Abstract
The uptake of iron into Pseudomonas aeruginosa is mediated by two major siderophores produced by the bacterium, pyoverdine and pyochelin. The bacterium is also able of utilize several heterologous siderophores of bacterial or fungal origin. In this work, we have investigated the iron uptake in P. aeruginosa PAO1 by the heterologous ferrichrome siderophore. 55Fe uptake assays showed that ferrichrome is transported across the outer membrane primarily (80%) by the FiuA receptor and to a lesser extent (20%) by a secondary transporter. Moreover, we demonstrate that like in the uptake of ferripyoverdine and ferripyochelin, the energy required for both pathways of ferrichrome uptake is provided by the inner membrane protein TonB1. Desferrichrome-55Fe uptake in P. aeruginosa was also dependent on the expression of the permease FiuB, suggesting that this protein is the inner membrane transporter of the ferrisiderophore. A biomimetic fluorescent analogue of ferrichrome, RL1194, was used in vivo to monitor the kinetics of iron release from ferrichrome in P. aeruginosa in real time. This dissociation involves acylation of ferrichrome and its biomimetic analogue RL1194 and recycling of both modified siderophores into the extracellular medium. FiuC, an N-acetyltransferase, is certainly involved in this mechanism of iron release, since its mutation abolished desferrichrome-55Fe uptake. The acetylated derivative reacts with iron in the extracellular medium and is able to be taken up again by the cells. All these observations are discussed in light of the current knowledge concerning ferrichrome uptake in P. aeruginosa and in Escherichia coli.
Iron is essential for life for practically all living organisms and plays a number of key roles in biology. DNA and RNA synthesis, glycolysis, energy generation by electron transport, nitrogen fixation, and photosynthesis are examples of processes in which iron-containing enzymes play vital roles. However, under physiological conditions iron forms highly insoluble ferric hydroxide complexes, which severely limits its bioavailability. To overcome the problem of iron inaccessibility, bacteria excrete high-affinity iron chelators termed siderophores, which are able to solubilize iron and deliver it into the cells (3, 64).
Pseudomonas aeruginosa is a ubiquitous environmental bacterium that is capable of infecting a wide variety of animal, insects, and plants. As a human pathogen, P. aeruginosa is the leading source of Gram-negative nosocomial infections (59) and causes chronic lung infections in approximately 90% of individuals suffering from cystic fibrosis (40). Under iron-limited conditions, P. aeruginosa produces two major siderophores, pyoverdine (PVD) (62) and pyochelin (PCH) (15). P. aeruginosa is also capable of utilizing numerous siderophores secreted by other microorganisms: pyoverdins from other pseudomonas, enterobactin (49), cepabactin (45), mycobactin and carboxymycobactin (38), fungal siderophores (ferrichrome [39]; deferrioxamines [39, 60]; and desferrichrysin, desferricrocin, and coprogen [44]), and natural occurring chelators such as citrate (14, 23) (for a review, see reference 47).
In Gram-negative bacteria, the uptake of ferrisiderophores always involves a specific transporter at the level of the outer membrane (4). The energy required for this process is provided by the proton motive force (PMF) of the inner membrane by means of an inner membrane complex comprising TonB, ExbB, and ExbD (21, 51, 63). In silico analysis of the P. aeruginosa genome (http://www.pseudomonas.com) revealed 32 genes encoding putative TonB-dependent transporters (13), of which only 12 are involved in metal (mostly iron) uptake (38). FpvA and FpvB are the outer membrane transporters involved in the uptake of PVD-Fe (19, 48), and FptA transports PCH-Fe (25). Concerning the heterologous siderophores, there are two transporters, FoxA and FiuA, involved in the transport of ferrioxamine B and ferrichrome (39). The mechanism involved in the translocation of ferrisiderophores across the outer membrane by the TonB-dependent transporters has been studied mostly in E. coli (for a review, see reference 5) and in the case of P. aeruginosa has been studied only for the FpvA/PVD and the FptA/PCH systems. The structures of FpvA (8, 11, 65) and FptA (12) have been solved and their interactions with PVD and PCH investigated at the molecular level (26, 27, 45, 53). Three tonB genes, encoding the energy coupler TonB, have been found in the P. aeruginosa genome, i.e., tonB1, tonB2, and tonB3. Disruption of tonB1 abrogates PVD- and PCH-mediated iron uptake (50, 58) and heme uptake (67). Inactivation of tonB2 has no adverse effect on iron or heme acquisition, but tonB1 tonB2 double mutants are more compromised with respect to growth in iron-restricted medium than is a single tonB1 knockout mutant (67). Mutation of tonB3 appears to result in defective twitching motility (28), and the gene product is most likely not involved in iron uptake.
In P. aeruginosa, many ferrisiderophore outer membrane transporters are also involved in a signaling cascade regulating the expression of genes involved in iron uptake. This is the case for FpvA (PVD uptake), FoxA (ferrioxamine), and FiuA (ferrichrome) (38, 39, 43, 61). Such a signaling cascade involves an extracytoplasmic function (ECF) sigma factor and an inner membrane anti-sigma factor. Equivalent cell surface signaling is present in Escherichia coli for ferricitrate uptake by FecA but not for ferrichrome, ferrioxamine, and enterobactin uptake by FhuA, FhuE, and FepA, respectively.
Little is known about the translocation of ferrisiderophores across the inner membrane in P. aeruginosa. In E. coli, this step involves a specific ABC transporter for almost every siderophore used by this bacterium: FhuBCD for the uptake of ferrichrome and ferrioxamine (33-36), FecBCD for the uptake of ferricitrate (6, 56) and, FepBCDG for the uptake of ferrienterobactin (9). In P. aeruginosa, the only characterized inner membrane siderophore transport protein is FptX, a proton motive force-dependent permease, which functions in PCH-Fe utilization (16). The inner membrane FoxB is involved in the utilization of ferrichrome and ferrioxamine B, but it remains to be determined whether this protein functions in the transport of ferrisiderophore or in the release of iron from ferrichrome or ferrioxamine (17). The genome clearly shows a fepBCDG homologue for the transport of ferrienterobactin. For the other iron uptake pathways present in P. aeruginosa, the transporters involved at the level of the inner membrane have not been identified. An import ABC transporter is present in the pvd locus (PA2407 to PA2410; http://www.pseudomonas.com), but its mutation does not affect PVD-Fe uptake (46).
In P. aeruginosa, the mechanism of ferrisiderophore dissociation has been investigated only for the PVD pathway. This step occurs in the periplasm by a mechanism involving no chemical siderophore modification but involving a reduction of iron and a recycling of the siderophore into the extracellular medium by the PvdRT-OpmQ efflux pump (20, 54, 66). In E. coli, the mechanism of ferrisiderophore dissociation has been investigated for the ferrichrome and ferrienterobactin pathways. Iron release from ferrichrome occurs in the cytoplasm and probably involves iron reduction (41) followed by acetylation of the siderophore and its recycling into the growth medium (24). For the ferrienterobactin pathway, a cytoplasmic esterase hydrolyzes the siderophore (7).
In the present work, we have investigated the ferrichrome pathway in P. aeruginosa using both ferrichrome and a fluorescently labeled biomimetic ferrichrome analogue. We evaluated the siderophore properties of the fluorescent analogue and identified the different transporters involved in the uptake across the outer and inner membranes. Furthermore, we demonstrated that following ferrichrome uptake, iron is released from the siderophore by a mechanism involving an acetylation of the chelator and the modified desferrichrome is secreted back into the growth medium.
MATERIALS AND METHODS
Chemicals and siderophores.
