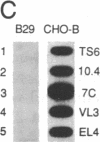
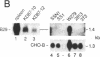
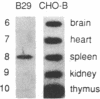
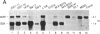Abstract
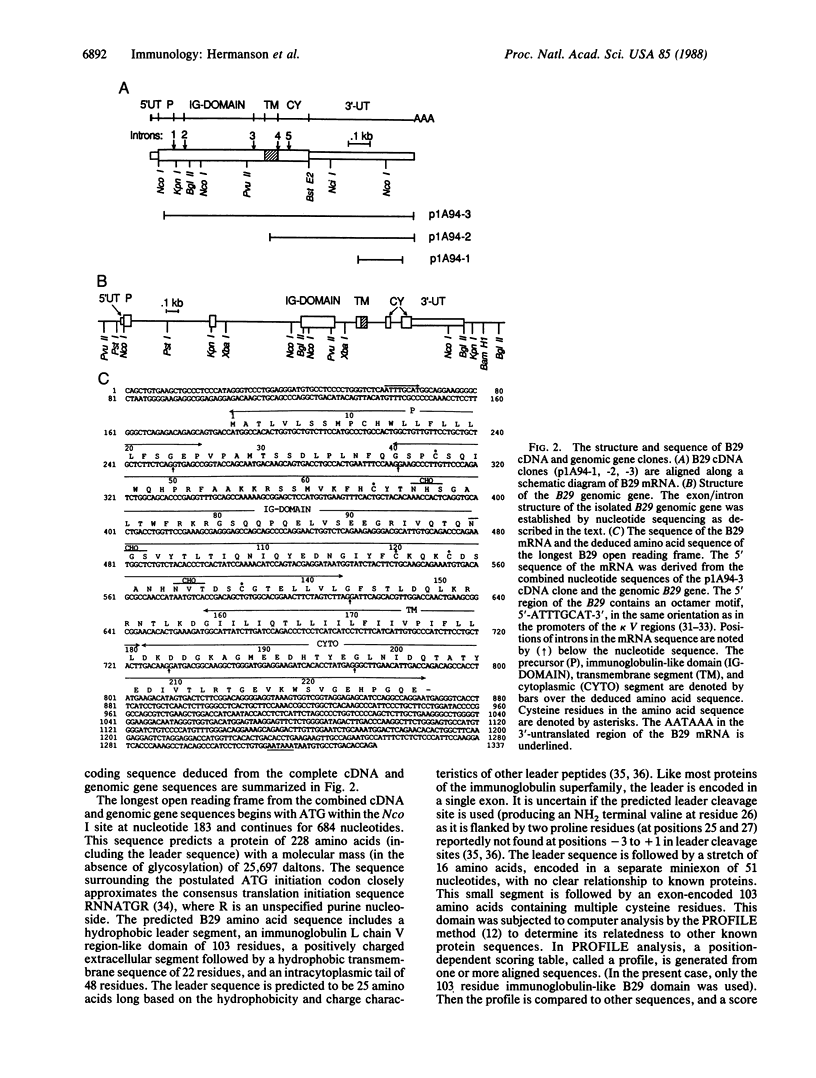
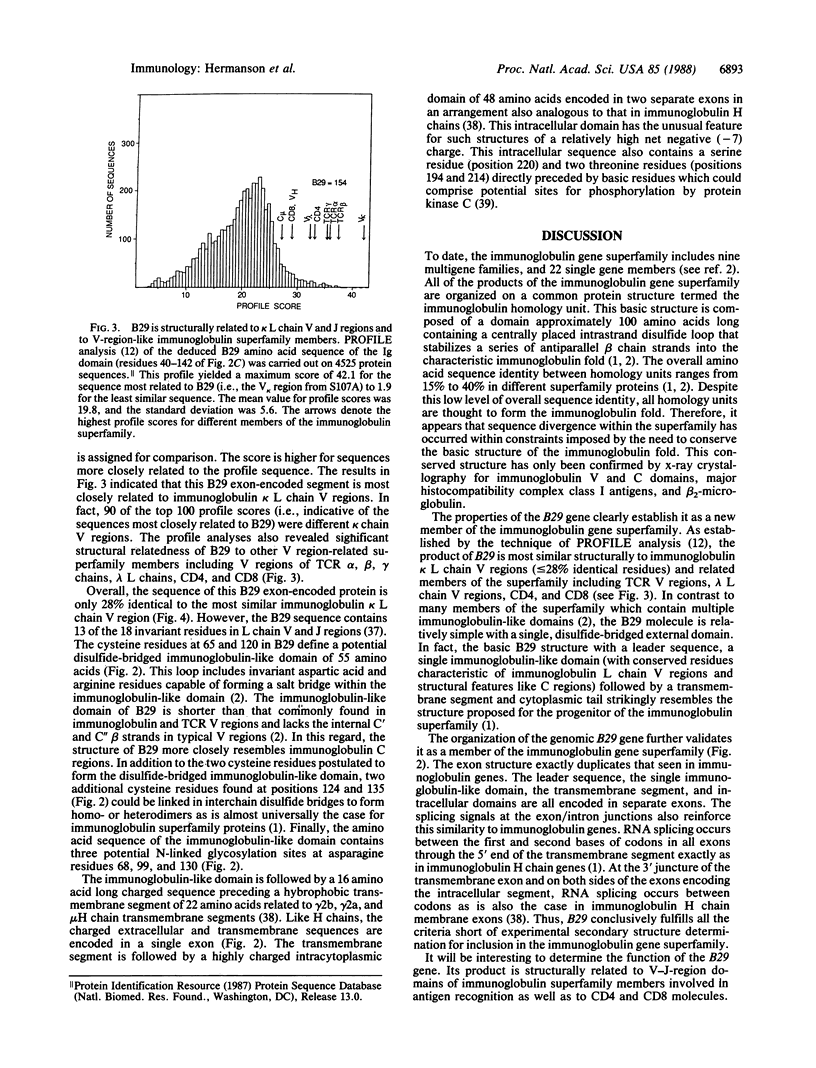
A number of the glycoproteins identified on the surfaces of cells of the immune response belong to the immunoglobulin superfamily. We have isolated and characterized cDNA clones and the complete genomic gene encoding a B-cell-specific member of the immunoglobulin superfamily called "B29." This isolate is expressed at all stages in B-cell development beginning with the earliest precursor B cells undergoing immunoglobulin heavy chain gene diversity region----joining region gene (DH----JH) rearrangements. The protein sequence predicted by the B29 coding region contains a leader sequence and a single extracellular immunoglobulin-like domain, followed by a hydrophobic transmembrane segment and a charged intracytoplasmic domain. The immunoglobulin-like domain contains cysteines and other conserved amino acids characteristic of light chain variable and joining regions, but overall the sequence is only distantly related to immunoglobulins. Each of these domains is encoded in separate exons in the B29 gene, in analogy to other members of the immunoglobulin superfamily. The conserved structural features of the immunoglobulin-like domain in the B29 gene product resemble those of other members of the immunoglobulin superfamily involved in cell recognition and adhesion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calame K. L. Mechanisms that regulate immunoglobulin gene expression. Annu Rev Immunol. 1985;3:159–195. doi: 10.1146/annurev.iy.03.040185.001111. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Cohen D. I., Nielsen E. A., Steinmetz M., Paul W. E., Hood L. Cell-type-specific cDNA probes and the murine I region: the localization and orientation of Ad alpha. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2194–2198. doi: 10.1073/pnas.81.