Abstract
Previously, we showed that laminin-binding to the dystrophin glycoprotein complex (DGC) of skeletal muscle causes a heterotrimeric G-protein, (Gαβγ) to bind, changing the activation state of the Gsα subunit. Others have shown that laminin-binding to the DGC also leads to Akt activation. Gβγ, released when Gsα is activated, is known to bind phosphatidylinositol 3-kinase (PI3K), which activates Akt in other cells. Here, we investigate whether muscle Akt activation results from Gβγ, using immunoprecipitation and immunoblotting, and purified Gβγ. In the presence of laminin, PI3K-binding to the DGC increases and Akt becomes phosphorylated and activated (pAkt), and glycogen synthase kinase is phosphorylated. Antibodies, which specifically block laminin-binding to α-dystroglycan, prevent PI3K-binding to the DGC. Purified bovine brain Gβγ also caused PI3K and Akt activation. These results show that DGC-Gβγ is binding PI3K and activating pAkt in a laminin-dependent manner. Mdx mice, which have greatly diminished amounts of DGC proteins, display elevated pAkt signaling and increased expression of integrin β1 compared to normal muscle. This integrin binds laminin, Gβγ, and PI3K. Collectively, these suggest that PI3K is an important target for the Gβγ, which normally binds to DGC syntrophin, and activates PI3K/Akt signaling. Disruption of the DGC in mdx mouse is causing dis-regulation of the laminin-DGC-Gβγ-PI3K-Akt signaling and is likely to be important to the pathogenesis of muscular dystrophy. Up-regulating integrin β1 expression and activating the PI3K/Akt pathway in muscular dystrophy may partially compensate for the loss of the DGC. The results suggest new therapeutic approaches to muscle disease.
Keywords: Duchenne muscular dystrophy (DMD), dystrophin–glycoprotein complex (DGC), phosphoinositide-3-kinase (PI3K), protein kinase B (Akt/PKB), laminin, integrin β1
Introduction
The association of the extracellular matrix with cell receptor proteins is central to maintaining muscle function and integrity. Defects in the protein complexes that mediate this linkage underlie the pathology in a variety of muscle diseases (Kanagawa and Toda, 2006; Lapidos et al., 2004; Rybakova et al., 2006). Several studies suggest that the structural linkage between the muscle extracellular matrix with receptors and receptor interactions with the cytoskeleton is crucial to prevent the progression of muscular dystrophy (Glass, 2005; Lansman and Franco-Obregon, 2006).
Cell-matrix contact is predominantly maintained through the interaction of muscle cell transmembrane receptors with laminin and other matrix proteins. The laminins, a large family of matrix proteins, are known to be multifunctional, performing key roles in development, differentiation, and migration through their ability to interact with cells via cell surface receptors (Gullberg et al., 1999). All laminin isoforms detected in skeletal muscle are recognized by α3β1, α6β1, and α7β1 integrins (Fujiwara et al., 2001; Kikkawa et al., 2000). Only two laminin receptor systems are present throughout the sarcolemma of mature skeletal muscle, namely the dystrophin glycoprotein complex (DGC) and α7β1 integrin (Mayer, 2003). Both have critical roles in the maintenance of muscle integrity (Guo et al., 2006).
The DGC provides a linkage between the cytoskeleton and the extracellular matrix. The loss-of-function mutations in DGC proteins were identified as the cause of DMD and other muscular dystrophies (Brown et al., 1999; Langenbach and Rando, 2002; Rooney et al., 2006; Watchko et al., 2002). Dystophin's N-terminal domain binds to F-actin while its C-terminal domain binds to β-dystroglycan, which in turn interacts with the laminin-binding subunit α-dystroglycan (αDG) (Campbell, 1995). The DGC also plays a role in cell signaling (Glass, 2005; Matsumura et al., 1997; Oak and Jarrett, 2003; Zhou et al., 2005; Zhou et al., 2006). Thus, αDG may be part of a signaling pathway for the maturation and maintenance of skeletal myofibres (Brown et al., 1999; Michele and Campbell, 2003).