55FeCl3 was purchased from Perkin-Elmer-Life and Analytical Sciences (Boston, MA). Ferrichrome and the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) were purchased from Sigma. PVD was purified and PVD-55Fe prepared as described previously (1, 55). PCH synthesis and PCH-55Fe preparation were as reported earlier (45). Tetrahydrofuran (THF) was dried by overnight treatment with KOH and subsequent filtration through basic alumina. Flash column chromatography separations were performed using Silica Gel 60 (230 to 400 mesh) (Merck, Darmstadt, Germany).
Organic synthesis.
Synthesis of RL1194 was performed as shown in Fig. S1 in the supplemental material, utilizing in part procedures described previously by Meijler et al. (42) and Dayan et al. (18). Sixty-five milligrams (0.3 mmol) of Boc-NHCH2-CONOHCH3 (compound 1) (18) was treated for 30 min at room temperature with a 2:1 mixture of methylene chloride and trifluoroacetic acid (TFA). Removal of excess TFA and solvent resulted in the salt of compound 2, which was used in the next step without further purification. Compound 3 was synthesized as described previously (42). The trifluoroacetate salt of H2N-CH2-CONOHCH3 dissolved in 5 ml dry THF was added to a solution of 112 mg (0.075 mmol) of compound 3 in 5 ml dry THF. The mixture was stirred at room temperature overnight. The mixture was purified by flash chromatography using chloroform followed by 7% methanol (MeOH) in chloroform as the eluting solvent, resulting in 35 mg (0.035 mmol, 47%) of RL1194 as a yellow oil: 1H nuclear magnetic resonance (NMR) (300 MHz, CDCl3), 8.58 (d, 1H), 8.51 (d, 1H), 8.40 (d, 1H), 7.72 (t, 1H), 7.48 (broad, 3H), 7.22 (d, 1H), 4.18 (m, 8H), 3.88 (d, 6H), 3.63 (m, 8H), 3.35 (s, 8H), 3.24 (s, 9H), 2.69 (d, 2H), 2.48 (d, 6H), 1.67 (m, 2H), 1.37 (m, 2H), 0.96 (t, 3H); mass spectrum (MS)-ES+, m/z 1024.52 [M + Na]+, MS-ES−, m/z 1000.53 [M − H]−.
Bacterial strains and growth media.
All of the strains used in this study are presented in Table 1. The strains were grown overnight in a succinate medium [the composition, in g/liter, is as follows: K2HPO4, 6.0; KH2PO4, 3.0; (NH4)2SO4, 1.0; MgSO4·7H2O, 0.2; sodium succinate, 4.0, with the pH adjusted to 7.0 by addition of NaOH] in the presence of 50 μg/ml tetracycline and 500 μg/ml streptomycin for PAD07 and PAD14; 50 μg/ml tetracycline for PA0470, PA0476, PA0478, and PAD08; and 50 μg/ml kanamycin and 10% Casamino Acids for BW25113entB::Kanr. To obtain expression of the ferrichrome uptake pathway in PAO1, PAD07, PAD08, PAD14, PA0470, PA0476, or PA0478 cells, the bacteria were grown in the presence of 5 μM desferrichrome.
TABLE 1.
Strains used in this studya
| Species and strain | Description | Reference |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Wild type | 52 |
| PAD07 | PVD− PCH− | 57 |
| PAD08 | PVD+ PCH+ ΔtonB1 | 57 |
| PAD14 | PVD− PCH− ΔtonB1 | 57 |
| PA0470 | PVD+ PCH+ ΔfiuA | 29 |
| PA0476 | PVD+ PCH+ ΔfiuB | 29 |
| PA0478 | PVD+ PCH+ ΔfiuC | 29 |
| E. coli BW25113entB::Kanr | Ent− | 2 |
PA0470, PA0476, and PA0478 were from the comprehensive P. aeruginosa transposon mutant library at the University of Washington Genome Center (29). The location of the mutation was confirmed by PCR with primers flanking the insertion sites. Further information can be found at http://www.genome.washington.edu/UWGC/Pseudomonas/index.cfm.
55Fe uptake.
To prepare the desferrichrome-55Fe and RL1194-55Fe complexes, 200 μM desferrichrome or RL1194 was incubated for 20 min in the presence of 10 μM 55Fe in 0.1N HCl. PVD-55Fe and PCH-55Fe were prepared as reported previously (45, 55). PVD-55Fe, desferrichrome-55Fe, and RL1194-55Fe uptake assays were carried out as reported previously for the FpvA/PVD system (55) and that for PCH-55Fe as described in reference 45. An overnight culture in iron-limited medium was harvested, and the bacteria were prepared at an optical density at 600 nm (OD600) of 1 for the PCH-55Fe uptake assay and of 3 for the other siderophore-55Fe uptake assays in 50 mM Tris-HCl (pH 8.0) and incubated at 37°C. Transport assays were initiated by adding 300 nM PVD-55Fe, desferrichrome-55Fe, or RL1194-55Fe. Aliquots (100 μl) of the suspension were removed at different times, filtered, and washed with 2 ml 0.5 N HCl, and the retained radioactivity was counted. For PCH-55Fe uptake (because of interaction of PCH with the filters), aliquots (300 μl) were removed and centrifuged at 12,000 × g for 2 min, and the supernatants containing the unbound ferrisiderophore were removed and radioactivity counted. The 55Fe uptake assays were repeated for all siderophores in the presence of 200 μM CCCP.
Fluorescence spectroscopy.
Fluorescence experiments were performed on PAD07 cells with a PTI (Photon Technology International TimeMaster; Bioritech) spectrofluorimeter. The cells were washed with 2 volumes of 50 mM Tris-HCl (pH 8.0) and resuspended in the same buffer to a final OD600 of 1. For all experiments, the sample was stirred at 29°C in a 1-ml cuvette, the excitation wavelength (λexc) was set at 400 nm, and the fluorescence emission (λem) was measured at 535 nm. RL1194-Fe at different concentrations was added and the fluorescence monitored every second for the duration of the experiment. As a control, the experiments were repeated in the absence of siderophores.
Siderophore recycling.
An overnight culture of PAD07 or BW25113entB::Kanr cells was resuspended in 50 mM Tris-HCl (pH 8.0) at a final OD600 of 1. The cells were then incubated at 30°C in the presence of 800 nM RL1194-Fe or desferrichrome-Fe. Aliquots of 1 ml were removed and centrifuged and the supernatant collected. The fluorescence of the supernatant was measured at 535 nm with the excitation wavelength set at 400 nm. The experiment was repeated at 0°C. At this temperature, no siderophore-Fe uptake occurs.
Iron chelation and uptake by the recycled siderophore.
An 800 nM concentration of FeCl3 was added to 800 nM RL1194-Fe or metal-free RL1194, both in 50 mM Tris-HCl solution (pH 8.0). The kinetics of iron chelation was monitored at 535 nM (excitation wavelength set at 400 nm). In parallel, an overnight culture of PAD07 cells was resuspended in 50 mM Tris-HCl (pH 8.0) at a final OD600 of 1. The cells were then incubated for 2 h at 30°C in the presence of 800 nM RL1194-Fe. Afterwards, the cells were pelleted and the supernatant containing the recycled RL1194 (RL1194recy) harvested. An 800 nM concentration of FeCl3 was added to 1 ml of this supernatant and the iron chelation monitored at 535 nm.
For the transport assay, 1 ml of the supernatant containing the recycled RL1194 or recycled desferrichrome (desferrichromerecy) was incubated with 80 nM 55FeCl3 for 20 min, and 500 μl of this mixture was incubated at 37°C with PAD07 cells prepared at an OD600 of 3 in 1 ml of 50 mM Tris-HCl (pH 8.0) buffer. After different times of incubation, 300-μl aliquots were removed and filtered and the radioactivity counted. The uptake experiment was repeated with PAD07 cells treated with 200 μM CCCP.