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Gribskov M., McLachlan A. D., Eisenberg D. Profile analysis: detection of distantly related proteins. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4355–4358. doi: 10.1073/pnas.84.13.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley-Hyde J. M., Lynch R. G. The physiology of B cells as studied with tumor models. Annu Rev Immunol. 1986;4:621–649. doi: 10.1146/annurev.iy.04.040186.003201. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hood L., Kronenberg M., Hunkapiller T. T cell antigen receptors and the immunoglobulin supergene family. Cell. 1985 Feb;40(2):225–229. doi: 10.1016/0092-8674(85)90133-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann Y., Berke G., Eshhar Z. Cytotoxic T lymphocyte hybridomas that mediate specific tumor-cell lysis in vitro. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2502–2506. doi: 10.1073/pnas.78.4.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. J., Weinbaum F. I., Mathieson B. J., McKeever P. E., Asofsky R. Characteristics of BALB/C T cell lymphomas grown as continuous in vitro lines. J Immunol. 1978 Jul;121(1):339–344. [PubMed] [Google Scholar]
- Kincade P. W. Experimental models for understanding B lymphocyte formation. Adv Immunol. 1987;41:181–267. doi: 10.1016/s0065-2776(08)60032-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Steinmetz M., Kobori J., Kraig E., Kapp J. A., Pierce C. W., Sorensen C. M., Suzuki G., Tada T., Hood L. RNA transcripts for I-J polypeptides are apparently not encoded between the I-A and I-E subregions of the murine major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5704–5708. doi: 10.1073/pnas.80.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A., Melchers F. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in pre-B lymphocytes. EMBO J. 1987 Aug;6(8):2267–2272. doi: 10.1002/j.1460-2075.1987.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A., Sakaguchi N., Melchers F. Organization of the murine Ig-related lambda 5 gene transcribed selectively in pre-B lymphocytes. EMBO J. 1987 Jan;6(1):103–107. doi: 10.1002/j.1460-2075.1987.tb04725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman D. R. The structure of the CD4 and CD8 genes. Annu Rev Immunol. 