the first country to introduce serogroup C meningococcal conjugate vaccines to control the growing burden of serogroup C disease. This program has been highly successful in reducing the incidence of serogroup C meningococcal disease in immunized persons [90, 91]. In addition, serogroup C meningococcal pharyngeal carriage has been reduced, leading to a reduction in serogroup C meningococcal disease in the unimmunized population, known as the herd immune effect [92, 93]. The reduction in serogroup C disease resulted in a substantial decrease in the overall incidence of meningococcal disease [94]. The success of this program led to the subsequent introduction of meningococcal conjugate vaccines into routine immunization programs in many European countries, as well as other parts of the world [95-97]. With the success of the immunization campaign in the UK, nearly 90% of invasive meningococcal disease in that country is now caused by serogroup B strains.
New Zealand Experience with Serogroup B Vaccine
In the US and many other parts of the world, a substantial proportion of meningococcal disease is caused by serogroup B N. meningitidis, which is antigenically highly variable in endemic settings. Unfortunately, no licensed vaccine exists that covers all serogroup B strains. However, serogroup B epidemics are caused by single clones, which has allowed for the development of tailor-made serogroup B vaccines. The incidence of meningococcal disease in New Zealand increased from around 1.6 per 100,000 incidence in 1990 to a peak of 17.4 per 100,000 in 2001. This was the result primarily of the emergence of an ST-41/44 clonal complex/lineage 3 serogroup B clone which accounted for 85% of disease by 2000 [11, 98]. The highest rates of disease occurred among young children and a disproportionate number of cases occurred in Pacific Islander and Maori children [11].
This epidemic led to the development and introduction of an outer-membrane vesicle vaccine against the epidemic strain [99]. The vaccine was introduced in mid-2004 and introduced across the country over a period of 2 years, starting with the high-incidence areas of North Island. The incidence of meningococcal disease declined to 2.6 per 100,000 by 2007, and the estimated effectiveness of the vaccine was 80% in fully immunized children aged 6 months to < 5 years old [100]. However, no data are available on the impact, if any, of this vaccine on meningococcal carriage in those who are immunized, or on the incidence of meningococcal disease in the unimmunized.
In summary, the epidemiology of meningococcal disease in the US is highly dynamic and constantly changing. The introduction of MCV4 into the routine immunization schedule for adolescents is a promising first step but additional work is needed. First, efforts must be made to increase vaccine coverage with MCV4, as well as the other vaccines that are now recommended for adolescents. Second, conjugate vaccines for infants, which are likely to be available soon, are required. Finally, broadly protective vaccines that prevent both endemic and epidemic serogroup B disease are needed.
Acknowledgments
Lee H. Harrison, MD, has received a career development award from the National Institute of Allergy and Infectious Diseases (NIAID) (K24 AI52788) and receives research funding from Sanofi Pasteur; he has received consulting fees and speaking honoraria from Sanofi Pasteur, Novartis, Merck, Wyeth, and GlaxoSmithKline.
The opinions expressed in this review are solely those of Dr. Harrison. Dr. Harrison reports that the content of this paper overlaps with the content of other reviews that he has written, either as the sole author [2] or with other authors [1, 3, 4].