RESULTS
Iron uptake by ferrichrome involves the outer membrane transporter FiuA.
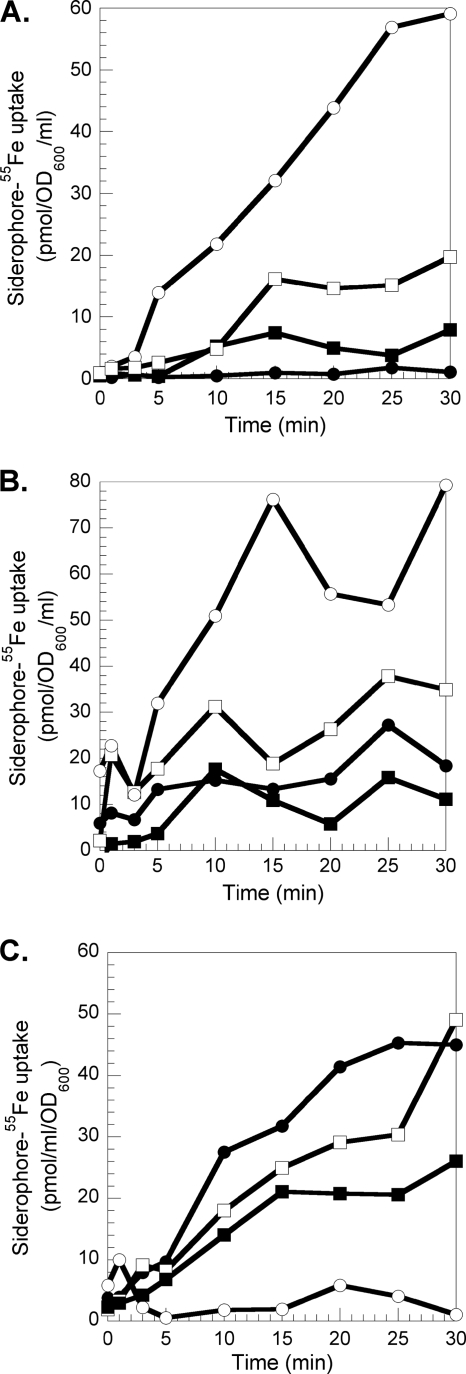
Previously, Llamas et al. have shown that the expression of FiuA and FoxA is regulated by the absence of iron and the presence of ferrichrome in the culture medium (39, 43). Moreover, growth experiments with a strain unable to produce PVD and PCH under iron-deficient conditions with or without ferrichrome indicated that FiuA and FoxA were the transporters involved in the uptake of ferrichrome (39). Here, we used a 55Fe uptake assay to investigate further the ferrichrome iron uptake pathway(s) in P. aeruginosa and to study its possible ability to transport, in addition to ferrichrome, the fluorescent analogue RL1194 (Fig. 1). In order to rule out any effect caused by the endogenous siderophores PVD and PCH, a P. aeruginosa mutant unable to produce either of these two siderophores, PAD07 (Table 1), was used. The bacteria were incubated in the presence of desferrichrome-55Fe (Fig. 2A) and RL1194-55Fe (Fig. 2B) and the radioactivity incorporated into the bacteria monitored. The experiment was repeated with cells pretreated with the protonophore CCCP. This compound inhibits the proton motive force (PMF) of the bacteria and therefore any TonB-dependent iron uptake (10). Figure 2A and B show PMF-dependent desferrichrome-55Fe (60 pmol/ml/OD600 unit) and RL1194-55Fe (80 pmol 55Fe/ml/OD600 unit) uptake in P. aeruginosa. When the fiuA gene was mutated (strain PA0470), a 70% decrease of the desferrichrome-55Fe uptake was observed, confirming again that FiuA is the ferrichrome outer membrane transporter in P. aeruginosa. A 70% decrease of RL1194-55Fe uptake was observed as well, indicating that FiuA is able to transport RL1194 with the same efficiency as ferrichrome. Since the 55Fe uptake was not completely abolished by the fiuA mutation for both chelators, P. aeruginosa may use FoxA as a second transporter for the uptake of ferrichrome. Such an observation has already been made by Llamas et al., who suggested FoxA as a second candidate (39). FoxA, like FiuA, seems to be able to transport RL1194-Fe. Competition uptake assays between desferrichrome-55Fe and increasing concentrations of RL1194-Fe (Fig. 2C) or RL1194-55Fe and increasing concentrations of desferrichrome-Fe (data not shown) also demonstrated that both siderophores are transported by the same TonB-dependent outer membrane receptors, FiuA and FoxA. Finally, the 55Fe uptake assays carried out with PAD07 cells in the presence of either desferrichrome, PVD, or PCH showed that all three iron uptake pathways (ferrichrome/FiuA, PVD/FpvA, and PCH/FptA) transport iron with the same efficiency (Fig. 3). Probably, as shown previously for E. coli (30), the limitation is not the amount of siderophore outer membrane receptor present but the amount of TonB.
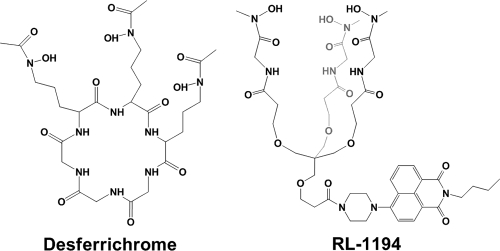
FIG. 1.
Structures of desferrichrome (left) and the fluorescent biomimetic analogue RL1194 (right).
FIG. 2.
Desferrichrome-55Fe and RL1194-55Fe uptake in PAD07 and PA0470. (A and B) PAD07 or PA0470 (ΔFiuA) cells at an OD600 of 3 were incubated for 10 min in 50 mM Tris-HCl (pH 8.0) buffer. Transport assays were started by adding 300 nM ferrichrome-55Fe (A) or RL1194-55Fe (B) (PAD07, ○; PA0470, □). Aliquots (100 μl) of the suspension were removed at different times and filtered, and the retained radioactivity was counted. The experiment was repeated in the presence of 200 μM CCCP (PAD07, •; PA0470, ▪). (C) PAD07 cells at an OD600 of 3 in 50 mM Tris-HCl (pH 8.0) buffer were incubated in the presence of 300 nM ferrichrome-55Fe (•). The experiment was repeated in the presence of 200 μM CCCP (○), 900 nM RL1194-Fe (▪), or 3 μM RL1194-Fe (□). The experiments were repeated three times with comparable results.
FIG. 3.
Desferrichrome-55Fe, PVD-55Fe, or PCH-55Fe uptake in PAD07. PAD07 cells were prepared at an OD600 of 3 in 50 mM Tris-HCl (pH 8.0). Transport assays were started by adding either 300 nM desferrichrome-55Fe (○), PVD-55Fe (□), or PCH-55Fe (▵). Aliquots (100 μl) of the suspension were removed at different times and the radioactivity counted. The uptake assays were repeated in the presence of 200 μM CCCP (desferrichrome-55Fe, •; Pvd-55Fe, ▪; PCH-55Fe, ▴). The experiments were repeated three times with comparable results.
Ferrichrome uptake by the FiuA transporter involves the energy coupler TonB1.
