Abstract
The homotrimeric enzyme Mt-Cam from Methanosarcina thermophila is the archetype of the γ class of carbonic anhydrases. A search of databases queried with Mt-Cam revealed that a majority of the homologs comprise a putative subclass (CamH) in which there is major conservation of all of the residues essential for the archetype Mt-Cam except Glu62 and an acidic loop containing the essential proton shuttle residue Glu84. The CamH homolog from M. thermophila (Mt-CamH) was overproduced in Escherichia coli and characterized to validate its activity and initiate an investigation of the CamH subclass. The Mt-CamH homotrimer purified from E. coli cultured with supplemental zinc (Zn-Mt-CamH) contained 0.71 zinc and 0.15 iron per monomer and had kcat and kcat/Km values that were substantially lower than those for the zinc form of Mt-Cam (Zn-Mt-Cam). Mt-CamH purified from E. coli cultured with supplemental iron (Fe-Mt-CamH) was also a trimer containing 0.15 iron per monomer and only a trace amount of zinc and had an effective kcat (kcateff) value normalized for iron that was 6-fold less than that for the iron form of Mt-Cam, whereas the kcat/Kmeff was similar to that for Fe-Mt-Cam. Addition of 50 mM imidazole to the assay buffer increased the kcateff of Fe-Mt-CamH more than 4-fold. Fe-Mt-CamH lost activity when it was exposed to air or 3% H2O2, which supports the hypothesis that Fe2+ has a role in the active site. The kcat for Fe-Mt-CamH was dependent on the concentration of buffer in a way that indicates that it acts as a second substrate in a “ping-pong” mechanism accepting a proton. The kcat/Km was not dependent on the buffer, consistent with the mechanism for all carbonic anhydrases in which the interconversion of CO2 and HCO3− is separate from intermolecular proton transfer.
Carbonic anhydrases (CAs) are metalloenzymes that catalyze the reversible hydration of carbon dioxide to bicarbonate (CO2 + H2O ⇆ HCO3− + H+) and are distributed among metabolically diverse species belonging to all three domains of life, reflecting the importance of these enzymes in biology (10, 26). Five classes (α, β, γ, δ, and ζ classes) of CAs that evolved independently and have no significant sequence identity have been identified. Our current understanding of the biochemistry and biological roles of CAs is based largely on several α and β class enzymes from mammals and plants. Two other CAs from members of the Eucarya domain, which belong to the δ and ζ classes and were isolated from a marine diatom, have been characterized (21). Although CAs are widely distributed in diverse prokaryotes (25), relatively few CAs belonging to the α and β classes from members of the Bacteria domain have been characterized. Remarkably, only two CAs from members of the Archaea domain, one each from the β and γ classes, have been characterized biochemically (35). Although the γ class is widely distributed in diverse species belonging to all three domains (26, 31), only the archetype γ class enzyme (Mt-Cam) from the anaerobic acetate-utilizing methane-producing species Methanosarcina thermophila in the Archaea domain has been characterized biochemically and shown to have CA activity (36). Mt-Cam belongs to a superfamily of proteins, comprised mainly of acyltransferases, that share a distinctive left-handed parallel β-helix fold predicted by a unique sequence motif (17, 19). Mt-Cam overproduced in Escherichia coli and purified aerobically contains zinc in the active site (2). However, overproduction in the closely related species Methanosarcina acetivorans yields an enzyme with 3-fold-greater CA activity and iron in the active site, establishing iron as the physiologically relevant metal (18).
The kinetic mechanism of Mt-Cam (1, 36) resembles that of all classes of CAs studied despite structural differences. The overall reaction occurs in two mechanistically distinct half-reactions shown in equations 1 and 2 and in equations 3 and 4, where M is a metal, E is the enzyme, and B is buffer.
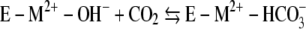 |
(1) |
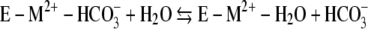 |
(2) |
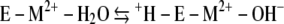 |
(3) |
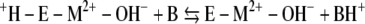 |
(4) |
The first half-reaction is a nucleophilic attack of metal-bound hydroxide on carbon dioxide, yielding bicarbonate, which is reflected in the steady-state parameter kcat/Km. The second half-reaction is the rate-determining proton transfer from metal-bound water to buffer, which is reflected in the steady-state parameter kcat. The proton extracted from the metal-bound water is transferred to the buffer, which acts as the second substrate in a ping-pong mechanism.
Kinetic analyses of single-amino-acid variants of Mt-Cam have identified Gln75, Asn73, and Asn202 as residues involved in a hydrogen bond network required for the first half-reaction and Glu62 and Glu84 as residues required for the second half-reaction (36). Also, analyses of single-amino-acid variants have identified Arg59, Asp61, and Asp76 as residues important for the integrity of the active site (31). The crystal structure identified a histidine motif (His81, His117, and His122) ligating the active site metal (17). Although Mt-Cam is the only γ class CA that has been characterized biochemically, homologs of Mt-Cam from Arabadopsis thaliana have also been investigated (20). Structural modeling and sequence analysis of the homologs have shown that there is conservation in Mt-Cam of the overall fold, metal ligand (Mt-Cam His81, His117, and His122), and residues important for catalysis (Mt-Cam Gln75 and Gln73) and active site structure (Mt-Cam Arg59, Asp61, and Asp76). However, Glu62 and Glu84 in Mt-Cam, which are required for the second half-reaction, are not conserved in the A. thaliana homologs, and CA activity was not detected in the proteins overproduced in E. coli. Furthermore, the proton shuttle residue (PSR) Glu84 in Cam is located on an acidic loop (30) that is not present in the A. thaliana homologs. A 2004 database search queried with one of the A. thaliana homologs retrieved sequences of putative γ class homologs from cyanobacteria, alpha- and gammaproteobacteria, plants, and green algae (20). With only one exception, all of the sequences showed conservation with the Mt-Cam residues essential for metal binding and catalysis except Glu62 and acidic loop residues that include Glu84. The crystal structure of an Mt-Cam homolog from the anaerobic archaeon Pyrococcus horikoshii that was overproduced in E. coli (14) has left-handed parallel β-helix fold and active site architecture similar to that of Mt-Cam (13) with bicarbonate bound to the active site zinc. However, the acidic loop and the PSR Glu84 of Mt-Cam are not conserved in the P. horikoshii enzyme, and CA activity was not examined. Interestingly, calcium was also shown to be present in the P. horikoshii structure. An Mt-Cam homolog from the marine alga Emiliania huxleyi was overproduced in E. coli and purified to homogeneity (27). The purified enzyme was not characterized, and CA activity was not examined, although 2-fold-greater CA activity was detected in extracts of E. coli expressing the gene. An alignment of the E. huxleyi homolog with Mt-Cam revealed the absence of residues essential for ligation of the active site metal (Mt-Cam His81, His117, and His122), residues important for activity (Mt-Cam Gln75, Gln73, and Glu62), and the acidic loop containing the PSR Glu84.