References
- 1.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27 2:B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev. 2006;19:142–64. doi: 10.1128/CMR.19.1.142-164.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granoff DM, Harrison LH, Borrow R. Meningococcal vaccines. In: Plotkin S, Orenstein WA, Offit PA, editors. Vaccines. Fifth. Philadelphia: Saunders Elsevier; 2008. pp. 399–434. [Google Scholar]
- 4.Harrison LH, Broome CV. The epidemiology of meningococcal meningitis in the U.S civilian population. In: Vedros NA, editor. Evolution of meningococcal disease. Vol. 1. Boca Raton, Fla: CRC Press; 1987. pp. 27–45. [Google Scholar]
- 5.Vogel U, Claus H, von Muller L, Bunjes D, Elias J, Frosch M. Bacteremia in an immunocompromised patient caused by a commensal Neisseria meningitidis strain harboring the capsule null locus (cnl) J Clin Microbiol. 2004;42:2898–901. doi: 10.1128/JCM.42.7.2898-2901.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoang LM, Thomas E, Tyler S, et al. Rapid and fatal meningococcal disease due to a strain of Neisseria meningitidis containing the capsule null locus. Clin Infect Dis. 2005;40:e38–42. doi: 10.1086/427875. [DOI] [PubMed] [Google Scholar]
- 7.Greenwood B. Manson Lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999;93:341–53. doi: 10.1016/s0035-9203(99)90106-2. [DOI] [PubMed] [Google Scholar]
- 8.Roberts L. Hitting early, epidemic meningitis ravages Nigeria and Niger. Science. 2009;324:20–1. doi: 10.1126/science.324.5923.20. [DOI] [PubMed] [Google Scholar]
- 9.Moore PS, Harrison LH, Telzak EE, Ajello GW, Broome CV. Group A meningococcal carriage in travelers returning from Saudi Arabia. JAMA. 1988;260:2686–9. [PubMed] [Google Scholar]
- 10.Diermayer M, Hedberg K, Hoesly F, et al. Epidemic serogroup B meningococcal disease in Oregon: the evolving epidemiology of the ET-5 strain. Jama. 1999;281:1493–7. doi: 10.1001/jama.281.16.1493. [DOI] [PubMed] [Google Scholar]
- 11.Baker MG, Martin DR, Kieft CE, Lennon D. A 10-year serogroup B meningococcal disease epidemic in New Zealand: descriptive epidemiology, 1991-2000. J Paediatr Child Health. 2001;37:S13–9. doi: 10.1046/j.1440-1754.2001.00722.x. [DOI] [PubMed] [Google Scholar]
- 12.Brooks R, Woods CW, Benjamin DK, Jr, Rosenstein NE. Increased case-fatality rate associated with outbreaks of Neisseria meningitidis infection, compared with sporadic meningococcal disease, in the United States, 1994-2002. Clin Infect Dis. 2006;43:49–54. doi: 10.1086/504804. [DOI] [PubMed] [Google Scholar]
- 13.Rosenstein NE, Perkins BA, Stephens DS, et al. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J Infect Dis. 1999;180:1894–901. doi: 10.1086/315158. [DOI] [PubMed] [Google Scholar]
- 14.Harrison LH, Jolley KA, Shutt KA, et al. Antigenic shift and increased incidence of meningococcal disease. J Infect Dis. 2006;193:1266–74. doi: 10.1086/501371. [DOI] [PubMed] [Google Scholar]
- 15.Boisier P, Nicolas P, Djibo S, et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007;44:657–63. doi: 10.1086/511646. [DOI] [PubMed] [Google Scholar]
- 16.Njanpop Lafourcade BM, Tamekloe TA, Snou O, et al. Serogroup X meningococcal meningitis in Togo during 2007 and 2008. International Pathogenic Neisseria Conference; Rotterdam, Netherlands. September 7-12; 2008. abstract P140. [Google Scholar]
- 17.Mutonga DM, Pimentel G, Muindi J, et al. Epidemiology and risk factors for serogroup X meningococcal meningitis during an outbreak in western Kenya, 2005-2006. Am J Trop Med Hyg. 2009;80:619–24. [PubMed] [Google Scholar]