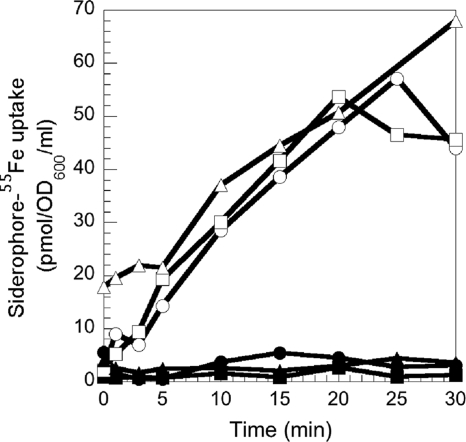
In P. aeruginosa, iron uptake by the PCH/FptA and PVD/FpvA pathways involves TonB1. To find out if this same TonB protein is involved in the uptake of iron by ferrichrome, desferrichrome-55Fe uptake by PAD08 and PAD14 was measured (Fig. 4). Both strains are tonB1 mutants, and PAD14 is also PVD and PCH deficient. With both strains, no more ferrichrome-55Fe uptake was observed, indicating that this iron uptake pathway in P. aeruginosa is TonB1 dependent.
FIG. 4.
(A) Desferrichrome-55Fe uptake in PAD08 (a tonB1 P. aeruginosa mutant). PAD07 (○) and PAD08 (×) cells were prepared at an OD600 of 3 in 50 mM Tris-HCl (pH 8.0). Transport assays were started by adding 300 nM desferrichrome-55Fe. Aliquots (100 μl) of the suspension were removed at different times and the radioactivity counted. The uptake assays were repeated in the presence of 200 μM CCCP (desferrichrome-55Fe uptake in PAD08 cells, •). The experiments were repeated with PAD14 (a tonB1 mutant unable to produce PVD and PCH) with comparable results. (B) Desferrichrome-55Fe uptake in PA0476 (a fiuB P. aeruginosa mutant) and PA0478 (a fiuC P. aeruginosa mutant). Transport assays were carried out with PAO1 (○), PA0476 (⋄), and PA0478 (▵) as for panel A and repeated in the presence of 200 μM CCCP (PAO1, •; PA0476, ♦; PA0478, ▴).
Ferrichrome uptake involves the inner membrane permease FiuB.
The fiuB (PA0476) gene is adjacent to genes required for ferrichrome uptake in P. aeruginosa PAO1, such as fiuA, fiuI, and fiuR (38, 39), and is predicted to encode an inner membrane permease (www.pseudomonas.com) with 11 membrane-spanning helices (determined with Phobius [31], available at http://www.ebi.ac.uk/Tools/phobius/). No ABC transporter is present in the proximity of fiuA, fiuI, and fiuR, suggesting that FiuB could be the transporter of ferrichrome at the level of the inner membrane. To investigate this hypothesis, desferrichrome-55Fe uptake assays were carried out with PA0476, a fiuA mutant (Fig. 4B). The data clearly showed an inhibition of desferrichrome-55Fe incorporation compared to the transport by wild-type PAO1, suggesting that FiuB is important for 55Fe uptake by desferrichrome.
Iron release from the siderophore in P. aeruginosa PAD07 cells.
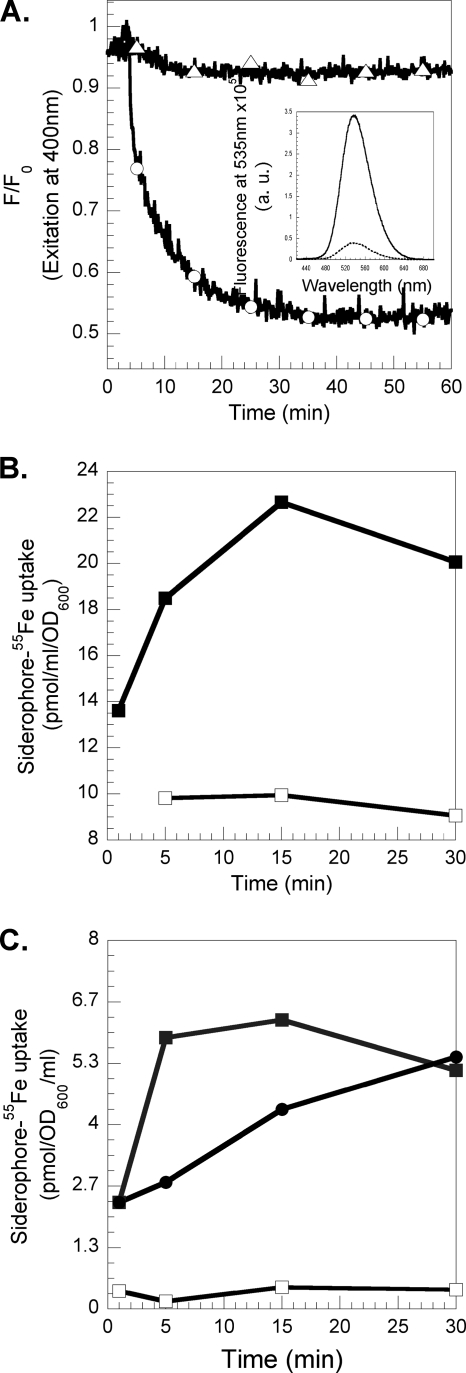
The fluorescence of RL1194 is quenched upon iron complexation and regained upon iron release. These spectral properties were used to follow in vivo and in real time the iron release from RL1194 in P. aeruginosa cells. PAD07 cells were incubated in the presence of increasing concentrations of RL1194-Fe and the fluorescence at 535 nm monitored (with the excitation wavelength set at 400 nm) (Fig. 5). In the presence of both RL1194-Fe and bacteria, an increase of fluorescence was observed, corresponding to iron release from the siderophore and formation of metal-free RL1194. This rise of fluorescence occurs immediately after addition of RL1194-Fe. The kinetics of metal release from the siderophore increased with the concentration of RL1194-Fe incubated with the bacteria, and saturation was reached at concentrations higher than 1.2 μM (data not shown).
FIG. 5.
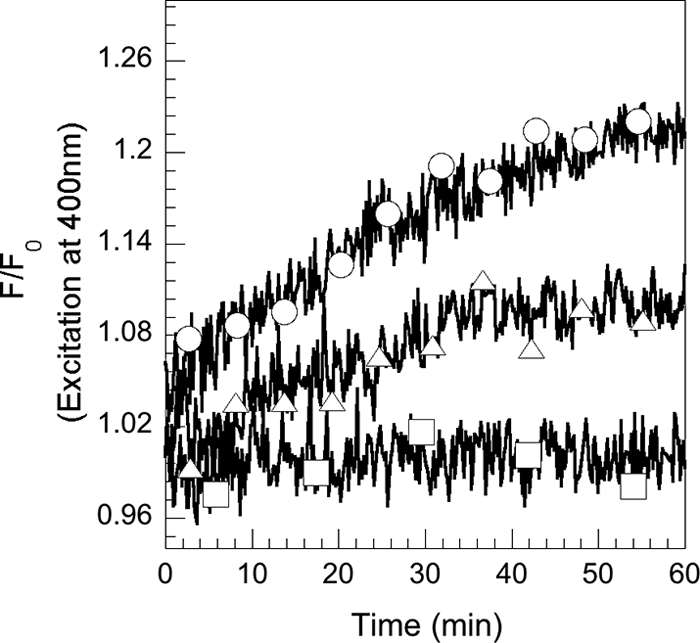
Kinetics of desferrichrome-55Fe dissociation in P. aeruginosa PAD07 cells. PAD07 cells were washed and resuspended at an OD600 of 1 in 50 mM Tris-HCl (pH 8.0) and incubated at 29°C. After addition of RL1194-Fe (400 nM [Δ] and 1,2 μM [○]), the fluorescence at 535 nm was monitored (with the excitation wavelength set at 400 nm). The experiment was repeated in the absence of siderophores (□). The experiments were repeated three times with comparable results.
RL1194 recycling after iron release into P. aeruginosa PAD07 cells.
