Abstract
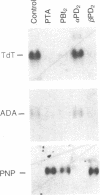
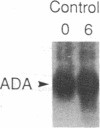
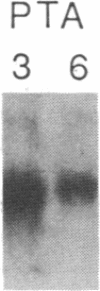
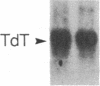
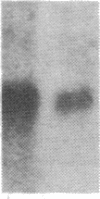
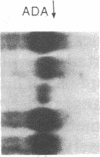
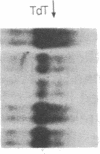
Incubation of human thymocytes in the presence of phorbol esters caused a reversible decrease in the mRNAs encoding terminal deoxynucleotidyltransferase (TdT; EC 2.7.7.31) and adenosine deaminase (ADA; EC 3.5.4.4) and an increase in the mRNA encoding purine nucleoside phosphorylase (PNP; EC 2.4.2.1). The effect of phorbol esters on TdT and ADA mRNA levels can be attributed to an apparent decrease in the stability of the mRNAs. The changes in ADA, TdT, and PNP mRNAs closely simulate changes in the activities of these enzymes that occur during T-cell differentiation in vivo, suggesting a role for protein kinase C activation in the regulation of the expression of these genes during intrathymic T-cell differentiation. A role for these purine degradation enzymes in the regulation of intracellular pools of the deoxynucleotide substrates of TdT is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Harkness R. A. Adenosine deaminase activity in thymus and other human tissues. Clin Exp Immunol. 1976 Dec;26(3):647–649. [PMC free article] [PubMed] [Google Scholar]
- Adrian G. S., Wiginton D. A., Hutton J. J. Structure of adenosine deaminase mRNAs from normal and adenosine deaminase-deficient human cell lines. Mol Cell Biol. 1984 Sep;4(9):1712–1717. doi: 10.1128/mcb.4.9.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton R., Martiniuk F., Hirschhorn R., Goldschneider I. Inverse relationship between adenosine deaminase and purine nucleoside phosphorylase in rat lymphocyte populations. Cell Immunol. 1980 Jan;49(1):208–214. doi: 10.1016/0008-8749(80)90071-4. [DOI] [PubMed] [Google Scholar]
- Belmont J. W., Henkel-Tigges J., Chang S. M., Wager-Smith K., Kellems R. E., Dick J. E., Magli M. C., Phillips R. A., Bernstein A., Caskey C. T. Expression of human adenosine deaminase in murine haematopoietic progenitor cells following retroviral transfer. Nature. 1986 Jul 24;322(6077):385–387. doi: 10.1038/322385a0. [DOI] [PubMed] [Google Scholar]
- Bevan M. J. In a radiation chimaera, host H-2 antigens determine immune responsiveness of donor cytotoxic cells. Nature. 1977 Sep 29;269(5627):417–418. doi: 10.1038/269417a0. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Molecular biology of terminal transferase. CRC Crit Rev Biochem. 1986;21(1):27–52. doi: 10.3109/10409238609113608. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen A., Barankiewicz J., Lederman H. M., Gelfand E. W. Purine and pyrimidine metabolism in human T lymphocytes. Regulation of deoxyribonucleotide metabolism. J Biol Chem. 1983 Oct 25;258(20):12334–12340. [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Friedman R. L. Expression of human adenosine deaminase using a transmissable murine retrovirus vector system. Proc Natl Acad Sci U S A. 1985 Feb;82(3):703–707. doi: 10.1073/pnas.82.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblett E. R., Ammann A. J., Wara D. W., Sandman R., Diamond L. K. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975 May 3;1(7914):1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Goddard J. M., Caput D., Williams S. R., Martin D. W., Jr Cloning of human purine-nucleoside phosphorylase cDNA sequences by complementation in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4281–4285. doi: 10.1073/pnas.80.14.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire K. E., Goldschneider I., Barton R. W., Bollum F. J. Ontogeny of terminal deoxynucleotidyl transferase-positive cells in lymphohemopoietic tissues of rat and mouse. J Immunol. 1979 Sep;123(3):1347–1352. [PubMed] [Google Scholar]
- Landau N. R., Schatz D. G., Rosa M., Baltimore D. Increased frequency of N-region insertion in a murine pre-B-cell line infected with a terminal deoxynucleotidyl transferase retroviral expression vector. Mol Cell Biol. 1987 Sep;7(9):3237–3243. doi: 10.1128/mcb.7.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Martinez-Valdez H., Doherty P. J., Thompson E., Benedict S. H., Gelfand E. W., Cohen A. Antagonistic effects of calcium ionophores and phorbol esters on T cell receptor mRNA levels in human thymocytes. J Immunol. 1988 Jan 15;140(2):361–366. [PubMed] [Google Scholar]
- Peterson R. C., Cheung L. C., Mattaliano R. J., Chang L. M., Bollum F. J. Molecular cloning of human terminal deoxynucleotidyltransferase. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4363–4367. doi: 10.1073/pnas.81.14.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Todaro G. J. Specific high affinity cell membrane receptors for biologically active phorbol and ingenol esters. Nature. 1980 Dec 4;288(5790):451–455. doi: 10.1038/288451a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyonaga B., Mak T. W. Genes of the T-cell antigen receptor in normal and malignant T cells. Annu Rev Immunol. 1987;5:585–620. doi: 10.1146/annurev.iy.05.040187.003101. [DOI] [PubMed] [Google Scholar]
- Tritsch G. L., Minowada J. Differences in purine metabolizing enzyme activities in human leukemia T-cell, B-cell and null cell lines. J Natl Cancer Inst. 1978 Jun;60(6):1301–1304. doi: 10.1093/jnci/60.6.1301. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Callahan G. N., Althage A., Cooper S., Klein P. A., Klein J. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978 Mar 1;147(3):882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]