- 18.Wu HM, Harcourt BH, Hatcher CP, et al. Emergence of ciprofloxacin-resistant Neisseria meningitidis in North America. N Engl J Med. 2009;360:886–92. doi: 10.1056/NEJMoa0806414. [DOI] [PubMed] [Google Scholar]
- 19.Andrews TD, Gojobori T. Strong positive selection and recombination drive the antigenic variation of the PilE protein of the human pathogen Neisseria meningitidis. Genetics. 2004;166:25–32. doi: 10.1534/genetics.166.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howell-Adams B, Seifert HS. Molecular models accounting for the gene conversion reactions mediating gonococcal pilin antigenic variation. Mol Microbiol. 2000;37:1146–58. doi: 10.1046/j.1365-2958.2000.02067.x. [DOI] [PubMed] [Google Scholar]
- 21.Saunders NJ, Jeffries AC, Peden JF, et al. Repeat-associated phase variable genes in the complete genome sequence of Neisseria meningitidis strain MC58. Mol Microbiol. 2000;37:207–15. doi: 10.1046/j.1365-2958.2000.02000.x. [DOI] [PubMed] [Google Scholar]
- 22.Claus H, Borrow R, Achtman M, et al. Genetics of capsule O-acetylation in serogroup C, W-135 and Y meningococci. Mol Microbiol. 2004;51:227–39. doi: 10.1046/j.1365-2958.2003.03819.x. [DOI] [PubMed] [Google Scholar]
- 23.Stephens DS, Swartley JS, Kathariou S, Morse SA. Insertion of Tn916 in Neisseria meningitidis resulting in loss of group B capsular polysaccharide. Infect Immun. 1991;59:4097–102. doi: 10.1128/iai.59.11.4097-4102.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolan-Livengood JM, Miller YK, Martin LE, Urwin R, Stephens DS. Genetic basis for nongroupable Neisseria meningitidis. J Infect Dis. 2003;187:1616–28. doi: 10.1086/374740. [DOI] [PubMed] [Google Scholar]
- 25.Uria MJ, Zhang Q, Li Y, et al. A generic mechanism in Neisseria meningitidis for enhanced resistance against bactericidal antibodies. J Exp Med. 2008;205:1423–34. doi: 10.1084/jem.20072577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh JW, Conley AM, Harrison LH. IS1301 insertion in opaD of an emergent Neisseria meningitidis ET-15 clone. International Pathogenic Neisseria Conference; Rotterdam, Netherlands. September 7-12; 2008. [Google Scholar]
- 27.Swartley JS, Marfin AA, Edupuganti S, et al. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci U S A. 1997;94:271–6. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguilera JF, Perrocheau A, Meffre C, Hahne S. Outbreak of serogroup W135 meningococcal disease after the Hajj pilgrimage, Europe, 2000. Emerg Infect Dis. 2002;8:761–7. doi: 10.3201/eid0808.010422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel U, Claus H, Frosch M. Rapid serogroup switching in Neisseria meningitidis. N Engl J Med. 2000;342:219–20. doi: 10.1056/NEJM200001203420319. [DOI] [PubMed] [Google Scholar]
- 30.Kertesz DA, Coulthart MB, Ryan JA, Johnson WM, Ashton FE. Serogroup B, electrophoretic type 15 Neisseria meningitidis in Canada. J Infect Dis. 1998;177:1754–7. doi: 10.1086/517439. [DOI] [PubMed] [Google Scholar]
- 31.Maiden MC, Malorny B, Achtman M. A global gene pool in the neisseriae. Mol Microbiol. 1996;21:1297–8. doi: 10.1046/j.1365-2958.1996.981457.x. [DOI] [PubMed] [Google Scholar]
- 32.Linz B, Schenker M, Zhu P, Achtman M. Frequent interspecific genetic exchange between commensal Neisseriae and Neisseria meningitidis. Mol Microbiol. 2000;36:1049–58. doi: 10.1046/j.1365-2958.2000.01932.x. [DOI] [PubMed] [Google Scholar]