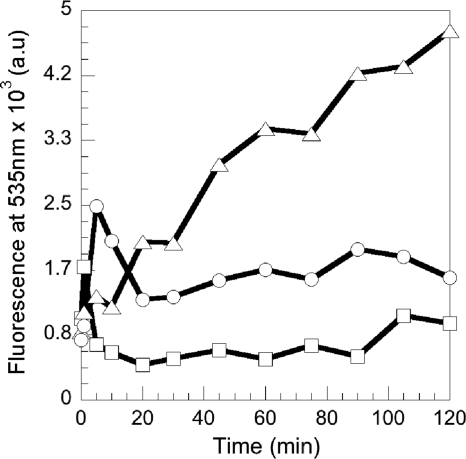
The fluorescence properties of RL1194 were used here to follow the fate of the siderophore after iron release into the bacteria. PAD07 cells were incubated in the presence of 800 nM nonfluorescent RL1194-Fe complex. At different times, aliquots were removed, the cells pelleted, and the fluorescence in the supernatant monitored at 535 nm (with the excitation wavelength set at 400 nm) (Fig. 6). An increase of fluorescence at 535 nm was observed in the extracellular medium, which was absent in the experiment without cells or when the cells were incubated at 0°C in order to avoid siderophore-Fe uptake. This increase of fluorescence at 535 nm suggests that metal-free RL1194 is recycled into the extracellular medium after having released its iron in the bacteria. The fluorescence spectrum of this recycled RL1194 was similar to that of RL1194 before iron uptake, with a maximum of fluorescence emission at 535 nm (data not shown).
FIG. 6.
Recycling of RL1194 after iron release into P. aeruginosa PAD07 cells. PAD07 cells at an OD600 of 1 in 50 mM Tris-HCl (pH 8.0) were incubated in the presence of 800 nM RL1194-Fe (at 0°C [○] or at 30°C [▵]). At different times, aliquots were removed, the cells pelleted, and the increase of fluorescence at 535 nm (λext = 400 nm) monitored in the supernatant. The experiment was repeated at 30°C in the absence of siderophores (□).
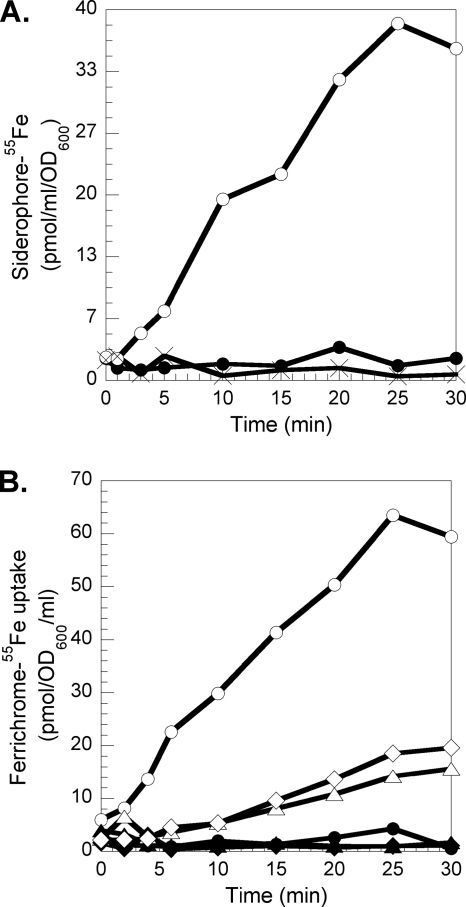
Chemical and biochemical characterization of recycled desferrichrome and recycled RL1194.
Mass spectrum analyses of RL1194recy indicated a triacetylation of the molecule (m/z 1129.24, MH+; calculated for triacetyl-RL1194-H+, 1129.19), and that for the desferrichromerecy indicated a diacetylation (m/z 772.45, MH+; calculated for diacetyl-desferrichrome-H+, 772.78). To test whether RL1194recy was still able to chelate iron, the molecule was incubated with one equivalent of FeCl3 and formation of RL1194recy-Fe was observed by the decrease in fluorescence (λexc = 400 nm, λem = 535 nm) (Fig. 7A). This clearly shows that despite the triacetylation of RL1194recy, the molecule is still able to chelate iron. In order to investigate the ability of RL1194recy and desferrichromerecy to transport iron into PVD- and PCH-deficient P. aeruginosa cells, RL1194recy was loaded with 55Fe and incubated in the presence of bacteria (Fig. 7B). 55Fe was incorporated into the cells, indicating that RL1194recy is, despite the acetylation, a fully functional siderophore able to chelate iron and to start a new iron uptake cycle. The same experiment was repeated with recycled desferrichrome (desferrichromerecy) harvested from the medium of P. aeruginosa and from E. coli cells (Fig. 7C) incubated with ferrichrome. Both desferrichromesrecy were able to chelate 55Fe and transport it into P. aeruginosa cells. In conclusion, the mechanism of iron release from ferrichrome in P. aeruginosa involves acetylation of the chelators, like in E. coli (24). Due to the ease of hydrolysis of the acetylated N-hydroxyl group by ferric chloride, a Lewis acid, the siderophore is still able to chelate iron and transport it into the bacteria like in E. coli (24).
FIG. 7.
(A) Iron chelation by RL1194recy. PAD07 cells at an OD600 of 1 in 50 mM Tris-HCl (pH 8.0) were incubated at 30°C in the presence of 800 nM RL1194-Fe. After 2 h of incubation, the cells were collected, the supernatant containing the recycled RL1194 (RL1194recy) incubated in the presence of 800 nM Fe (○; iron was added after 2 min), and the fluorescence at 535 nm monitored (λext = 400 nm). The experiment was repeated with supernatant of PAD07 cells incubated in the presence of 800 nM RL1194 at 0°C (▵). F0, fluorescence at time zero; F, fluorescence at time t. The inset shows the fluorescence emission spectra of metal-free RL1194recy (solid line) and of RL1194recy-Fe (dashed line). (B) 55Fe uptake into P. aeruginosa PAD07 cells by RL1194recy. PAD07 at an OD600 of 3 in 50 mM Tris-HCl (pH 8.0) buffer at 37°C was incubated in the presence of 800 nM RL1194recy loaded with 55Fe (▪), and 300 μl of cell suspension was removed at different times and filtered and the radioactivity retained counted. The experiment was repeated in the presence of 200 μM CCCP (□). (C) 55Fe uptake into P. aeruginosa PAD07 cells by desferrichromerecy. PAD07 at an OD600 of 3 in 50 mM Tris-HCl (pH 8.0) buffer at 37°C was incubated in the presence of 800 nM desferrichromerecy from P. aeruginosa (▪) and from E. coli (•) loaded with 55Fe, and 300 μl of cell suspension was removed at different times and filtered and the radioactivity retained counted. The experiment was repeated in the presence of 200 μM CCCP (data shown only for desferrichromerecy from P. aeruginosa) (□).
Ferrichrome uptake involves FiuC, a cytoplasmic N-acetyltransferase.
The fiuC (PA0478) gene is, like fiuB, adjacent to fiuA in P. aeruginosa PAO1. This gene is predicted to encode a cytoplasmic N-acetyltransferase (www.pseudomonas.com). Mutation of this gene completely abolished desferrichrome-55Fe uptake (Fig. 4B), indicating that this protein is necessary for P. aeruginosa to gain access to iron by the ferrichrome pathway.