Since only the archetype of the γ class CAs has been characterized biochemically, our overall understanding of the γ class is vague. Sequence analyses of putative homologs have suggested that there is substantial diversity similar to that in the α class (28). In particular, the sequence analyses indicated that many homologs lack the acidic loop and the essential PSR Glu84 of Mt-Cam, suggesting that these homologs belong to a subclass of the γ class. However, the absence of CA activity reported for the two purified proteins belonging to the putative subclass (14, 27) calls into question the catalytic activity, metal content, and other properties. Here we describe a more recent sequence analysis of the γ class and biochemical characterization of a representative of the putative subclass, Mt-CamH from M. thermophila, that was overproduced in E. coli and had substantial CA activity, although it has properties that distinguish it from Mt-Cam.
MATERIALS AND METHODS
Cloning of the gene and heterologous overproduction of Mt-CamH from M. thermophila.
The 570-bp region encoding Mt-CamH was amplified from M. thermophila genomic DNA with the Expand high-fidelity PCR system (Roche Applied Sciences) using a sense primer (5′-GGTGGTCATATGAAGAGGAATTTTAAAATGC) that partially corresponds to nucleotides encoding amino acids 1 to 7 and an antisense primer (5′-ACCACCAAGCTTTCATCAACTCTCTTC-3′) that partially corresponds to nucleotides 551 to 568 downstream of the beginning of the gene that generated NdeI and HindIII restriction sites. The PCR product was blunt ended with Expand high-fidelity polymerase mixture (Roche Applied Sciences), digested with NdeI and HindIII, and cloned into the digested pet22b vector (Novagen), which yielded pCamHT13A. This plasmid was transformed into E. coli strain Rosetta(DE3)(pLysI) (Novagen), which was inoculated into Luria-Bertani broth containing 100 μg/ml ampicillin and 34 μg/ml chloramphenicol. Cells were grown at 37°C to an A600 of 0.6 to 0.8 and then chilled to 16°C. The chilled cultures were induced to overproduce Mt-CamH by addition of isopropyl thiogalactopyranoside (IPTG) to a final concentration of 1 mM. Simultaneously, either 500 μM ZnSO4 or 200 μM ferric ammonium citrate was added to the medium. Then the induced cultures were allowed to grow at 16°C for 16 to 20 h. Cells were harvested by centrifugation and stored frozen at −80°C until they were lysed.
Purification of Mt-CamH and Mt-Cam.
Mt-CamH purified from iron-supplemented cultures (Fe-Mt-CamH) was anaerobically purified by employing an glove bag (Coy Laboratory Products, Ann Arbor, MI) with an inert atmosphere at 25°C and anaerobic procedures to maintain all solutions under an N2 atmosphere. All buffers were made anaerobic by vacuum degassing and replacement with N2. Frozen cells were thawed in the glove bag on ice and resuspended in an equal volume of ice-chilled 50 mM N-Tris(hydroxymethyl)methyl-3-aminopropanesulfonic acid buffer (pH 8.5) containing 1 mM dithiothreitol (DTT) and 1 μM DNase I, which was followed by two passes through a chilled French press at a pressure of 6.9 × 103 kPa. Crude lysate was then centrifuged at 29,000 × g for 60 min, which was followed by passage of the supernatant through a 0.45-μm filter. The filtrate was loaded onto an SP-Sepharose Fast Flow (Amersham Pharmacia Biotech) cation-exchange column (bed volume, 66 ml) and washed with 1 bed volume of buffer A (50 mM TAPS [pH 8.5] containing 1 mM DTT) to remove unbound proteins. Weakly bound proteins were removed by washing the column with 1.5 bed volumes of buffer A containing 0.11 M NaCl. Six bed volumes of a gradient of 0.13 to 0.55 M NaCl in buffer A was applied. Fractions eluting between 0.15 and 0.30 M NaCl were pooled, immediately diluted so that they contained less than 12 mg protein/ml with buffer A to avoid aggregation, and then stored in serum bottles with stoppers containing an N2 atmosphere on ice for immediate use without freezing. The method used for purification of Mt-CamH from cells supplemented with zinc (Zn-Mt-CamH) was the same as the method used for purification of Fe-Mt-CamH except that anaerobic procedures were not used. Overproduction of Mt-Cam in E. coli, purification, and reconstitution with Zn2+ (Zn-Mt-Cam) or Fe2+ (Fe-Mt-Cam) were performed as previously described (13).
Steady-state kinetic measurements.
Carbon dioxide hydration activity was assayed by the pH indicator method (16) with a model SF-2001 KinTek stopped-flow instrument (KinTek Corp., Austin, TX). The enzyme monomer concentrations ranged from 400 nM to 1 μM. The buffer-indicator dye pairs used were (i) morpholineethanesulfonic acid (MES) and chlorophenol red (at pH 5.7 to 6.9) with measurement at a wavelength of 574 nm, (ii) morpholinepropanesulfonic acid (MOPS) or imidazole and 4-nitrophenol (at pH 6.5 to 7.7) with measurement at a wavelength of 400 nm, and (iii) TAPS and m-cresol purple (at pH 7.7 to 9.1) with measurement at a wavelength of 578 nm. The buffer concentration was 50 mM, the total ionic strength was adjusted to 50 mM with Na2SO4, and the final pH indicator concentration was 50 μM. Saturated solutions of CO2 (32.9 mM in H2O) were prepared by bubbling CO2 gas into deionized water at 25°C. The final experimental concentrations after mixing with CO2 ranged from 7.9 to 24.7 mM. The initial 5 to 10% of the total absorbance changes were used to calculate initial steady-state kinetic data used for kinetic analysis, using the average for 10 to 15 reaction traces per experiment. The initial rate data were fitted to the Michaelis-Menten equation to obtain experimental values for kcat and Km. The pH-independent values for the CO2 hydration reaction was determined by fitting the experimental pH-dependent Michaelis-Menten parameters to equations 5 and 6
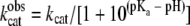 |
(5) |
 |
(6) |
where kcatobs is the observed kcat.