- 33.Harrison LH, Shutt KA, Marsh JW, et al. Evidence of capsular switching in invasive Neisseria meningitidis isolates in the pre-meningococcal conjugate vaccine era, United States, 2000-2005. International Pathogenic Neisseria Conference; Rotterdam, Netherlands. September 7-12; 2008. [Google Scholar]
- 34.Mayer LW, Reeves MW, Al-Hamdan N, et al. Outbreak of W135 meningococcal disease in 2000: not emergence of a new W135 strain but clonal expansion within the electophoretic type-37 complex. J Infect Dis. 2002;185:1596–605. doi: 10.1086/340414. [DOI] [PubMed] [Google Scholar]
- 35.Zombre S, Hacen MM, Ouango G, et al. The outbreak of meningitis due to Neisseria meningitidis W135 in 2003 in Burkina Faso and the national response: main lessons learnt. Vaccine. 2007;25 1:A69–71. doi: 10.1016/j.vaccine.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 36.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196:1346–54. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 37.Hsu HE, Shutt KA, Moore MR, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009;360:244–56. doi: 10.1056/NEJMoa0800836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trotter CL, Ramsay ME, Gray S, Fox A, Kaczmarski E. No evidence for capsule replacement following mass immunisation with meningococcal serogroup C conjugate vaccines in England and Wales. Lancet Infect Dis. 2006;6:616–7. doi: 10.1016/S1473-3099(06)70584-9. author reply 617-8. [DOI] [PubMed] [Google Scholar]
- 39.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–48. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson LA, Wenger JD. Laboratory-based surveillance for meningococcal disease in selected areas, United States, 1989-1991. MMWR CDC Surveill Summ. 1993;42:21–30. [PubMed] [Google Scholar]
- 42.Jones IR, Urwin G, Feldman RA, Banatvala N. Social deprivation and bacterial meningitis in north east Thames region: three year study using small area statistics. Bmj. 1997;314:794–5. doi: 10.1136/bmj.314.7083.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Platonov AE, Vershinina IV, Kuijper EJ, Borrow R, Kayhty H. Long term effects of vaccination of patients deficient in a late complement component with a tetravalent meningococcal polysaccharide vaccine. Vaccine. 2003;21:4437–47. doi: 10.1016/s0264-410x(03)00440-7. [DOI] [PubMed] [Google Scholar]
- 44.Stephens DS, Hajjeh RA, Baughman WS, Harvey RC, Wenger JD, Farley MM. Sporadic meningococcal disease in adults: results of a 5-year population-based study. Ann Intern Med. 1995;123:937–40. doi: 10.7326/0003-4819-123-12-199512150-00007. [DOI] [PubMed] [Google Scholar]
- 45.Holmes FF, Weyandt T, Glazier J, Cuppage FE, Moral LA, Lindsey NJ. Fulminant Meningococcemia after splenectomy. Jama. 1981;246:1119–20. [PubMed] [Google Scholar]
- 46.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–95. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francke EL, Neu HC. Postsplenectomy infection. Surg Clin North Am. 1981;61:135–55. doi: 10.1016/s0039-6109(16)42339-x. [DOI] [PubMed] [Google Scholar]
- 48.Salimans MM, Bax WA, Stegeman F, van Deuren M, Bartelink AK, van Dijk H. Association between familial deficiency of mannose-binding lectin and mutations in the corresponding gene and promoter region. Clin Diagn Lab Immunol. 2004;11:806–7. doi: 10.1128/CDLI.11.4.806-807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuipers S, Aerts PC, Cluysenaer OJ, et al. A case of familial meningococcal disease due to deficiency in mannose-binding lectin (MBL) Adv Exp Med Biol. 2003;531:351–5. doi: 10.1007/978-1-4615-0059-9_29. [DOI] [PubMed] [Google Scholar]