Integrins are heterodimeric laminin receptors that are transmembrane, link to the cytoskeleton and mediate cell-cell and cell-matrix interactions (Hynes, 2002; Rooney et al., 2006). Eighteen α and eight β chains have been identified (Gullberg et al., 1999). Among these, the β1 integrin family forms the largest group of receptors for extracellular matrix proteins (Mayer, 2003) and all skeletal muscle integrins contain β1. Recent studies using function-blocking antibodies have revealed the β1 associated integrins to be important in mediating cell adhesion and differentiation (Ekstrom et al., 2003; Farias et al., 2005; Stawowy et al., 2005; Tian et al., 2002; Urbich et al., 2000). Integrins provide a similar link between the cytoskeleton and the extracellular matrix as does the DGC (Sotgia et al., 2003). Previous papers suggest that the DGC and α7β1 integrin are independently controlled laminin receptors. Increased expression of the α7β1 integrin has been observed in DMD patients and mdx mice, and raised the possibility that integrin may functionally compensate for the loss of the DGC in disease (Burkin et al., 2001; Cohn et al., 1999; Hodges et al., 1997; Vachon et al., 1997). Integrins are capable of stabilizing muscle against destruction and ameliorating the dystrophin-deficient phenotype (Mayer, 2003).
Myogenic differentiation is a highly regulated process that is controlled by multiple factors, including extracellular matrix, transmembrane receptors, and intracellular signaling molecules. Therefore, one model of the pathogenesis, which leads to cell apoptosis or necrosis in the muscle dystrophies, is through interruption of the DGC’s interaction with the extracellular matrix resulting in a loss of cellular signaling (Langenbach and Rando, 2002).
The PI3K/Akt pathway is necessary to prevent apoptosis in a wide variety of cells. The PI3K/Akt pathway also has a role in the process of myotube differentiation (Ananthanarayanan et al., 2005; Glass, 2005; Lai et al., 2004; Peter and Crosbie, 2006). Activation of the PI3K/Akt signaling pathway is a key modulator of skeletal muscle hypertrophy both in vitro and in vivo (Takahashi et al., 2002). Gβγ can activate PI3K following binding of GTP or cholera toxin (CT) (Brock et al., 2003; Gilman, 1987; Schnitzler et al., 2007), and thereby initiate Gβγ mediated signal transduction pathways. Activation of PI3K and formation of its lipid products in vivo lead to activation of Akt and downstream inhibition of glycogen synthase kinase-3 (GSK-3), which are involved in cell survival and protein synthesis pathways (Baar and Esser, 1999; Pap and Cooper, 1998).
An understanding of the relevant signal transduction pathways and of the interactions between these pathways in the skeletal muscle cell will facilitate efforts to elucidate the pathogenesis of muscular dystrophies. To understand the role of the PI3K-Akt signaling in muscular dystrophies, we perform a detailed analysis of the protein interactions between the DGC and PI3K/Akt signaling in skeletal muscle, and then we investigated the role of Gβγ-dimers, laminin and its receptors in the activation of PI3K/Akt. We also investigated whether perturbation of these interactions could lead to the disruption of PI3K/Akt signaling in muscle cells. The results demonstrate the existence of a specific link between the laminin-DGC-Gβγ-PI3K-Akt signaling in skeletal muscle. Gβγ binding activates PI3K/Akt signaling in a laminin-dependent manner, and phosphorylation of Akt and GSK result from activation of PI3K. This reveals further details of how the PI3K/Akt pathway becomes activated upon binding of the DGC to the extracellular matrix. This laminin-DGC-Gβγ-PI3K-Akt signaling is likely to be important to the pathogenesis of muscular dystrophies. Up-regulating integrin β1 expression and its signaling may partially compensate for the loss of dystrophin in mdx mice.