DISCUSSION
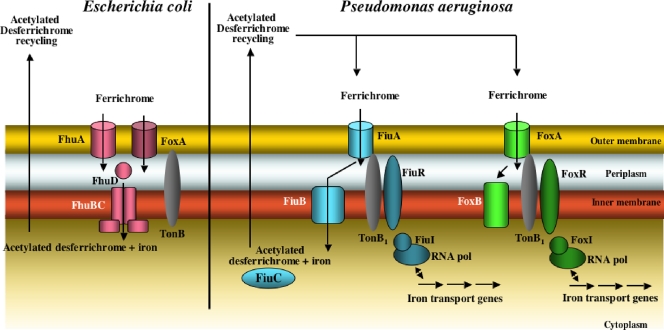
The ferrichrome pathway in E. coli has been the subject of extensive investigation during the last 20 years and became the archetype in the field (for a review, see reference 4). P. aeruginosa uses the heterologous siderophore ferrichrome (39), but little is known concerning the mechanisms involved in the ferrichrome pathway in this bacterium. As shown by a previous proteomic approach and here by 55Fe uptake assays, ferrichrome is transported mostly by FiuA and to a lesser extent by FoxA. The same was observed with the fluorescently labeled biomimetic ferrichrome analogue RL1194: 80% of RL1194 was transported by FiuA and 20% by the second transporter (Fig. 2B). A second transporter for ferrichrome, FhuE, is also present in E. coli (22, 32) and transports ferrichrome with a lower efficiency than FhuA, the major transporter. Surprisingly, RL1194 was unable to transport iron to E. coli (data not shown), indicating that the siderophore specificities of FiuA and FoxA of P. aeruginosa seem to be significantly different from those of FhuA and FhuE in E. coli. Both transporters, FiuA and FoxA, are (like FpvA and FptA) regulated by TonB1 and the proton motive force of the inner membrane (Fig. 4).
The use of the fluorescently labeled biomimetic ferrichrome analogue RL1194 allowed us to follow in vivo and in real time the kinetics of iron release from ferrichrome in P. aeruginosa (Fig. 5). This mechanism of iron release involves acetylation of the siderophore and the recycling of the modified molecule to the extracellular medium (Fig. 6). Actually, both RL1194recy and desferrichromerecy were acetylated; mass spectrometry analyses have shown triacetylation for the first chelator and diacetylation for the second. In order to bind iron(III), the acetylated chelators should be hydrolyzed. Ferric chloride acts both as a Lewis acid, hydrolyzing the acetyl group, and as the source of iron(III). The combined data indicate that the mechanism involved in the iron release from ferrichrome in P. aeruginosa is very similar to that described previously for E. coli, where iron is released from ferrichrome in the cytoplasm by a mechanism involving iron reduction (41) followed by acetylation of the desferrisiderophore (24) (Fig. 8). The acetylation is probably the mechanism by which cells remove potentially deleterious metal chelators from the cytoplasm.
FIG. 8.
The ferrichrome pathways in E. coli and P. aeruginosa. In E. coli the transport involves two TonB outer membrane transporters, FhuA and FhuE, and an inner membrane ABC transporter, FhuBCD. Iron is released by a mechanism probably involving metal reduction and acetylation of the desferrichrome, followed by siderophore recycling in the extracellular medium. In P. aeruginosa, transport involves two TonB1 outer membrane transporters, FiuA and FoxA, and a permease, FiuB, for transport across the inner membrane. Iron is released from the siderophore by a mechanism similar to that in E. coli. FiuC is probably the N-acetyltransferase. FoxB seems also to be involved in ferrichrome transport; however, its function is so far unknown. In P. aeruginosa, both outer membrane transporters, FiuA and FoxA, are also involved in a signaling cascade regulating the expression of the genes involved in the ferrichrome pathway.
Our data also highlighted two new genes essential for ferrichrome uptake in P. aeruginosa. Both genes (encoding FiuB and FiuC, for PA0476 and PA0478, respectively) are located in the vicinity of fiuA in the P. aeruginosa genome. Their mutation almost completely abolished ferrichrome incorporation into the bacteria (Fig. 4B), indicating that these proteins, like FhuA and TonB1, are essential for ferrichrome uptake. FiuC is predicted to be a cytoplasmic N-acetyltransferase which is probably involved in the acetylation of ferrichrome during the iron release step. FiuB is predicted to be a permease with 11 membrane-spanning helices. Since FiuC is predicted to be cytoplasmic and FiuB a permease, it is tempting to suppose that FiuB is the transporter of ferrichrome across the inner membrane. In E. coli, this step is achieved by FhuBCD, an ABC transporter (33, 35-37). No homologue of this ABC transporter could be found in the P. aeruginosa genome (http://www.pseudomonas.com).
Mutation of FiuB and FiuC abolished desferrichrome-55Fe uptake with the same efficiency as mutation of the outer membrane transporter FiuA. Uptake occurs only if all the proteins necessary in the pathway are expressed, in order to avoid any ferrichrome accumulation in either the periplasm or cytoplasm of the bacteria. In E. coli, similarly, mutation of FhuBCD abolishes ferrichrome uptake. The inner membrane protein FoxB is also involved in the utilization of ferrichrome by P. aeruginosa (17). Mutagenesis of this membrane protein did not abolish ferrichrome utilization, suggesting that this function is redundant (17). FoxB is predicted to be an inner membrane protein with four membrane-spanning helices. In this study, it was not determined whether FoxB is involved in the transport of ferrichrome or in iron release from the siderophore.
Taken together, the data show that the ferrichrome pathway in P. aeruginosa has many similarities to the ferrichrome pathway described previously for E. coli (Fig. 8), indicating conservation among bacterial species. Like in E. coli, the transport of ferrichrome across the outer membrane involves a primary transporter, FiuA, and a secondary one, FoxA (FhuA and FhuE in E. coli). In both bacteria, iron release from ferrichrome involves acetylation of the chelator and probably occurs in the cytoplasm. Consequently, acylated ferrichrome is recycled to the extracellular medium. One major difference between the two pathways is the type of transporter involved in the translocation of ferrichrome across the inner membrane: it is an ABC transporter in the case of E. coli and a permease in the case of P. aeruginosa. A second difference is the involvement of the outer membrane transporters FiuA and FoxA of P. aeruginosa in a signaling cascade regulating the expression of fiuA or foxA (38, 39, 43). These signaling cascades involve an ECF sigma factor (FiuI and FoxI) and an anti-sigma factor (FiuR and FoxR). No equivalent cell surface signaling is present in E. coli for ferrichrome uptake by FhuA or FhuE.
In conclusion, the E. coli ferrichrome and enterobactin pathways have been the archetypes in the field of ferrisiderophore transport for years, and it was believed that the mechanisms involved in these two pathways were probably common to all siderophore pathways in Gram-negative bacteria. The data presented here indicate that diversity can be found in the mechanisms involved in iron uptake by siderophores. Further studies have to be carried out to better understand all these diversities and their possible significance, depending on the specific microorganisms and their siderophores.
Supplementary Material
Acknowledgments
We thank Rachel Lazar for her skillful synthetic work.
This work was partially supported by the Centre National de la Recherche Scientifique (CNRS) and a grant from the ANR (Agence Nationale de Recherche, ANR-08-BLAN-0315-01) (I.J.S.) and by the Helen and Martin Kimmel Center for Molecular Design and the Kimmelman Center for Macromolecular Assemblies (A.S.). M. Hannauer had a fellowship from the French Ministère de la Recherche et de la Technologie. A.S. holds the Siegfried and Irma Ullman Professorial Chair.