- 50.Emonts M, Hazelzet JA, de Groot R, Hermans PW. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect Dis. 2003;3:565–77. doi: 10.1016/s1473-3099(03)00740-0. [DOI] [PubMed] [Google Scholar]
- 51.Smirnova I, Mann N, Dols A, et al. Assay of locus-specific genetic load implicates rare Toll-like receptor 4 mutations in meningococcal susceptibility. Proc Natl Acad Sci U S A. 2003;100:6075–80. doi: 10.1073/pnas.1031605100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olcen P, Kjellander J, Danielsson D, Lindquist BL. Epidemiology of Neisseria meningitidis; prevalence and symptoms from the upper respiratory tract in family members to patients with meningococcal disease. Scand J Infect Dis. 1981;13:105–9. doi: 10.3109/inf.1981.13.issue-2.05. [DOI] [PubMed] [Google Scholar]
- 53.Young LS, LaForce FM, Head JJ, Feeley JC, Bennett JV. A simultaneous outbreak of meningococcal and influenza infections. N Engl J Med. 1972;287:5–9. doi: 10.1056/NEJM197207062870102. [DOI] [PubMed] [Google Scholar]
- 54.Harrison LH, Armstrong CW, Jenkins SR, et al. A cluster of meningococcal disease on a school bus following epidemic influenza. Arch Intern Med. 1991;151:1005–9. [PubMed] [Google Scholar]
- 55.Moore PS, Hierholzer J, DeWitt W, et al. Respiratory viruses and mycoplasma as cofactors for epidemic group A meningococcal meningitis. Jama. 1990;264:1271–5. [PubMed] [Google Scholar]
- 56.Krasinski K, Nelson JD, Butler S, Luby JP, Kusmiesz H. Possible association of mycoplasma and viral respiratory infections with bacterial meningitis. Am J Epidemiol. 1987;125:499–508. doi: 10.1093/oxfordjournals.aje.a114556. [DOI] [PubMed] [Google Scholar]
- 57.Cartwright KA, Jones DM, Smith AJ, Stuart JM, Kaczmarski EB, Palmer SR. Influenza A and meningococcal disease. Lancet. 1991;338:554–7. doi: 10.1016/0140-6736(91)91112-8. [DOI] [PubMed] [Google Scholar]
- 58.Greenwood BM, Bradley AK, Cleland PG, et al. An epidemic of meningococcal infection at Zaria, Northern Nigeria. 1. General epidemiological features. Trans R Soc Trop Med Hyg. 1979;73:557–62. doi: 10.1016/0035-9203(79)90052-x. [DOI] [PubMed] [Google Scholar]
- 59.Brundage JF, Zollinger WD. Evolution of meningococcal disease epidemiology in the US army. In: Vedros NA, editor. Evolution of Meningococcal Disease. I. Boca Raton, FL: CRC Press; 1987. pp. 5–25. [Google Scholar]
- 60.Tappero JW, Reporter R, Wenger JD, et al. Meningococcal disease in Los Angeles County, California, and among men in the county jails. N Engl J Med. 1996;335:833–40. doi: 10.1056/NEJM199609193351201. [DOI] [PubMed] [Google Scholar]
- 61.Imrey PB, Jackson LA, Ludwinski PH, et al. Outbreak of serogroup C meningococcal disease associated with campus bar patronage. Am J Epidemiol. 1996;143:624–30. doi: 10.1093/oxfordjournals.aje.a008792. [DOI] [PubMed] [Google Scholar]
- 62.Imrey PB, Jackson LA, Ludwinski PH, et al. Meningococcal carriage, alcohol consumption, and campus bar patronage in a serogroup C meningococcal disease outbreak. J Clin Microbiol. 1995;33:3133–7. doi: 10.1128/jcm.33.12.3133-3137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischer M, Hedberg K, Cardosi P, et al. Tobacco smoke as a risk factor for meningococcal disease. Pediatr Infect Dis J. 1997;16:979–83. doi: 10.1097/00006454-199710000-00015. [DOI] [PubMed] [Google Scholar]
- 64.Cookson ST, Corrales JL, Lotero JO, et al. Disco fever: epidemic meningococcal disease in northeastern Argentina associated with disco patronage. J Infect Dis. 1998;178:266–9. doi: 10.1086/517450. [DOI] [PubMed] [Google Scholar]