Analytical procedures.
Protein concentrations were determined by measuring the A280 of solutions and using a theoretical monomer extinction coefficient of 6,990 cm−1 M−1 and a computed monomer molecular mass of 20,335 Da.
The native molecular mass was estimated with a Superdex 75 high-resolution gel filtration column (Amersham Pharmacia Biotech, Piscataway, NJ) equilibrated with buffer A containing 150 mM KCl and eluted with a flow rate of 1.0 ml/min. The eluting holoenzyme was detected by measuring the absorbance at 280 nm. The procedure was performed anaerobically in the inert glove bag.
The N-terminal sequence of Mt-CamH was determined by using an Applied Biosystems Procise 491 sequencer at the Macro Core Facility of the Pennsylvania State University College of Medicine.
A comprehensive metal analysis of Mt-CamH and Mt-Cam was conducted using inductively coupled plasma atomic emission spectroscopy at the Chemical Analysis Laboratory, University of Georgia, Athens. The iron content was also determined using the Ferene S Fe assay (34) in triplicate.
TaqMan quantitative reverse transcription (RT)-PCR (Perkin-Elmer Applied Biosystems) was performed at the Nucleic Acid Facility of the Pennsylvania State University. Total RNA was isolated from acetate-, methanol-, or trimethylamine-grown cells harvested at mid-exponential phase using a modified protocol from an RNeasy mini kit (Qiagen, Valencia, CA) that involved two additional DNase I steps. The relative abundances of the gene encoding Mt-CamH were determined using the cycle threshold method (Perkin-Elmer Applied Biosystems) with the 16S rRNA gene used for normalization. Primers and probes are listed in Table S1 in the supplemental material.
Nucleotide sequence accession number.
The DNA sequence of the 570-bp region encoding Mt-CamH has been deposited in the GenBank database under accession number FJ905299 (http://www.ncbi.nlm.nih.gov/GenBank/).
RESULTS
Phylogenetic analyses.
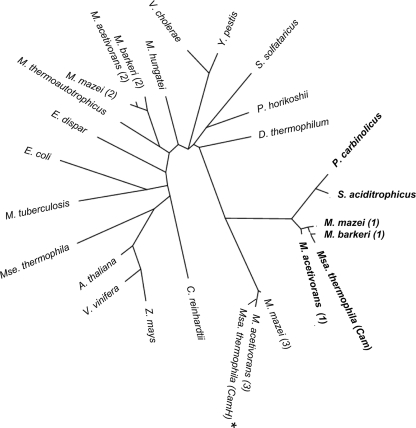
Mt-Cam homologues were retrieved from a BLAST search performed with the GenBank NR database with an expect cutoff of e-5. Duplicates and highly redundant proteins (95% identical) were removed, and proteins between 150 and 230 amino acids long were extracted. The resulting 694 proteins were aligned with Clustal W, and 690 of these proteins showed strict conservation of all three histidine residues in Mt-Cam that coordinate the active site metal (see Table S2 in the supplemental material). All but 2 of these 690 sequences showed strict conservation of Arg59 of Mt-Cam. The majority of the 690 sequences also showed strict conservation of both Asp61 and Asp76 of Mt-Cam, while all of the remaining sequences showed conservative replacement with Glu at both positions consistent with roles of these residues in the putative proteins similar to Mt-Cam. However, 640 of the 690 sequences, including all or part of the acidic loop of Mt-Cam, were missing Glu84, consistent with the trend observed in previous database searches of fewer sequences (8, 19). Of the 640 sequences missing Glu84, 601 were also missing Glu62. Another 59 sequences were missing Mt-Cam residue Asn73, 7 sequences were missing Gln75, and 601 sequences were missing Asn202. The results suggest that the majority of the γ class is comprised of a subclass of proteins distinct from Mt-Cam, whose the catalytic activity is uncertain. The great majority of the 640 putative CAs belonging to this subclass were from diverse species of the domain Bacteria (581 CAs), and fewer CAs were from species belonging to the Archaea (50 CAs) and Eukarya (9 CAs) domains. These results indicate that the subclass dominates the γ class and is widespread in nature. Figure 1A shows a phylogenetic tree of representatives of each of the domains along with the archetype Mt-Cam from M. thermophila and Mt-Cam homologs that contain the acidic loop and a full complement of the essential residues of Mt-Cam.
FIG. 1.
Phylogenetic tree of selected Cam and CamH homologs. Cam homologs are indicated by bold type. The CamH homolog from M. thermophila is indicated by an asterisk. The members of the Eukarya included are Vitis vinifera (accession no. gi|157335308), Zea mays (gi|219362885), Entamoeba dispar (gi|167389375), Arabidopsis thaliana (gi|10177532), Chlamydomonas reinhardtii (gi|159490549), and Yersinia pestis (gi|22127899). The members of the Bacteria included are Syntrophus aciditrophicus (gi|85859775), Pelobacter carbinolicus (gi|77919438), Vibrio cholerae (gi|153217568), Mycobacterium tuberculosis (gi|215432497), Dictyoglomus thermophilum (gi|206900416), and Escherichia coli (gi|563866). The members of the Archaea included are Sulfolobus solfataricus (gi|15897307), Methanospirillum hungatei (gi|88601918), Pyrococcus horikoshii (gi|14591369), Methanothermobacter thermoautotrophicus (gi|15679583), three Methanosarcina acetivorans strains (indicated by 2 [gi|20090000], 1 [gi|20091364], and 3 [gi|20089968]), three Methanosarcina mazei strains (indicated by 2 [gi|21228263], 1 [gi|21229190], and 3 [gi|21228253]), two Methanosarcina barkeri strains (indicated by 2 [gi|73669360] and 1 [gi|73670479]), Methanosarcina thermophila (gi|1827571) (Cam), and Methanosaeta thermophila (gi|116754979). The tree was constructed as described in reference 5.