MATERIALS AND METHODS
Materials
Rabbit antibodies against Gβ, PI3Kp110, Akt1, Akt 1/2, actin (C-2), Na+,K+-ATPase (H- 300) and integrin β1 and mouse monoclonal antibodies against PI3Kp85 and pAkt were from Santa Cruz Biotechnology Inc. (Califonia, USA). PI3K inhibitors LY294002, wortmannin and rabbit polyclonal antibodies against phospho-GSK3β were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). GTP-γS, GDP and cholera toxin were from Sigma Chemical, Inc. (St. Louis, Mo, USA). Mouse laminin-1 was from BD Biosciences, Inc. (Bedford, MA, USA). Mouse monoclonal αDG antibodies VIA4-1 and IIH6 were the generous gifts from Dr. Kevin P. Campbell (University of Iowa, USA). Affinity purified rabbit polyclonal antibody against β-dystroglycan was a generous gift from Dr. Tamara C. Petrucci (Laboratorio di Biologia Cellulare, Instituto Superiore di Sanita, Via le Regina Elena, Roma, Italia). Goat anti-mouse IgG (H+L)-horseradish peroxidase conjugate and goat anti-rabbit IgG (H+L)-horseradish peroxidase conjugate were from Bio-Rad Laboratories, Inc. (USA). Phosphatidylinositol (cat. # P0639) and phosphatidylinositol-3-phosphate (P0474) and histone (H4524) were from Sigma. γ-32P-ATP was from MP Biomedical. The mouse C2C12 cell line was obtained from the American Type Culture Collection (Rockville, MD). All other chemicals were of the highest purity available commercially.
Preparation of Microsomes from Rabbit Skeletal Muscle
Frozen rabbit skeletal muscle (3 g back muscle) was homogenized in 21 ml pyrophosphate buffer (20 mM sodium pyrophosphate, 20 mM phosphate, 1 mM MgCl2, 0.303 M sucrose, and 0.5 mM EDTA, pH 7.0) in the presence of a cocktail of protease inhibitors. The homogenate was centrifuged at 13000 g for 15 min at 4°C. The supernatant was then centrifuge for 30 min at 32500 g to pellet muscle microsomes. The pellets were suspended in 0.6 ml buffer A (50 mM Tris, pH 7.5, 100 mM NaCl).
Laminin Depletion
Laminins were depleted from skeletal muscle membrane using heparin-Sepharose as previously described (Oak and Jarrett, 2003). Rabbit muscle microsomes were incubated with either Sepharose 4B as a negative control (retaining endogenous laminins) or heparin-Sepharose (to deplete laminins) for 1 h at 4°C, with gentle mixing. After incubation, the resin was removed by slow speed centrifugation (2000 rpm) and the supernatant microsomes were used. Microsome were adjusted to equal protein concentration.
Bovine brain Gβγ was purified as described (Katada et al., 1986; Zhou et al., 2005).
PI3K assays
Immunoprecipitates with either PI3K-p110 or VIA4 antibodies were prepared and mixed with purified Gβγ and added to a PI3K assay previously described (Dogra et al., 2006). The assay uses phosphatidylinositol as substrate and γ-32P-ATP to follow the production of phosphatidylinositol-3-32PO4, separated by silica thin layer chromatography and detected by autoradiography. Phosphatidylinositol and phosphatidylinositol-3-PO4 were also run on the same plate and stained (Dittmer and Lester, 1964) to determine the Rf for substrate and product, respectively.
Akt assay
Immunoprecipitates with either Akt1/2 or VIA4 antibodies were prepared and mixed with purified Gβγ and added to an Akt assay previously described (Dogra et al., 2006) using histone H2B as substrate.
