Abstract
Superdormant spores of Bacillus cereus and Bacillus subtilis germinated just as well as dormant spores with pressures of 150 or 500 MPa and with or without heat activation. Superdormant B. subtilis spores also germinated as well as dormant spores with peptidoglycan fragments or bryostatin, a Ser/Thr protein kinase activator.
Spores of Bacillus species are formed in sporulation, a process that is generally triggered by starvation for one or more nutrients (13, 19). These spores are metabolically dormant and extremely resistant to a large variety of environmental stresses, including heat, radiation, and toxic chemicals, and as a consequence of these properties, these spores can remain viable in their dormant state for many years (13, 18, 19). However, spores are constantly sensing their environment, and if nutrients return, the spores can rapidly return to growth through the process of spore germination (17). Spore germination is generally triggered by specific nutrients that bind to nutrient germinant receptors, with this binding alone somehow triggering germination. However, spore germination can also be triggered by many non-nutrient agents, including cationic surfactants such as dodecylamine, a 1:1 complex of Ca2+ with pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA], a major spore small molecule), very high pressures, specific peptidoglycan fragments, and bryostatin, an activator of Ser/Thr protein kinases (17, 19, 20). For nutrient germinants in particular, spore germination is also potentiated by a prior sublethal heat treatment termed heat activation (17).
While normally the great majority of spores in populations germinate relatively rapidly in response to nutrient germinants, a small percentage of spores germinate extremely slowly. These spores that are refractory to nutrient germination have been termed superdormant spores and are a major concern for the food industry (8). Recently superdormant spores of three Bacillus species have been isolated by repeated germination of spore populations with specific nutrient germinants and isolation of remaining dormant spores (5, 6). These superdormant spores germinate extremely poorly with the nutrient germinants used in superdormant spore isolation, as well as with other nutrient germinants. All of the specific defects leading to spore superdormancy are not known, although an increased level of receptors for specific nutrient germinants decreases levels of superdormant spores obtained with the nutrients that are ligands for these receptors (5). Superdormant spores also have significantly higher temperature optima for heat activation of nutrient germination than the spore population as a whole (7).
In contrast to the poor germination of superdormant spores with nutrient germinants, superdormant spores germinate normally with dodecylamine and Ca-DPA (5, 6). This is consistent with possible roles of nutrient germinant receptor levels and/or heat activation temperature optima in affecting spore superdormancy, since neither dodecylamine nor Ca-DPA triggers Bacillus spore germination through nutrient germinant receptors, and germination with these agents is also not stimulated by heat activation (11, 15, 17). However, the effects of high pressures, peptidoglycan fragments, and bryostatin, all of which almost certainly trigger spore germination by mechanisms at least somewhat different than triggering of germination by nutrients, dodecylamine, and Ca-DPA (2, 3, 11, 15, 20, 22, 23), have not been tested for their effects on superdormant spores. Consequently, we have compared the germination of dormant and superdormant spores of two Bacillus species by high-pressures, peptidoglycan fragments, and bryostatin.
The spores used in this work were from Bacillus subtilis PS533 (16), a derivative of strain 168 that also carries plasmid pUB110, providing resistance to kanamycin (10 μg/ml), and Bacillus cereus T (originally obtained from H. O. Halvorson). Spores of these strains were prepared and purified as described previously (6, 10, 12). Superdormant spores of B. subtilis were prepared by germination following heat activation at 75°C for 30 min by two germination treatments at 37°C with 10 mM l-valine for 2 h, followed by isolation of remaining dormant spores, all as described previously (5, 10, 12). These superdormant spores germinated extremely poorly with 10 mM valine at 37°C, giving ≤10% germination in 2 h at 37°C, while the initial spore population exhibited >95% germination under the same conditions (data not shown). Superdormant B. cereus spores were isolated similarly, although heat activation was at 65°C for 30 min and the germinant was 5 mM inosine as described previously (6). These superdormant B. cereus spores exhibited <5% germination with inosine in 2 h at 37°C compared to the >95% germination of the initial dormant spores under the same conditions (data not shown).
High-pressure germination of dormant and superdormant spores.