Footnotes
Published ahead of print on 4 January 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Albrecht-Gary, A. M., S. Blanc, N. Rochel, A. Z. Ocacktan, and M. A. Abdallah. 1994. Bacterial iron transport: coordination properties of pyoverdin PaA, a peptidic siderophore of Pseudomonas aeruginosa. Inorg. Chem. 33:6391-6402. [Google Scholar]
- 2.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boukhalfa, H., and A. L. Crumbliss. 2002. Chemical aspects of siderophore mediated iron transport. Biometals 15:325-339. [DOI] [PubMed] [Google Scholar]
- 4.Braun, V. 2003. Iron uptake by Escherichia coli. Front. Biosci. 8:1409-1421. [DOI] [PubMed] [Google Scholar]
- 5.Braun, V., and F. Endriss. 2007. Energy-coupled outer membrane transport proteins and regulatory proteins. Biometals 20:219-231. [DOI] [PubMed] [Google Scholar]
- 6.Braun, V., and C. Herrmann. 2007. Docking of the periplasmic FecB binding protein to the FecCD transmembrane proteins in the ferric citrate transport system of Escherichia coli. J. Bacteriol. 189:6913-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brickman, T. J., and M. A. McIntosh. 1992. Overexpression and purification of ferric enterobactin esterase from Escherichia coli. Demonstration of enzymatic hydrolysis of enterobactin and its iron complex. J. Biol. Chem. 267:12350-12355. [PubMed] [Google Scholar]
- 8.Brillet, K., L. Journet, H. Celia, L. Paulus, A. Stahl, F. Pattus, and D. Cobessi. 2007. A β-strand lock-exchange for signal transduction in TonB-dependent transducers on the basis of a common structural motif. Structure 15:1383-1391. [DOI] [PubMed] [Google Scholar]
- 9.Chenault, S. S., and C. F. Earhart. 1991. Organization of genes encoding membrane proteins of the Escherichia coli ferrienterobactin permease. Mol. Microbiol. 5:1405-1413. [DOI] [PubMed] [Google Scholar]
- 10.Clément, E., P. J. Mesini, F. Pattus, M. A. Abdallah, and I. J. Schalk. 2004. The binding mechanism of pyoverdin with the outer membrane receptor FpvA in Pseudomonas aeruginosa is dependent on its iron-loaded status. Biochemistry 43:7954-7965. [DOI] [PubMed] [Google Scholar]
- 11.Cobessi, D., H. Célia, N. Folschweiller, I. J. Schalk, M. A. Abdallah, and F. Pattus. 2005. The crystal structure of the pyoverdin outer membrane receptor FpvA from Pseudomonas aeruginosa at 3.6 Å resolution. J. Mol. Biol. 347:121-134. [DOI] [PubMed] [Google Scholar]
- 12.Cobessi, D., H. Celia, and F. Pattus. 2004. Crystallization and X-ray diffraction analyses of the outer membrane pyochelin receptor FptA from Pseudomonas aeruginosa. Acta Crystallogr. D Biol. Crystallogr. 60:1919-1921. [DOI] [PubMed] [Google Scholar]
- 13.Cornelis, P., and S. Matthijs. 2002. Diversity of siderophore-mediated iron uptake systems in fluorescent pseudomonads: not only pyoverdines. Environ. Microbiol. 4:787-798. [DOI] [PubMed] [Google Scholar]
- 14.Cox, C. D. 1980. Iron uptake with ferripyochelin and ferriccitrate by Pseudomonas aeruginosa. J. Bacteriol. 142:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox, C. D., K. L. Rinehart, Jr., M. L. Moore, and J. C. Cook, Jr. 1981. Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 78:4256-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuiv, P. O., P. Clarke, D. Lynch, and M. O'Connell. 2004. Identification of rhtX and fptX, novel genes encoding proteins that show homology and function in the utilization of the siderophores rhizobactin 1021 by Sinorhizobium meliloti and pyochelin by Pseudomonas aeruginosa, respectively. J. Bacteriol. 186:2996-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuiv, P. O., D. Keogh, P. Clarke, and M. O'Connell. 2007. FoxB of Pseudomonas aeruginosa functions in the utilization of the xenosiderophores ferrichrome, ferrioxamine B, and schizokinen: evidence for transport redundancy at the inner membrane. J. Bacteriol. 189:284-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dayan, I., J. Libman, Y. Agi, and A. Shanzer. 1993. Chiral siderophore analogs—ferrichrome. Inorg. Chem. 32:1467-1475. [Google Scholar]
- 19.Ghysels, B., B. T. Dieu, S. A. Beatson, J. P. Pirnay, U. A. Ochsner, M. L. Vasil, and P. Cornelis. 2004. FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology 150:1671-1680. [DOI] [PubMed] [Google Scholar]
- 20.Greenwald, J., F. Hoegy, M. Nader, L. Journet, G. L. A. Mislin, P. L. Graumann, and I. J. Schalk. 2007. Real-time FRET visualization of ferric-pyoverdine uptake in Pseudomonas aeruginosa: a role for ferrous iron. J. Biol. Chem. 282:2987-2995. [DOI] [PubMed] [Google Scholar]
- 21.Gumbart, J., M. C. Wiener, and E. Tajkhorshid. 2007. Mechanics of force propagation in TonB-dependent outer membrane transport. Biophys. J. 93:496-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hantke, K. 1983. Identification of an iron uptake system specific for coprogen and rhodotorulic acid in Escherichia coli K12. Mol. Gen. Genet 191:301-306. [DOI] [PubMed] [Google Scholar]
- 23.Harding, R. A., and P. W. Royt. 1990. Acquisition of iron from citrate by Pseudomonas aeruginosa. J. Gen. Microbiol. 136:1859-1867. [DOI] [PubMed] [Google Scholar]
- 24.Hartman, A., and V. Braun. 1980. Iron transport in Escherichia coli: uptake and modification of ferrichrome. J. Bacteriol. 143:246-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinrichs, D. E., L. Young, and K. Poole. 1991. Pyochelin-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. Infect. Immun. 59:3680-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoegy, F., H. Celia, G. L. Mislin, M. Vincent, J. Gallay, and I. J. Schalk. 2005. Binding of iron-free siderophore, a common feature of siderophore outer membrane transporters of Escherichia coli and Pseudomonas aeruginosa. J. Biol. Chem. 280:20222-20230. [DOI] [PubMed] [Google Scholar]
- 27.Hoegy, F., X. Lee, S. Noël, G. L. Mislin, D. Rognan, C. Reimmann, and I. J. Schalk. 2009. Stereospecificity of the siderophore pyochelin outer membrane transporters in fluorescent Pseudomonads. J. Biol. Chem. 284:14949-14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, B., K. Ru, Z. Yuan, C. B. Whitchurch, and J. S. Mattick. 2004. tonB3 is required for normal twitching motility and extracellular assembly of type IV pili. J. Bacteriol. 186:4387-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs, M. A., A. Alwood, I. Thaipisuttikul, D. Spencer, E. Haugen, S. Ernst, O. Will, R. Kaul, C. Raymond, R. Levy, L. Chun-Rong, D. Guenthner, D. Bovee, M. V. Olson, and C. Manoil. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 100:14339-14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadner, R. J., and K. J. Heller. 1995. Mutual inhibition of cobalamin and siderophore uptake systems suggests their competition for TonB function. J. Bacteriol. 177:4829-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kall, L., A. Krogh, and E. L. Sonnhammer. 2007. Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res. 35:W429-W432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Killmann, H., and V. Braun. 1998. Conversion of the coprogen transport protein FhuE and the ferrioxamine B transport protein FoxA into ferrichrome transport proteins. FEMS Microbiol. Lett. 161:59-67. [DOI] [PubMed] [Google Scholar]