Among the 640 subclass sequences were sequences from Methanosarcina mazei and M. acetivorans (Fig. 1), annotated as CamH (http://cmr.tigr.org/tigr-scripts/CMR/CmrHomePage.cgi); the genomes of these organisms also encode Mt- Cam homologs (9, 11; http://cmr.tigr.org/tigr-scripts/CMR/CmrHomePage.cgi). The sequenced genome of M. thermophila also contains a gene encoding a putative CamH homolog (Mt-CamH) (Fig. 2). Although the acidic loop including the essential residue Glu84 of Mt-Cam is not conserved, Mt-CamH shares 46% identity with Mt-Cam and contains other conserved Mt-Cam residues essential for the left-handed β-helix fold, metal ligation, stability of the active site, and catalysis (Fig. 2) (13, 17, 30, 32, 36). Based on the CamH designation for the subclass homolog in M. acetivorans (http://cmr.tigr.org/tigr-scripts/Clin.Microbiol.Rev./CmrHomePage.cgi), the subclass is referred to below as the CamH subclass.
FIG. 2.
Sequence alignment of Mt-Cam and Mt-CamH. +, catalytically relevant residues in Mt-Cam; %, metal ligands in Mt-Cam; #, structurally relevant residues in Mt-Cam. Acidic loop residues, including the proton shuttle residue Glu84 in Mt-Cam, are underlined. The sequences were aligned using the CLUSTAL 2.0.10 multiple-sequence alignment program.
Heterologous production of Mt-CamH from M. thermophila.
The results of sequence analyses and the lack of CA activity of purified CamH homologs (14, 27) have called into question the catalytic potential and other properties of the CamH subclass. Thus, Mt-CamH was overproduced in E. coli and characterized to begin to investigate the CamH subclass and increase our overall understanding of the γ class and the physiological role of CAs in Methanosarcina species. Mt-CamH was overproduced in media supplemented with either ZnSO4 or FeSO4 and was purified to homogeneity as determined by SDS-PAGE. The N terminus of the proteins overproduced with either metal supplement was identical to the first 15 amino acid residues of the sequence deduced from the gene encoding Mt-CamH. The two proteins overproduced with a metal supplement migrated to the same position in SDS-PAGE, which indicated that the monomer molecular mass is 20 kDa (data not shown). As estimated by sIze exclusion chromatography, the native molecular mass was 62 kDa for both preparations, suggesting that Mt-CamH is a homotrimer resembling Mt-Cam (data not shown). Mt-CamH purified aerobically from E. coli cultured with a zinc supplement (Zn-Mt-CamH) contained less than a full complement of metal, which was predominantly zinc along with smaller amounts of iron (Table 1). Mt-CamH purified anaerobically from E. coli cultured with supplemental iron (Fe-Mt-CamH) contained similar amounts of iron (Table 1) and only trace amounts of zinc (0.009/monomer). No metals other than zinc or iron were detected in either preparation. Mt-Cam from M. thermophila was overproduced, and the enzyme was reconstituted with a full complement of either zinc (Mt-Zn-Cam) or iron (Mt-Fe-Cam) (Table 1) by unfolding the protein to release the active site metal and then refolding it in the presence of Zn2+ or Fe2+, as previously described (13, 29). Using the same procedure with Mt-CamH was unsuccessful due to the greater instability of Mt-CamH than of Mt-Cam when the proteins were unfolded. Unfolding of Mt-CamH led to irreversible precipitation that precluded refolding in the presence of metal.
TABLE 1.
Michaelis-Menten steady-state kinetic parameters for Cam and CamHa
| Enzyme | Km (mM) | kcat (10−3 s−1) | kcat/Km (10−6 M−1 s−1) | kcateff (10−3 s−1)b | kcat/Kmeff (10−6 M−1 s−1)b | Metal/ monomer ratio |
|---|---|---|---|---|---|---|
| Zn-Mt-Cam | 15.6 ± 0.8 | 116.4 ± 3.0 | 7.5 ± 0.6 | 116.4 ± 3.0 | 7.5 ± 0.6 | 1.2 (Zn) |
| Fe-Mt-Cam | 33.3 ± 6.2 | 231.4 ± 20.4 | 6.9 ± 1.9 | 235.1 ± 22.8 | 7.0 ± 2.1 | 0.97 (Fe) |
| Zn-Mt-CamH | 3.1 ± 0.1 | 0.493 ± 0.001 | 0.16 ± 0.03 | NAc | NA | 0.71 (Zn) |
| 0.15 (Fe) | ||||||
| Fe-Mt-CamH | 3.0 ± 0.7 | 5.2 ± 0.02 | 1.7 ± 0.01 | 40.0 ± 1.23 | 13.1 ± 1.86 | 0.13 (Fe) |
| Fe-Mt-CamHd | 8.0 ± 1.7 | 24.2 ± 1.9 | 3.03 ± 0.9 | 186.1 ± 17.0 | 23.3 ± 5.5 | 0.13 (Fe) |
Assays were performed using stopped-flow spectroscopy at pH 7.5 and 25°C in 50 mM HEPES.
Effective kcat (kcateff) and kcat/Kmeff values were obtained by dividing apparent kcat and kcat/Km values by the molar ratio of metal to monomer to reflect parameters for only the metal-containing holoenzyme fraction. For this calculation, the metal-to-monomer ratio was assumed to be 1.0.
NA, not applicable.
Assayed in the presence of 50 mM imidazole.
Kinetic parameters.
Mt-Cam from M. thermophila reconstituted with zinc (Zn-Mt-Cam) or iron (Fe-Mt-Cam) in the active site was prepared, and the steady-state kinetic parameters for CO2 hydration activity were compared with those of Zn-Mt-CamH and Fe-Mt-CamH (Table 1). The metal content and steady-state kinetic parameters for CO2 hydration activity obtained for the reconstituted Zn-Mt-Cam and Fe-Mt-Cam proteins were comparable to those published previously (13, 29). Both the Zn-Mt-CamH and Fe-Mt-CamH enzymes had significant activity, a result that validated the conclusion that Mt-CamH is a member of the γ class of CAs. The kcat and kcat/Km values in the direction of CO2 hydration for both Zn-Mt-CamH and Fe-Mt-CamH were only a fraction of those recorded for Zn-Mt-Cam and Fe-Mt-Cam, although the proportions of activity due to zinc and to iron cannot be determined for Zn-Mt-CamH. When normalized to the content of iron, the effective kcat (kcateff) value for Fe-Mt-CamH increased substantially, albeit ∼6-fold less than that for Fe-Mt-Cam, and the kcat/Kmeff for Fe-Mt-CamH was comparable to that for Fe-Mt-Cam. These results are consistent with the hypothesis that iron is the in vivo physiologically relevant metal for Mt-CamH, as previously shown for Mt-Cam (18). They are also consistent with the proposed absence of a PSR in Mt-CamH compared to Mt-Cam. Since kcat is related to PSR efficiency and kcat/Km is limited to steps up to and including the release of bicarbonate in the CO2 hydration reaction, a much larger reduction would be expected for kcateff of Mt-CamH than for kcateff of Mt-Cam along with relatively little difference in the kcat/Kmeff values, which was observed (Table 1). Thus, the Km for CO2 hydration should be lower for proton transfer-impaired CAs as it merely reflects the kcat/(kcat/Km) ratio. Indeed, the Km (CO2) values for Zn-Mt-CamH and Fe-Mt-CamH were substantially less than those for Zn-Mt-Cam and Fe-Mt-Cam (Table 1). It should be noted that the Km for CAs is a product of multiple kinetic constants and has little or no significance for substrate binding affinity.