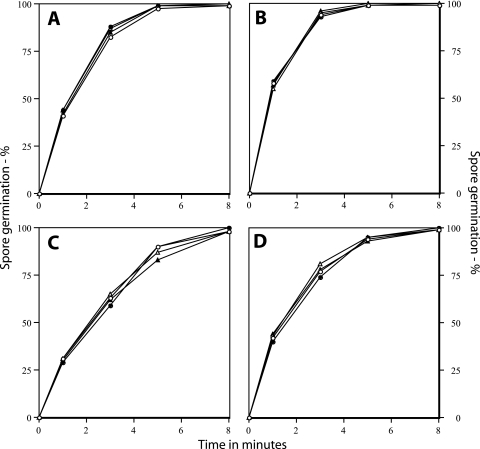
For high-pressure treatment, 1.5 ml spores at an optical density at 600 nm (OD600) of 1 in 50 mM Tris-HCl buffer (pH 7.4) that were or were not heat activated as described above shortly before pressure treatment were added to multiple sterile pouches (VWR International, Mississagua, ON, Canada), and the pouches were heat sealed after expelling as much air as possible. Pressure treatment was carried out at either 37°C and 150 MPa of pressure or 50°C and 500 MPa of pressure in a PT-1 Research System pressure unit (Avure Technologies, Kent, WA) using water as the hydrostatic medium (21). Pouches were removed after various times of pressure treatment as well as after no pressure treatment and opened, and samples were frozen prior to analyses. Control experiments showed that neither the incubation temperatures used nor the freezing had effects on either spore viability or germination (data not shown). To assess the germination of the pressure-treated spore samples, the spores recovered from pouches were centrifuged in a microcentrifuge, the pellet resuspended in 25 μl H2O, and 300 to 400 spores were examined by phase-contrast microscopy to determine the percentage of spores that had gone through germination. The results of this experiment were clear: dormant and superdormant spores of either B. cereus or B. subtilis exhibited almost identical rates of germination with a pressure of 150 MPa, and this was also the case when the pressure treatment was at 500 MPa (Fig. 1A to D). In addition, prior heat activation had no notable effect on the rates of spore germination with pressures of either 150 or 500 MPa.
FIG. 1.
(A to D) Pressure germination of dormant and superdormant B. cereus (A and B) and B. subtilis (C and D) spores. Dormant spores of B. cereus and B. subtilis were prepared and purified, and superdormant spores were isolated after two germination treatments with l-valine (B. subtilis) or inosine (B. cereus) as described in the text. Spores at an OD600 of 1 in 50 mM Tris-HCl (pH 7.4), either without or with heat activation as described in the text, were treated with either 150 MPa of pressure at 37°C or 500 MPa of pressure at 50°C. At various times of pressure treatment, 1-ml samples were taken, rapidly frozen and subsequently thawed, and centrifuged in a microcentrifuge; pellet fractions were suspended in 25 μl water; and spores were examined by phase-contrast microscopy to determine the percentage of spore germination. ○, dormant spores, no heat activation; •, superdormant spores, no heat activation; ▵, dormant spores, heat activated; and ▴, superdormant spores, heat activated.
Germination of dormant and superdormant spores with peptidoglycan fragments and bryostatin.
Dormant and superdormant B. subtilis spores were assayed for germination with peptidoglycan fragments, either purified fragments or those released from growing B. subtilis cells, and bryostatin as previously described (20). Spores at a concentration of 107 per ml were incubated for 60 min at 37°C with either nongerminating buffer, purified B. subtilis peptidoglycan fragments (50 μM), cell-free supernatant fluid obtained from a culture of B. subtilis grown to an A600 of ∼1.0 in Tris-Spizizen-salts (TSS) medium and containing released peptidoglycan fragments (20), or bryostatin (10 μM) in 50-μl reaction mixtures. The extent of germination was determined by measuring CFU following heat treatment of the spores for 20 min at 80°C and growth overnight on plates at 37°C. Superdormant spores and dormant spores germinated similarly in response to purified peptidoglycan fragments and bryostatin. Why germination with these molecules is not complete is not known, although the extent of germination is not different between the two types of spores. The dormant and superdormant spores do germinate to different extents with cell-free supernatant fluid from a B. subtilis culture (Table 1), but it is not clear if this difference is a reflection of differences in the manner in which the two types of spores were isolated, or some intrinsic difference in these spores' properties.