- 65.Froeschle JE. Meningococcal disease in college students. Clin Infect Dis. 1999;29:215–6. doi: 10.1086/520166. [DOI] [PubMed] [Google Scholar]
- 66.Harrison LH, Dwyer DM, Maples CT, Billmann L. Risk of meningococcal infection in college students. Jama. 1999;281:1906–10. doi: 10.1001/jama.281.20.1906. [DOI] [PubMed] [Google Scholar]
- 67.Bruce MG, Rosenstein NE, Capparella JM, Shutt KA, Perkins BA, Collins M. Risk factors for meningococcal disease in college students. JAMA. 2001;286:688–93. doi: 10.1001/jama.286.6.688. [DOI] [PubMed] [Google Scholar]
- 68.MacLennan J, Kafatos G, Neal K, et al. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis. 2006;12:950–7. doi: 10.3201/eid1206.051297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neal KR, Nguyen-Van-Tam JS, Jeffrey N, et al. Changing carriage rate of Neisseria meningitidis among university students during the first week of term: cross sectional study. Bmj. 2000;320:846–9. doi: 10.1136/bmj.320.7238.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrison LH, Kreiner CJ, Shutt KA, et al. Risk factors for meningococcal disease in students in grades 9-12. Pediatr Infect Dis J. 2008;27:193–9. doi: 10.1097/INF.0b013e31815c1b3a. [DOI] [PubMed] [Google Scholar]
- 71.Schuchat A, Hilger T, Zell E, et al. Active bacterial core surveillance of the emerging infections program network. Emerg Infect Dis. 2001;7:92–9. doi: 10.3201/eid0701.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McNabb SJ, Jajosky RA, Hall-Baker PA, et al. Summary of notifiable diseases--United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;55:1–92. [PubMed] [Google Scholar]
- 73.McEllistrem MC, Kolano JA, Pass MA, et al. Correlating epidemiologic trends with the genotypes causing meningococcal disease, Maryland. Emerg Infect Dis. 2004;10:451–456. doi: 10.3201/eid1003.020611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Band JD, Chamberland ME, Platt T, Weaver RE, Thornsberry C, Fraser DW. Trends in meningococcal disease in the United States, 1975-1980. J Infect Dis. 1983;148:754–8. doi: 10.1093/infdis/148.4.754. [DOI] [PubMed] [Google Scholar]
- 75.Galaid EI, Cherubin CE, Marr JS, Schaefler S, Barone J, Lee W. Meningococcal disease in New York City, 1973 to 1978. Recognition of groups Y and W-135 as frequent pathogens. JAMA. 1980;244:2167–71. [PubMed] [Google Scholar]
- 76.Jackson LA, Schuchat A, Reeves MW, Wenger JD. Serogroup C meningococcal outbreaks in the United States. An emerging threat Jama. 1995;273:383–9. [PubMed] [Google Scholar]
- 77.Zangwill KM, Schuchat A, Riedo FX, et al. School-based clusters of meningococcal disease in the United States. Descriptive epidemiology and a case-control analysis. Jama. 1997;277:389–95. [PubMed] [Google Scholar]
- 78.Finn R, Groves C, Coe M, Pass M, Harrison LH. Cluster of serogroup C meningococcal disease associated with attendance at a party. South Med J. 2001;94:1192–4. [PubMed] [Google Scholar]
- 79.Whalen CM, Hockin JC, Ryan A, Ashton F. The changing epidemiology of invasive meningococcal disease in Canada, 1985 through 1992. Emergence of a virulent clone of Neisseria meningitidis. Jama. 1995;273:390–4. [PubMed] [Google Scholar]
- 80.Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54:1–21. [PubMed] [Google Scholar]
- 81.Vaccination coverage among adolescents aged 13-17 years - United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:1100–3. [PubMed] [Google Scholar]
- 82.Harrison LH, Pass MA, Mendelsohn AB, et al. Invasive meningococcal disease in adolescents and young adults. JAMA. 2001;286:694–9. doi: 10.1001/jama.286.6.694. [DOI] [PubMed] [Google Scholar]