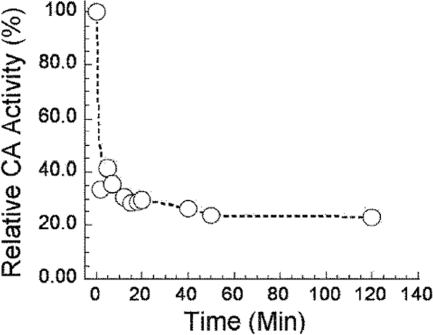
The effect of oxidation of Mt-Fe-CamH on activity was evaluated to further characterize the enzyme and the role of iron in the active site. It was shown previously that exposure of Fe-Mt-Cam to air or hydrogen peroxide oxidizes Fe2+ to Fe3+ with loss of the active site metal and CA activity (29). More than 65% of the CA activity was lost within 10 min after exposure of Fe-Mt-CamH to air (Fig. 3), whereas no significant loss of activity was observed for Zn-Mt-CamH over a period of 2 h (data not shown). Incubation of Fe-Mt-CamH (subunit concentration, 1 μM) with 3% H2O2 for 10 min prior to the assay resulted in loss of 80% of the activity, whereas no significant loss of activity was observed for Zn-Mt-CamH when it was treated in the same way (data not shown). The residual activity is ascribed to loss of iron from the active site that allows scavenging of zinc from contaminated buffer, as described previously for Cam (29). The cumulative results for Fe-Mt-Cam are similar to those reported for Fe-Mt-Cam (29), supporting the hypothesis that iron is present in the active site of Fe-Mt-CamH and plays a catalytic role. Overall, the kinetic parameters indicate that there are substantial differences between the γ class archetype Mt-Cam and Mt-CamH representing the CamH subclass.
FIG. 3.
Effect of oxidation on Fe-Mt-CamH activity. The enzyme (subunit concentration, 400 nM) was exposed to 1 atm air and assayed at the times indicated using the stopped-flow method at 25°C in 50 mM HEPES (pH 7.5). The CO2 concentration used was 24.7 mM. A control incubated anaerobically exhibited 100% activity over the time period shown (data not shown).
The sequence comparison in Fig. 2 shows that Mt-CamH is missing the acidic loop of Mt-Cam that includes the PSR Glu84. The lower kcat values for Mt-CamH than for Mt-Cam (Table 1) are consistent with the absence of a PSR in Mt-CamH, contributing to the low kcat values. To determine if the absence of a PSR is rate determining in Mt-CamH, the enzyme activity was assayed in the presence of imidazole. Imidazole is a chemically reactive (pKa ∼6.8) buffer that facilitates intramolecular proton transfer in vitro in CAs that lack a PSR, such as HCAIII, or in CAs in which the PSR is replaced by an unreactive residue by site-directed mutagenesis (7, 22, 24, 30, 33). The small imidazole molecule enters the active site and acts as a proton acceptor. The ability of imidazole to increase the kcat of CAs is considered strong evidence that intramolecular proton transfer is rate limiting. The kcat for Fe-Mt-CamH increased nearly 5-fold when a preparation was assayed in the presence of 50 mM imidazole (Table 1), indicating that intramolecular proton transfer is rate limiting. Indeed, the kcateff approached that of Fe-Mt-Cam. The Km increased with imidazole like the Km reported for variants of Mt-Cam (30) in which the PSR Glu84 is replaced by unreactive residues. This result suggests that imidazole enters and rescues the active site PSR function by extracting a proton from metal-bound water and transferring it to buffer.
Catalytic mechanism.
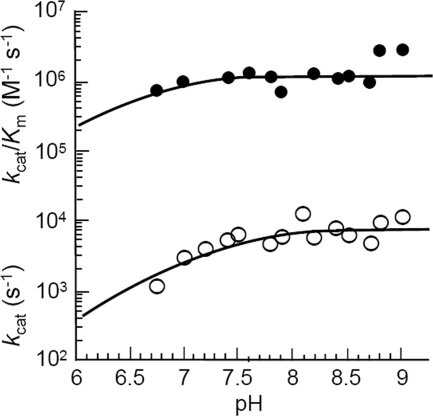
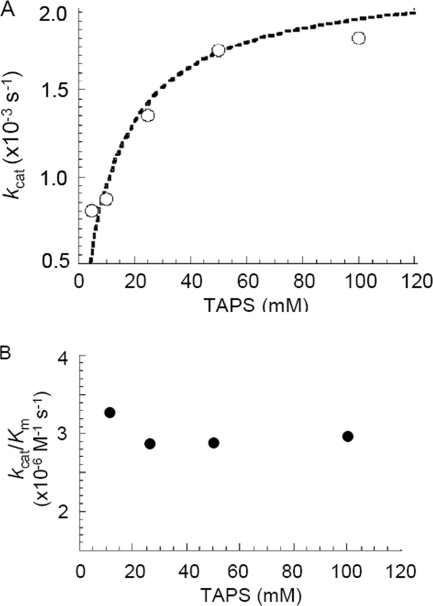
The pH dependence of CO2 hydration was measured by stopped-flow spectroscopy using the changing pH indicator assay (Fig. 4). The steady-state values for kcat and kcat/Km were pH dependent, with a single pKa of 7.3 ± 0.02 for kcat and one reliable pKa discernible for kcat/Km (pKa 6.6 ± 0.4). The steady-state parameters kcat and kcat/Km increased with increasing pH, suggesting that an unprotonated form of the enzyme is required for activity, which is consistent with the metal-hydroxide mechanism of catalysis. Indeed, the pKa obtained for kcat/Km is expected to reflect an estimate for the metal-bound hydroxide. Since the proton transfer step is the rate-determining step, the pH profile of kcat most likely tracks with the pKa of intramolecular proton acceptor groups and not with that of metal-bound hydroxide. However, in the absence of a clearly identifiable efficient proton transfer group, it is difficult to attribute the observed pKa for kcat to a single intramolecular PSR. The dependence of CO2 hydration catalyzed by Fe-Mt-CamH on the concentration of TAPS buffer was determined at pH 8.2 (Fig. 5). The kcat was dependent on the concentration of buffer in a saturable manner, with an apparent Km for TAPS of 10.3 ± 0.2 mM. This result indicates that buffer acts as the second substrate in a ping-pong mechanism, accepting a proton from the enzyme during CO2 hydration. The Km value also increased with increasing buffer concentration, resulting in a kcat/Km value that was independent of the buffer concentration. These results are consistent with the metal-hydroxide mechanism in which the interconversion of CO2 and HCO3− (equations 1 and 2) reflected by kcat/Km is separate from the intermolecular proton transfer reflected by kcat (equations 3 and 4).