TABLE 1.
Germination of superdormant spores with peptidoglycan fragments, cell-free supernatant fluid, and bryostatina
| Incubation agent | % Spore germination (mean± SE) |
|
|---|---|---|
| Dormant spores | Superdormant spores | |
| Nongerminating buffer | 0 | 0 |
| B. subtilis peptidoglycan fragments (50 μM) | 28 ± 5 | 25 ± 3 |
| B. subtilis cell-free supernatant fluid | 55 ± 2 | 32 ± 6 |
| Bryostatin (10 μM) | 42 ± 2 | 35 ± 3 |
Dormant and superdormant spores were incubated with the indicated agents for 60 min at 37°C prior to heat inactivation of germinated spores as described in the text and plating on Luria broth agar plates (20). The percentage of spore germination was calculated based on CFU obtained using nongerminating buffer as a negative control (0% germination). The data are from three different experiments, and averages are from multiple experiments with the same spore preparation.
Conclusions.
The results in this study allow a number of new conclusions about superdormant spores of Bacillus species. First, superdormant B. subtilis spores germinate relatively normally with both bryostatin and peptidoglycan fragments. These two agents appear to trigger B. subtilis spore germination by activating a eukaryotic-like membrane-bound Ser/Thr protein kinase that contains an extracellular domain capable of binding peptidoglycan (20). As noted above, the only mediators of spore superdormancy identified to date are levels of nutrient germinant receptors and the temperature of heat activation (5, 7), and neither heat activation nor nutrient germinant receptors are involved in spore germination triggered by bryostatin or peptidoglycan fragments (20; unpublished observations).
The second major conclusion is that superdormant spores of at least two Bacillus species also germinate normally with a pressure of 500 MPa. Again this is not completely surprising, since spore germination triggered by this level of pressure does not involve the nutrient germinant receptors and does not appear to require heat activation prior to spore germination (2, 22, 23), as was confirmed in the current work for dormant and superdormant spores of B. cereus and B. subtilis.
The third major conclusion is that superdormant spores of B. cereus and B. subtilis also germinate normally with a pressure of 150 MPa. This was somewhat surprising for two reasons. First, spore germination triggered by this level of pressure depends on the nutrient germinant receptors, and at least large increases (20- to 200-fold) in levels of nutrient germinant receptors result in significant increases in rates of spore germination with 150 MPa of pressure (2, 22, 23). Second, there are at least some reports that spore germination by this level of pressure is increased by prior heat activation (4, 9). However, there are no data on effects of small decreases in nutrient germinant receptor levels on germination by 150 MPa of pressure, and perhaps these effects on pressure germination are minimal compared to effects on nutrient germination, especially if there is significant cooperativity between nutrient germinant receptors in nutrient germination (as has been suggested in reference 1), but not in pressure germination (2). It is also by no means proven that superdormant spores have decreased levels of nutrient germinant receptors, although this has been proposed to be the case (7). With regard to our observation of the absence of an effect of heat activation on spore germination with 150 MPa of pressure, a number studies have also found that the great majority of individual spores in populations of B. subtilis and B. cereus germinated well with pressures of ∼150 MPa, even without heat activation (2, 14, 22, 23). In contrast, the extent of nutrient germination of the B. cereus and B. subtilis spore preparations used in the current work was increased from 2.5-fold (B. subtilis with l-valine) to fourfold (B. cereus with inosine) by heat activation for 30 min at 65°C (B. cereus) or 75°C (B. subtilis) (data not shown).
The result that spores of two Bacillus species that were superdormant for nutrient germination still germinated normally under high-pressure conditions may have important implications for the well-documented phenomenon that pressure germination of spores of Bacillus species is often not complete. Indeed, this latter observation is a major reason that high-pressure treatment has not achieved wider utilization in the food industry, as a small percentage of spores often fail to germinate readily upon high-pressure treatment and are thus very difficult to kill. The factors that cause some spores in populations not to germinate with high pressure are not clear. However, the current work suggests that these factors are not identical to those responsible for spore superdormancy for nutrient germination. Consequently, it might be fruitful to isolate and characterize spores that are superdormant with regard to germination by pressures of both 150 and 500 MPa.