Immunoprecipitation
Rabbit muscle microsomes were isolated and incubated for 30 min in buffer A containing 1mM CaCl2, 1mM MgCl2, 1mM GTP-γS, 1mM ATP and with or without 3 µg laminin-1. After pre-clearing with protein G-Sepharose, 5 µg of specific antibodies was added and incubated for 1 h at 4°C with gentle mixing. The membranes were solubilized in 1% digitonin, and incubation was continued for another 1 h. The immunoprecipitates were collected by adding protein G-Sepharose, and incubating for 2 h at 4°C. After washing with buffer K (20 mM Hepes, pH 7.5, 10 mM MgCl2, 100 mM KCl), bound protein was eluted with SDS-PAGE sample buffer.
Western Blotting
Samples were separated by 12% SDS-PAGE, and transferred to nitrocellulose membranes at 4°C. Membranes were blocked with 5% nonfat milk and incubated with the indicated primary antibodies. Primary antibodies VIA4, PI3Kp85, pAkt, Akt, p-GSK-3β, integrin β1 and β-dystroglycan were used at a 1:1000 dilution. Immunoreactive bands were then detected with horseradish peroxidase-conjugated secondary antibodies. Anti-rabbit IgG was diluted 1: 10000, and anti-mouse IgG were used at a 1:5000 dilution. Membranes were then stripped by incubation at 50°C in 2% SDS, 100 mM 2-mercaptoethanol, 62.5 mM Tris, pH 6.8. The blots were then blocked again with milk and probed with additional primary antibodies, typically to provide a loading control. Blots were developed using enhanced chemiluminescence (ECL) as previously described (Oak and Jarrett, 2003) and exposure to X-ray film.
Cell Culture
Mouse C2C12 myoblasts were maintained in Dulbecco's modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere of 5% CO2 in air. Differentiation to myotubes was initiated at 80% confluence by replacing the growth medium with DMEM containing 1% FBS and maintaining the cells in this medium for 5 days. On the fifth day, myotubes were washed with PBS and harvest by adding 1 ml of trypsin-EDTA solution (0.05% trypsin, 0.02% EDTA in Hank’s Balanced Salt Solution, HBSS) for 5 min at 37°C. The cells were then immediately suspended in DMEM containing 10% FBS and removed from the plate. Myotubes (106/ml, 1 ml) were cultured in six well plates for 24 h in the absence or presence of increasing concentrations of LY294002 or wortmannin. At the termination of the inhibition experiment, cells on the plate were washed with PBS and used for the MTS assay of cell viability using the CellTiter 96® AQueous reagent and protocols (Promega, Madison WI) or were then lysed in 0.3 ml/well of modified RIPA lysis buffer (1% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% SDS in PBS) supplemented with protease inhibitors (1 µg/ml pepstatin A, 5 µg/ml aprotinin, 1 µg/ml leupeptin, 0.75 mM benzamidine, and 0.1 mM phenylmethylsulfonyl fluoride). Following incubation for 30 min at 4°C, cell lysates were clarified by centrifugation at 15000 g for 15 min, the supernatant mixed with SDS-PAGE sample buffer, and Western blotted.
In other experiments, myotubes were cultured in the presence of 10 µg/ml of VIA4 or IIH6 antibodies and microsomes prepared from the myotubes (Fig. 2C).
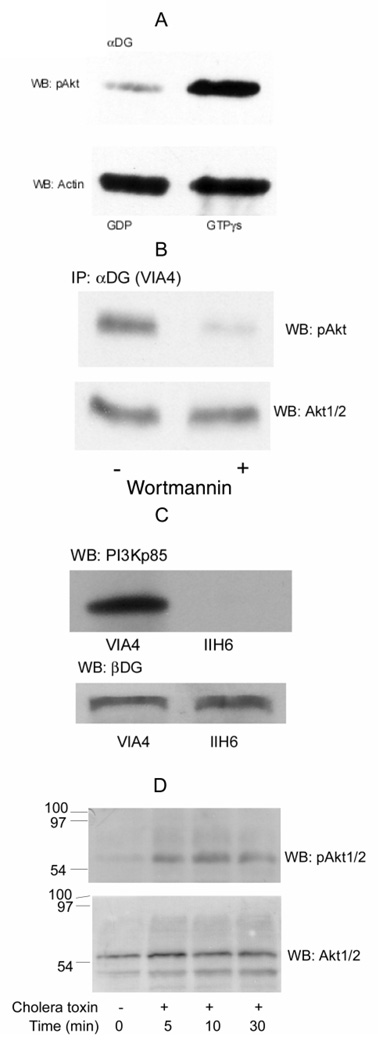
Fig. 2. Activation of PI3K/Akt by Gβγ-dimers in skeletal muscles.