FIG. 4.
pH dependence of CO2 hydration catalyzed by Fe-Mt-CamH. Activities were measured in 50 mM buffer at an ionic strength of 150 mM and 25°C. The subunit concentration used was 1 μM. The CO2 concentration was varied from 7.9 to 24.7 mM. Data were weighted based on the standard errors determined by fitting the observed initial rates to the Michaelis-Menten equation.
FIG. 5.
Buffer dependence of CO2 hydration catalyzed by Fe-Mt-Cam. The CO2 hydration activity (subunit concentration, 1.0 μM) was measured at pH 8.2, an ionic strength of 150 mM, and 25°C. The CO2 concentration was varied from 7.9 to 24.7 mM, and the TAPS concentration was varied from 10 to 100 mM. The Km for TAPS was 10.3 ± 0.2 mM, and the maximum kcat was 1.9 × 103 ± 0.01 × 103 s−1. Both kinetic parameters were determined by fitting the observed initial rates to the Michaelis-Menten equation.
Expression of Mt-Cam and Mt-CamH genes.
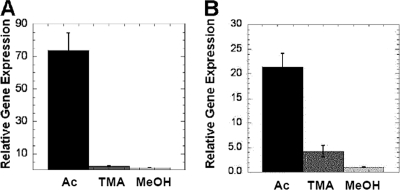
Quantitative RT-PCR (Fig. 6) showed that the expression of the gene encoding Mt-Cam was substantially greater in acetate-grown M. thermophila cells than in methanol- or trimethylamine-grown cells, consistent with previously reported results (3). A similar pattern of expression was obtained for the gene encoding Mt-CamH.
FIG. 6.
Relative expression of the genes encoding Mt-Cam and Mt-CamH in acetate-, trimethylamine-, and methanol-grown M. acetivorans determined by quantitative RT-PCR. (A) Mt-Cam gene. (B) Mt-CamH gene. Ac, acetate; TMA, trimethylamine; MeOH, methanol.
DISCUSSION
The results presented here describe the biochemical and kinetic characterization of Mt-CamH from M. thermophila, the first characterization for a large subclass of the γ class of CAs widely distributed in nature and only the second γ subclass of CAs characterized. The results are expected to encourage and direct future studies of the CamH subclass that should increase our general understanding of the γ class.
The properties of Mt-CamH distinguish it from the archetype Mt-Cam from M. thermophila. A particularly distinguishing property is the reactivity of Mt-CamH with zinc and iron, suggesting that there are differences in the active site architecture compared to that of Mt-Cam. The Fe/Zn ratio (0.2) in Fe-Mt-CamH was 5-fold less than that reported for Mt-Cam (1.0) overproduced and purified like Fe-Mt-CamH (29). A possible explanation for the difference is weaker binding of iron in Mt-CamH that was lost during purification, although purification was performed anaerobically to prevent oxidation of Fe2+ with loss of Fe3+ like that for Mt-Cam. Another possibility is that maturation of Fe-Mt-CamH produced in M. thermophila requires accessory proteins not present in E. coli to incorporate Fe2+ in the active site. This possibility is consistent with the high occupancy of zinc compared to iron in Mt-CamH overproduced in E. coli cultured with supplemental zinc. The affinity of complexed zinc for the active site nitrogen ligands is expected to be at least an order of magnitude greater than that of iron predicted by the Irving-Williams series (12). Thus, in the absence of accessory proteins to incorporate iron, zinc would be expected to occupy the active site of Mt-CamH when it is overproduced in E. coli cultured with supplemental zinc. Despite the presence of zinc in the crystal structure of the CamH homolog from the anaerobe P. horikoshii (14), the results presented here suggest that a role for iron in the active site needs to be examined considering that the enzyme was overproduced in E. coli.
Other distinguishing features of Mt-CamH are the absence of the essential PSR in Mt-Cam and effective kcat values substantially less than those of Mt-Cam, analogous to the α class isozymes HCAIII and HCAII. The HCAIII isozyme lacks the PSR present in HCAII, resulting in a kcat 300-fold less than that of HCAII (23). Furthermore, the presence of imidazole increases the kcat of HCAIII 5-fold (4), similar to the increase reported here for the kcat of Fe-Mt-CamH. These results are consistent with the absence of a PSR in Mt-CamH that contributes to the lower kcat compared to that of Mt-Cam, although additional characterization and crystal structures are necessary to address this question. Clearly, the intramolecular proton transfer to buffer for Mt-CamH is distinct from that for Mt-Cam and is of great interest.
The results presented here also indicate that Mt-Cam and Mt-CamH have features in common, including subunit composition, the fundamental reaction mechanism, and utilization of Fe2+ in the active site. The kinetic analyses indicate that, although there may be differences in the active site architecture and intramolecular proton transfer, the mechanism for the Mt-CamH reaction consists of two kinetically distinct half-reactions (equations 1 to 4), like that for Mt-Cam. We found that iron is the more catalytically active metal for Mt-CamH, and this enzyme is only the second CA shown to utilize this metal for catalysis. Mt-Cam contains Fe2+ in the active site when it is overproduced in the closely related species M. acetivorans (18), a result that establishes that iron is the physiologically relevant metal in Mt-Cam and also supports the hypothesis that iron is the physiologically relevant metal in Mt-CamH.