Acknowledgments
This work was supported by grants from the National Institutes of Health, GM19698 (P.S.) and GM81368 (J.D.), and a Multidisciplinary University Research Initiative from the Department of Defense (P.S.).
Footnotes
Published ahead of print on 4 January 2010.
REFERENCES
- 1.Atluri, S., K. Ragkousi, D. E. Cortezzo, and P. Setlow. 2006. Co-operativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this co-operativity by alterations in the GerB receptor. J. Bacteriol. 188:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, E. P., K. Koziol-Dube, D. Guan, J. Wei, B. Setlow, D. E. Cortezzo, D. G. Hoover, and P. Setlow. 2005. Factors influencing the germination of Bacillus subtilis spores via the activation of nutrient receptors by high pressure. Appl. Environ. Microbiol. 71:5879-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, E. P., J. Wei, S. Atluri, D. E. Cortezzo, K. Koziol-Dube, D. G. Hoover, and P. Setlow. 2007. Analysis of factors influencing the rate of germination of spores of Bacillus subtilis by very high pressure. J. Appl. Microbiol. 102:65-76. [DOI] [PubMed] [Google Scholar]
- 4.Clouston, J. G., and P. A. Wills. 1969. Initiation of germination and inactivation of Bacillus pumilus spores by hydrostatic pressure. J. Bacteriol. 97:684-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh, S., and P. Setlow. 2009. Isolation and characterization of superdormant spores of Bacillus species. J. Bacteriol. 191:1787-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh, S., and P. Setlow. 2010. The preparation, germination properties and stability of superdormant spores of Bacillus cereus. J. Appl. Microbiol. 108:582-590. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh, S., P. Zhang, Y.-Q. Li, and P. Setlow. 2009. Superdormant spores of Bacillus species have elevated wet-heat resistance and temperature requirements for heat activation. J. Bacteriol. 191:5584-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould, G. W. 1970. Germination and the problem of dormancy. J. Appl. Bacteriol. 33:34-49. [DOI] [PubMed] [Google Scholar]
- 9.Gould, G. W., and A. J. H. Sale. 1970. Initiation of germination of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:335-346. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
- 11.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piggot, P. J., and D. W. Hilbert. 2004. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 7:579-586. [DOI] [PubMed] [Google Scholar]
- 14.Raso, J., M. Marcela Gongora-Nieto, G. V. Barbosa-Canovas, and B. G. Swanson. 1998. Influence of several environmental factors on the initiation of germination and inactivation of Bacillus cereus by high hydrostatic pressure. Int. J. Food Microbiol. 44:125-132. [DOI] [PubMed] [Google Scholar]
- 15.Setlow, B., A. E. Cowan, and P. Setlow. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95:637-648. [DOI] [PubMed] [Google Scholar]
- 16.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 18.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Appl. Microbiol. 101:514-525. [DOI] [PubMed] [Google Scholar]
- 19.Setlow, P., and E. A. Johnson. 2007. Spores and their significance, p. 35-67. In M. P. Doyle and L. R. Beuchat (ed.), Food microbiology: fundamentals and frontiers, 3rd ed. ASM Press, Washington, DC.
- 20.Shah, I. M., M. H. Laaberki, D. L. Popham, and J. Dworkin. 2008. A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell 135:486-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei, J., P. Setlow, and D. G. Hoover. 2009. Effects of moderately high pressure plus heat on the germination and inactivation of Bacillus cereus spores lacking proteins involved in germination. Lett. Appl. Microbiol. 49:646-651. [DOI] [PubMed] [Google Scholar]
- 22.Wuytack, E. Y., S. Boven, and C. W. Michiels. 1998. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl. Environ. Microbiol. 64:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wuytack, E. Y., J. Soons, F. Poschet, and C. W. Michiels. 2000. Comparative study of pressure- and nutrient-induced germination of Bacillus subtilis spores. Appl. Environ. Microbiol. 66:257-261. [DOI] [PMC free article] [PubMed] [Google Scholar]