1987;5:561–584. doi: 10.1146/annurev.iy.05.040187.003021. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Parslow T. G., Blair D. L., Murphy W. J., Granner D. K. Structure of the 5' ends of immunoglobulin genes: a novel conserved sequence. Proc Natl Acad Sci U S A. 1984 May;81(9):2650–2654. doi: 10.1073/pnas.81.9.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N. W., Marrack P., Kappler J. W. Antigen-specific, H-2-restricted helper T cell hybridomas. J Exp Med. 1982 Jul 1;156(1):191–204. doi: 10.1084/jem.156.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Wall R. Immunoglobulin RNA rearrangements in B lymphocyte differentiation. Adv Immunol. 1984;35:39–59. doi: 10.1016/s0065-2776(08)60573-8. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Berger C. N., Melchers F. Isolation of a cDNA copy of an RNA species expressed in murine pre-B cells. EMBO J. 1986 Sep;5(9):2139–2147. doi: 10.1002/j.1460-2075.1986.tb04477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986 Dec 11;324(6097):579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C. H., Ewald S. J., Kehry M. R., Douglas R. H., Raschke W. C., Hood L. E. Characterization of multiple immunoglobulin mu-chains synthesized by two clones of a B cell lymphoma. J Immunol. 1980 Nov;125(5):2097–2105. [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Toyonaga B., Mak T. W. Genes of the T-cell antigen receptor in normal and malignant T cells. Annu Rev Immunol. 1987;5:585–620. doi: 10.1146/annurev.iy.05.040187.003101. [DOI] [PubMed] [Google Scholar]
- Whitlock C., Denis K., Robertson D., Witte O. In vitro analysis of murine B-cell development. Annu Rev Immunol. 1985;3:213–235. doi: 10.1146/annurev.iy.03.040185.001241. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Wirth T., Staudt L., Baltimore D. An octamer oligonucleotide upstream of a TATA motif is sufficient for lymphoid-specific promoter activity. Nature. 1987 Sep 10;329(6135):174–178. doi: 10.1038/329174a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]