The finding that the genes encoding both Mt-Cam and Mt-CamH were upregulated in acetate-grown M. thermophila compared with trimethylamine- or methanol-grown M. thermophila is consistent with a role for both CAs in the pathway for conversion of acetate to methane. The deduced amino acid sequence encoded by the Mt-Cam gene identified an additional 34 N-terminal residues of the protein purified from M. thermophila with properties characteristic of signal peptides in secretory proteins, suggesting that Mt-Cam resides outside the cytoplasmic membrane, where it is proposed to function in removal of CO2 that is produced in the conversion of acetate to equal amounts of CO2 and methane (2). The gene encoding CamH from M. thermophila does not encode additional N-terminal residues consistent with a cytosolic location. Based on the putative locations of Mt-Cam and Mt-CamH, a function for both Mt-Cam and Mt-CamH during growth on acetate has been proposed (6), in which the enzymes facilitate the exchange of acetate for bicarbonate, analogous to the system that was previously proposed for acetate-utilizing species producing CO2 as an end product of metabolism (15).
Conclusions.
Overproduction and characterization of Mt-CamH showed that it has CA activity, and this is only the second time that this has been demonstrated for any member of the γ class of CAs and the first that it has been demonstrated for the CamH subclass. Clearly, more research is necessary to determine if other representatives of the CamH subclass, particularly those that are missing residues other than the Glu84 residue that has been shown to be essential for Mt-Cam activity, also have CA activity. The properties of Mt-CamH distinguish it from Mt-Cam, the archetype of the γ class, in important ways. Most importantly, Mt-CamH is missing the acidic loop and essential Glu84 PSR of Mt-Cam which potentially contributes to the lower kcat for Mt-CamH. The reactivity of Mt-CamH with zinc and iron suggested that there are differences in the active site architecture compared to that of Mt-Cam. Our results establish that Mt-CamH and Mt-Cam have fundamentally similar reaction mechanisms and are able to utilize iron in the active site, which is consistent with a physiological role for iron, as established for Mt-Cam. Mt-CamH is only the second γ class CA characterized biochemically that advances an understanding of the biochemistry and physiology of the γ class and also contributes to our general understanding of prokaryotic CAs.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Science Foundation and the Thomas H. Maren Foundation to J.G.F.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 18 December 2009.
REFERENCES
- 1.Alber, B. E., C. M. Colangelo, J. Dong, C. M. V. Stålhandske, T. T. Baird, C. Tu, C. A. Fierke, D. N. Silverman, R. A. Scott, and J. G. Ferry. 1999. Kinetic and spectroscopic characterization of the gamma-carbonic anhydrase from the methanoarchaeon Methanosarcina thermophila. Biochemistry 38:13119-13128. [DOI] [PubMed] [Google Scholar]
- 2.Alber, B. E., and J. G. Ferry. 1994. A carbonic anhydrase from the archaeon Methanosarcina thermophila. Proc. Nat. Acad. Sci. U. S. A. 91:6909-6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alber, B. E., and J. G. Ferry. 1996. Characterization of heterologously produced carbonic anhydrase from Methanosarcina thermophila. J. Bacteriol. 178:3270-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An, H., C. Tu, D. Duda, I. Montanez-Clemente, K. Math, P. J. Laipis, R. McKenna, and D. N. Silverman. 2002. Chemical rescue in catalysis by human carbonic anhydrases II and III. Biochemistry 41:3235-3242. [DOI] [PubMed] [Google Scholar]
- 5.Dereeper, A., V. Guignon, G. Blanc, S. Audic, S. Buffet, F. Chevenet, J. F. Dufayard, S. Guindon, V. Lefort, M. Lescot, J. M. Claverie, and O. Gascuel. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36:W465-W469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferry, J. G. The γ class of carbonic anhydrase. Biochim. Biophys. Acta, in press. [DOI] [PMC free article] [PubMed]
- 7.Fisher, Z., J. A. H. Prada, C. Tu, D. Duda, C. Yoshioka, H. Q. An, L. Govindasamy, D. N. Silverman, and R. McKenna. 2005. Structural and kinetic characterization of active-site histidine as a proton shuttle in catalysis by human carbonic anhydrase II. Biochemistry 44:1097-1105. [DOI] [PubMed] [Google Scholar]
- 8.Fu, X., L. J. Yu, L. Mao-Teng, L. Wei, C. Wu, and M. Yun-Feng. 2008. Evolution of structure in gamma-class carbonic anhydrase and structurally related proteins. Mol. Phylogenet. Evol. 47:211-220. [DOI] [PubMed] [Google Scholar]
- 9.Galagan, J. E., C. Nusbaum, A. Roy, M. G. Endrizzi, P. Macdonald, W. FitzHugh, S. Calvo, R. Engels, S. Smirnov, D. Atnoor, A. Brown, N. Allen, J. Naylor, N. Stange-Thomann, K. DeArellano, R. Johnson, L. Linton, P. McEwan, K. McKernan, J. Talamas, A. Tirrell, W. Ye, A. Zimmer, R. D. Barber, I. Cann, D. E. Graham, D. A. Grahame, A. M. Guss, R. Hedderich, C. Ingram-Smith, H. C. Kuettner, J. A. Krzycki, J. A. Leigh, W. Li, J. Liu, B. Mukhopadhyay, J. N. Reeve, K. Smith, T. A. Springer, L. A. Umayam, O. White, R. H. White, E. C. de Macario, J. G. Ferry, K. F. Jarrell, H. Jing, A. J. Macario, I. Paulsen, M. Pritchett, K. R. Sowers, R. V. Swanson, S. H. Zinder, E. Lander, W. W. Metcalf, and B. Birren. 2002. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 12:532-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewett-Emmett, D. 2000. Evolution and distribution of the carbonic anhydrase gene families, p. 29-76. In W. R. Chegwidden, N. D. Carter, and Y. H. Edwards (ed.), The carbonic anhydrases. Birkhauser Verlag, Basel, Switzerland. [DOI] [PubMed]