- 33.Koster, W. 2001. ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12. Res. Microbiol. 152:291-301. [DOI] [PubMed] [Google Scholar]
- 34.Koster, W. 1991. Iron(III) hydroxamate transport across the cytoplasmic membrane of Escherichia coli. Biol. Met. 4:23-32. [DOI] [PubMed] [Google Scholar]
- 35.Koster, W., and V. Braun. 1986. Iron hydroxamate transport of Escherichia coli: nucleotide sequence of the fhuB gene and identification of the protein. Mol. Gen. Genet 204:435-442. [DOI] [PubMed] [Google Scholar]
- 36.Koster, W., and V. Braun. 1989. Iron-hydroxamate transport into Escherichia coli K12: localization of FhuD in the periplasm and of FhuB in the cytoplasmic membrane. Mol. Gen. Genet 217:233-239. [DOI] [PubMed] [Google Scholar]
- 37.Koster, W., A. Gudmundsdottir, M. D. Lundrigan, A. Seiffert, and R. J. Kadner. 1991. Deletions or duplications in the BtuB protein affect its level in the outer membrane of Escherichia coli. J. Bacteriol. 173:5639-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llamas, M. A., M. J. Mooij, M. Sparrius, C. M. Vandenbroucke-Grauls, C. Ratledge, and W. Bitter. 2008. Characterization of five novel Pseudomonas aeruginosa cell-surface signalling systems. Mol. Microbiol. 67:458-472. [DOI] [PubMed] [Google Scholar]
- 39.Llamas, M. A., M. Sparrius, R. Kloet, C. R. Jimenez, C. Vandenbroucke-Grauls, and W. Bitter. 2006. The heterologous siderophores ferrioxamine B and ferrichrome activate signaling pathways in Pseudomonas aeruginosa. J. Bacteriol. 188:1882-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 41.Matzanke, B. F., S. Anemuller, V. Schunemann, A. X. Trautwein, and K. Hantke. 2004. FhuF, part of a siderophore-reductase system. Biochemistry 43:1386-1392. [DOI] [PubMed] [Google Scholar]
- 42.Meijler, M. M., R. Arad-Yellin, Z. I. Cabantchik, and A. Shanzer. 2002. Synthesis and evaluation of iron chelators with masked hydrophilic moieties. J. Am. Chem. Soc. 124:12666-12667. [DOI] [PubMed] [Google Scholar]
- 43.Mettrick, K. A., and I. L. Lamont. 2009. Different roles for anti-sigma factors in siderophore signalling pathways of Pseudomonas aeruginosa. Mol. Microbiol. 74:1257-1271. [DOI] [PubMed] [Google Scholar]
- 44.Meyer, J. M. 1992. Exogenous siderophore-mediated iron uptake in Pseudomonas aeruginosa: possible involvement of porin OprF in iron translocation. J. Gen. Microbiol. 138:951-958. [DOI] [PubMed] [Google Scholar]
- 45.Mislin, G. L. A., F. Hoegy, D. Cobessi, K. Poole, D. Rognan, and I. J. Schalk. 2006. Binding properties of pyochelin and structurally related molecules to FptA of Pseudomonas aeruginosa. J. Mol. Biol. 357:1437-1448. [DOI] [PubMed] [Google Scholar]
- 46.Ochsner, U. A., P. J. Wilderman, A. I. Vasil, and M. L. Vasil. 2002. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol. Microbiol. 45:1277-1287. [DOI] [PubMed] [Google Scholar]
- 47.Poole, K., and G. A. McKay. 2003. Iron acquisition and its control in Pseudomonas aeruginosa: many roads lead to Rome. Front. Biosci. 8:d661-d686. [DOI] [PubMed] [Google Scholar]
- 48.Poole, K., S. Neshat, K. Krebes, and D. E. Heinrichs. 1993. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J. Bacteriol. 175:4597-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poole, K., L. Young, and S. Neshat. 1990. Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J. Bacteriol. 172:6991-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poole, K., Q. Zhao, S. Neshat, D. E. Heinrichs, and C. R. Dean. 1996. The Pseudomonas aeruginosa tonB gene encodes a novel TonB protein. Microbiology 142:1449-1458. [DOI] [PubMed] [Google Scholar]
- 51.Postle, K., and R. J. Kadner. 2003. Touch and go: tying TonB to transport. Mol. Microbiol. 49:869-882. [DOI] [PubMed] [Google Scholar]
- 52.Royle, P. L., H. Matsumoto, and B. W. Holloway. 1981. Genetic circularity of the Pseudomonas aeruginosa PAO chromosome. J. Bacteriol. 145:145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schalk, I. J. 2008. Metal trafficking via siderophores in Gram-negative bacteria: specificities and characteristics of the pyoverdine pathway. J. Inorg. Biochem. 102:1159-1169. [DOI] [PubMed] [Google Scholar]
- 54.Schalk, I. J., M. A. Abdallah, and F. Pattus. 2002. Recycling of pyoverdin on the FpvA receptor after ferric pyoverdin uptake and dissociation in Pseudomonas aeruginosa. Biochemistry 41:1663-1671. [DOI] [PubMed] [Google Scholar]
- 55.Schalk, I. J., C. Hennard, C. Dugave, K. Poole, M. A. Abdallah, and F. Pattus. 2001. Iron-free pyoverdin binds to its outer membrane receptor FpvA in Pseudomonas aeruginosa: a new mechanism for membrane iron transport. Mol. Microbiol. 39:351-360. [DOI] [PubMed] [Google Scholar]
- 56.Staudenmaier, H., B. Van Hove, Z. Yaraghi, and V. Braun. 1989. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J. Bacteriol. 171:2626-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takase, H., H. Nitanai, K. Hoshino, and T. Otani. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun. 68:1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takase, H., H. Nitanai, K. Hoshino, and T. Otani. 2000. Requirement of the Pseudomonas aeruginosa tonB gene for high-affinity iron acquisition and infection. Infect. Immun. 68:4498-4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Delden, C., and B. H. Iglewski. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasil, M. L., and U. A. Ochsner. 1999. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol. Microbiol. 34:399-413. [DOI] [PubMed] [Google Scholar]
- 61.Visca, P., L. Leoni, M. J. Wilson, and I. L. Lamont. 2002. Iron transport and regulation, cell signalling and genomics: lessons from Escherichia coli and Pseudomonas. Mol. Microbiol. 45:1177-1190. [DOI] [PubMed] [Google Scholar]
- 62.Wendenbaum, S., P. Demange, A. Dell, J. M. Meyer, and M. A. Abdallah. 1983. The structure of pyoverdin Pa, the siderophore of Pseudomonas aeruginosa. Tetrahedron Lett. 24:4877-4880. [Google Scholar]
- 63.Wiener, M. C. 2005. TonB-dependent outer membrane transport: going for Baroque? Curr. Opin. Struct. Biol. 15:394-400. [DOI] [PubMed] [Google Scholar]
- 64.Winkelmann, G. 2002. Microbial siderophore-mediated transport. Biochem. Soc. Trans. 30:691-696. [DOI] [PubMed] [Google Scholar]
- 65.Wirth, C., W. Meyer-Klaucke, F. Pattus, and D. Cobessi. 2007. From the periplasmic signaling domain to the extracellular face of an outer membrane signal transducer of Pseudomonas aeruginosa: crystal structure of the ferric pyoverdine outer membrane receptor. J. Mol. Biol. 68:398-406. [DOI] [PubMed] [Google Scholar]
- 66.Yeterian, E., L. W. Martin, I. L. Lamont, and I. J. Schalk. A resistance-division-nodulation (RND) efflux pump is required for siderophore recycling by Pseudomonas aeruginosa. Environ. Microbiol. Rep., in press. [DOI] [PubMed]
- 67.Zhao, Q., and K. Poole. 2000. A second tonB gene in Pseudomonas aeruginosa is linked to the exbB and exbD genes. FEMS Microbiol. Lett. 184:127-132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.