- 83.Lingappa JR, Rosenstein N, Zell ER, Shutt KA, Schuchat A, Perkins BA. Surveillance for meningococcal disease and strategies for use of conjugate meningococcal vaccines in the United States. Vaccine. 2001;19:4566–75. doi: 10.1016/s0264-410x(01)00209-2. [DOI] [PubMed] [Google Scholar]
- 84.Snape MD, Perrett KP, Ford KJ, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA. 2008;299:173–84. doi: 10.1001/jama.2007.29-c. [DOI] [PubMed] [Google Scholar]
- 85.Harrison LH. A multivalent conjugate vaccine for prevention of meningococcal disease in infants. JAMA. 2008;299:217–9. doi: 10.1001/jama.2007.57-c. [DOI] [PubMed] [Google Scholar]
- 86.Jackson LA, Baxter R, Reisinger K, et al. Phase III Comparison of an Investigational Quadrivalent Meningococcal Conjugate Vaccine with the Licensed Meningococcal ACWY Conjugate Vaccine in Adolescents. Clin Infect Dis. 2009 doi: 10.1086/599117. [DOI] [PubMed] [Google Scholar]
- 87.Nolan T, Lambert S, Roberton D, et al. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus-toxoid conjugate vaccine is immunogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine. 2007;25:8487–99. doi: 10.1016/j.vaccine.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 88.Giuliani MM, Adu-Bobie J, Comanducci M, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006;103:10834–9. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fletcher LD, Bernfield L, Barniak V, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun. 2004;72:2088–100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–7. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 91.Trotter CL, Ramsay ME, Kaczmarski EB. Meningococcal serogroup C conjugate vaccination in England and Wales: coverage and initial impact of the campaign. Commun Dis Public Health. 2002;5:220–5. [PubMed] [Google Scholar]
- 92.Maiden MC, Stuart JM. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet. 2002;359:1829–31. doi: 10.1016/S0140-6736(02)08679-8. [DOI] [PubMed] [Google Scholar]
- 93.Maiden MC, Ibarz-Pavon AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gray SJ, Trotter CL, Ramsay ME, et al. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol. 2006;55:887–96. doi: 10.1099/jmm.0.46288-0. [DOI] [PubMed] [Google Scholar]
- 95.Trotter CL, Ramsay ME. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol Rev. 2007;31:101–7. doi: 10.1111/j.1574-6976.2006.00053.x. [DOI] [PubMed] [Google Scholar]
- 96.Tapsall J. Annual report of the Australian Meningococcal Surveillance Programme, 2007. Commun Dis Intell. 2008;32:299–307. [PubMed] [Google Scholar]
- 97.De Wals P. Meningococcal C vaccines: the canadian experience. Pediatr Infect Dis J. 2004;23:S280–4. [PubMed] [Google Scholar]
- 98.O'Hallahan J, Lennon D, Oster P, et al. From secondary prevention to primary prevention: a unique strategy that gives hope to a country ravaged by meningococcal disease. Vaccine. 2005;23:2197–201. doi: 10.1016/j.vaccine.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 99.Oster P, Lennon D, O'Hallahan J, Mulholland K, Reid S, Martin D. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine. 2005;23:2191–6. doi: 10.1016/j.vaccine.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 100.Galloway Y, Stehr-Green P, McNicholas A, O'Hallahan J. Use of an observational cohort study to estimate the effectiveness of the New Zealand group B meningococcal vaccine in children aged under 5 years. Int J Epidemiol. 2008 doi: 10.1093/ije/dyn228. Epub ahead of print. [DOI] [PubMed] [Google Scholar]