- 11.Hovey, R., S. Lentes, A. Ehrenreich, K. Salmon, K. Saba, G. Gottschalk, R. P. Gunsalus, and U. Deppenmeier. 2005. DNA microarray analysis of Methanosarcina mazei Go1 reveals adaptation to different methanogenic substrates. Mol. Genet. Genomics 273:225-239. [DOI] [PubMed] [Google Scholar]
- 12.Irving, H., and R. J. P. Williams. 1948. Order of stability of metal complexes. Nature 162:746-747. [Google Scholar]
- 13.Iverson, T. M., B. E. Alber, C. Kisker, J. G. Ferry, and D. C. Rees. 2000. A closer look at the active site of γ-carbonic anhydrases: high resolution crystallographic studies of the carbonic anhydrase from Methanosarcina thermophila. Biochemistry 39:9222-9231. [DOI] [PubMed] [Google Scholar]
- 14.Jeyakanthan, J., S. Rangarajan, P. Mridula, S. P. Kanaujia, Y. Shiro, S. Kuramitsu, S. Yokoyama, and K. Sekar. 2008. Observation of a calcium-binding site in the γ-class carbonic anhydrase from Pyrococcus horikoshii. Acta Crystallogr. Sect. D Biol. Crystallogr. 64:1012-1019. [DOI] [PubMed] [Google Scholar]
- 15.Karrasch, M., M. Bott, and R. K. Thauer. 1989. Carbonic anhydrase activity in acetate grown Methanosarcina barkeri. Arch. Microbiol. 151:137-142. [Google Scholar]
- 16.Khalifah, R. G. 1971. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 246:2561-2573. [PubMed] [Google Scholar]
- 17.Kisker, C., H. Schindelin, B. E. Alber, J. G. Ferry, and D. C. Rees. 1996. A left-handed beta-helix revealed by the crystal structure of a carbonic anhydrase from the archaeon Methanosarcina thermophila. EMBO J. 15:2323-2330. [PMC free article] [PubMed] [Google Scholar]
- 18.Macauley, S. R., S. A. Zimmerman, E. E. Apolinario, C. Evilia, Y. Hou, J. G. Ferry, and K. R. Sowers. 2009. The archetype γ-class carbonic anhydrase (Cam) contains iron when synthesized in vivo. Biochemistry 48:817-819. [DOI] [PubMed] [Google Scholar]
- 19.Parisi, G., M. Fornasari, and J. Echave. 2000. Evolutionary analysis of γ-carbonic anhydrase and structurally related proteins. Mol. Phylogenet. Evol. 14:323-334. [DOI] [PubMed] [Google Scholar]
- 20.Parisi, G., M. Perales, M. Fornasari, A. Colaneri, N. Schain, D. Casati, S. Zimmermann, A. Brennicke, A. Araya, J. G. Ferry, J. Echave, and E. Zabaleta. 2004. Gamma carbonic anhydrases in plant mitochondria. Plant Mol. Biol. 55:193-207. [DOI] [PubMed] [Google Scholar]
- 21.Park, H., B. Song, and F. M. Morel. 2007. Diversity of the cadmium-containing carbonic anhydrase in marine diatoms and natural waters. Environ. Microbiol. 9:403-413. [DOI] [PubMed] [Google Scholar]
- 22.Rowlett, R. S., C. Tu, M. M. McKay, J. R. Preiss, R. J. Loomis, K. A. Hicks, R. J. Marchione, J. A. Strong, G. S. Donovan, and J. E. Chamberlin. 2002. Kinetic characterization of wild-type and proton transfer-impaired variants of beta-carbonic anhydrase from Arabidopsis thaliana. Arch. Biochem. Biophys. 404:197-209. [DOI] [PubMed] [Google Scholar]
- 23.Silverman, D. N., C. K. Tu, X. Chen, S. M. Tanhauser, A. J. Kresge, and P. J. Laipis. 1993. Rate-equilibria relationships in intramolecular proton transfer in human carbonic anhydrase-III. Biochemistry 32:10757-10762. [DOI] [PubMed] [Google Scholar]
- 24.Silverman, D. N., and S. H. Vincent. 1983. Proton transfer in the catalytic mechanism of carbonic anhydrase. Crit. Rev. Biochem. 14:207-255. [DOI] [PubMed] [Google Scholar]
- 25.Smith, K. S., and J. G. Ferry. 2000. Procaryotic carbonic anhydrases. FEMS Microbiol. Rev. 24:335-366. [DOI] [PubMed] [Google Scholar]
- 26.Smith, K. S., C. Jakubzick, T. C. Whittam, and J. G. Ferry. 1999. Carbonic anhydrase is an ancient enzyme widespread in prokaryotes. Proc. Natl. Acad. Sci. U. S. A. 96:15184-15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soto, A. R., H. Zheng, D. Shoemaker, J. Rodriguez, B. A. Read, and T. M. Wahlund. 2006. Identification and preliminary characterization of two cDNAs encoding unique carbonic anhydrases from the marine alga Emiliania huxleyi. Appl. Environ. Microbiol. 72:5500-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Supuran, C. T. 2007. Carbonic anhydrases as drug targets—an overview. Curr. Top. Med. Chem. 7:825-833. [DOI] [PubMed] [Google Scholar]
- 29.Tripp, B. C., C. B. Bell, F. Cruz, C. Krebs, and J. G. Ferry. 2004. A role for iron in an ancient carbonic anhydrase. J. Biol. Chem. 279:6683-6687. [DOI] [PubMed] [Google Scholar]
- 30.Tripp, B. C., and J. G. Ferry. 2000. A structure-function study of a proton transport pathway in a novel γ-class carbonic anhydrase from Methanosarcina thermophila. Biochemistry 39:9232-9240. [DOI] [PubMed] [Google Scholar]
- 31.Tripp, B. C., K. Smith, and J. G. Ferry. 2001. Carbonic anhydrase: new insights for an ancient enzyme. J. Biol. Chem. 276:48615-48618. [DOI] [PubMed] [Google Scholar]
- 32.Tripp, B. C., C. Tu, and J. G. Ferry. 2002. Role of arginine 59 in the γ-class carbonic anhydrases. Biochemistry 41:669-678. [DOI] [PubMed] [Google Scholar]
- 33.Tu, C., R. S. Rowlett, B. C. Tripp, J. G. Ferry, and D. N. Silverman. 2002. Chemical rescue of proton transfer in catalysis by carbonic anhydrases in the β- and γ-class. Biochemistry 41:15429-15435. [DOI] [PubMed] [Google Scholar]
- 34.Zabinski, R., E. Munck, P. M. Champion, and J. M. Wood. 1972. Kinetic and Mossbauer studies on the mechanism of protocatechuic acid 4,5-oxygenase. Biochemistry 11:3212-3219. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman, S. A., and J. G. Ferry. 2008. The beta and gamma classes of carbonic anhydrase. Curr. Pharm. Des. 14:716-721. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman, S. A., and J. G. Ferry. 2006. Proposal for a hydrogen bond network in the active site of the prototypic γ-class carbonic anhydrase. Biochemistry 45:5149-5157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.