A, Rabbit skeletal muscle microsomes were isolated and incubated for 1 h at 37° in buffer K containing 1 mM CaCl2 and 1 mM ATP and either 1 mM GTP-γ S o r 1 m M G D P, and then were immunoprecipitated with VIA4-1 α-dystroglycan (α-DG) antibody and Western blotted for pAkt. Membrane were then stripped and re-probed with an actin antibody, as indicated. pAkt was decreased in the presence of GDP compared to GTP-γS, but actin was not significantly altered and provides a loading control. B, C2C12 myotubes were pretreated with 200 nM wortmannin overnight at 37°C (+) or with buffer alone (−). The myotubes were counted and adjusted to equal cell counts, microsomes prepared and immunoprecipited with αDG antibody (VIA4). After SDS-PAGE and electroblotting, the blot was probed with antibody against pAkt. The same samples loaded on adjacent wells of the same gel was probed for total Akt1/2 as a loading control. C, the myotube, grown in either antibody, were used to prepare microsomes, which were then pretreated with the same antibody against αDG (VIA4 or IIH6). After immunoprecipitation with protein G-Sepharose, the proteins were separated on SDS-PAGE and transferred to nitrocellulose membranes. The membrane was probed with antibody against PI3Kp85 (upper panel). The same blot was stripped and re-probed with a βDG antibody (lower panel) to confirm equal loading. D, An equal number of C2C12 cells were cultured with 100 ng/ml cholera toxin for 0, 5, 10, 30, 60 min at 37°. Clarified cell lysates were adjusted to the same protein concentration (Bradford, 1976) and equal amounts separated on 12% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were probed with antibodies against pAkt (upper panel). The stripped blot from was reprobed for total Akt1/2 to confirm equal loading (lower panel).
Preparation of Laminin- Sepharose
CNBr-preactivated Sepharose (Pharmacia) was suspended in ice-cold 1 mM HCl for 15 min and washed with 1 mM HCl on a sintered glass filter. Laminin-1 (1 mg) in coupling buffer (0.1 M NaHCO3, 0.5 M NaCl, pH 8.3) was mixed with 0.5 ml of the activated Sepharose in a screwcap plastic tube on a wheel rotator mixer overnight at 4°C. The laminin-Sepharose was washed with blocking buffer (0.1 M Tris-HCl, 0.5 M NaCl, pH 8.0), and mixing continued in this buffer overnight. The resin was stored in blocking buffer containing 10 mM NaN3 as a 1:1 slurry.
Pull-Down Assay
Laminin-Sepharose (100 µL of a 50% slurry) was washed with buffer K containing 1 mM CaCl2. Microsomes in buffer K containing 1 mM CaCl2, 1 mM GTP-γS and 1 mM ATP were added and incubated for 1 h at 4°C. The microsomes were solubilized by addition of digitonin to 1% and incubation continued for 1 h at 4°C. The samples were centrifuged for 1 min at 2000 rpm in a microcentrifuge. The resins were washed three times with buffer K. Bound protein was eluted with 60 µL of twice concentrated SDS-PAGE sample buffer. Samples were heated for 5 min at 95°C and centrifuge for 5 min to remove the resins. Supernatants were applied to 12% SDS-PAGE and electroblotted to nitrocellulose. The blots were blocked with 5% nonfat milk in TTBS (20 mM Tris, 0.1 M NaCl, 0.2% Tween-20, pH 7.5). Blots were incubated with 1:1000 diluted antibodies against integrin β1 or β-dystroglycan. Goat anti-rabbit IgG (H+L)-horseradish peroxidase conjugate (1: 10000) was used as a secondary antibody. The blots were developed using ECL.
Animal Models
Mdx and wild-type (C57BL/6) breeder pairs were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in the light- and temperature-controlled quarters where they received food and water ad libitum. Mice were anesthetized with isofluorane for surgery and tissue removal follow by exsanguination. To examine mdx disease progression, four mice were analyzed at 4 weeks, and six adult mice were analyzed at 6 month of age. Skeletal muscles (gastrocnemius) were harvested and snap frozen in liquid nitrogen. All tissues were stored at −80°C until analysis. All procedures were carried in accordance with guidelines set by the UTSA Institutional Animal Care and Use Committee.
Cholera Toxin Experiment
C2C12 myoblasts (106/ml, 1 ml) in DMEM medium supplemented with 10% fetal bovine serum were placed in a 24 well plate and maintained at 37°C and 5% CO2 for 30 min in a humidified incubator. Cholera toxin was added to 100 ng/ml for 0, 5, 10, and 30 min. The cells were then washed with PBS, and dissolved in RIPA buffer as previously described. The clarified cell lysates were separated on 12% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were probed with antibodies against phosphorylated Akt followed by ECL. The membranes were then stripped as previously described and probed for total Akt.
Loading Controls
Several specific loading controls are specified in the figure legends. All blots were stained for protein with Ponceau Red (0.2% Ponceau Red, 3% trichloroacetic acid, 3% 5-sulfosalicylic acid), a reversible stain, prior to blocking with milk to observe whether loading was equal. Also blots were usually stripped after the first western blot, western blotting was repeated with a different primary antibody to detect an unrelated protein, typically β-actin, total Akt or β-dystroglycan, confirming equal loading. For each experiment, 2–3 different exposures of each film were taken. In most cases, a longer exposure time is shown in the figures for clarity but shorter exposure time films were used for the densitometry analysis reported in the text, since these are less prone to film saturation errors. In addition, each experiment was repeated at least 3 times.
Statistical
Unless otherwise stated, densitometry was performed on two independent experiments where the ECL or autoradiographic films were underexposed, to avoid problems with film saturation. Data are reported as the mean ± standard deviation. Statistical significance was assessed using Student’s t-test (1 tail with unequal sample variance).
RESULTS
Interactions between the DGC-Gβγ and the PI3K /Akt pathway
For most of the experiments here, we use antibodies from different species (e.g., mouse and rabbit) for immunoprecipitation and western blotting, which allows us to perform western blots without the interference of observing immunoglobulin heavy chain. This allows us to observe Akt (60 kDa.) clearly.
Previously, we had shown that heterotrimeric Gαβγ was bound by DGC syntrophin in a laminindependent manner (Zhou et al., 2005). To examine whether the DGC, Gβγ, PI3K (the p85 or p110 subunits) and Akt form a complex in skeletal muscle, four different immunoprecipitation experiments were performed, and then interactions were analyzed by western blot. The results show that regardless of whether antibodies against Akt (Fig. 1A), Gβ (Fig. 1B), PI3K (Fig. 1C), or DGC α-dystroglycan (Fig. 1D) are used for immunopreciptiation, the other components are detected by western blots, showing that all are associated with one another. These results confirm that the DGC is associated with several signaling molecules, including Gβγ, PI3K and Akt, which are co-localized at the skeletal muscle membrane. In Fig. 1A–D, an equivalent amount of the crude microsomes (without immunoprecipitation) is also shown. Essentially all of the microsomal pAkt immunopreciptitate and much of the α-dystroglycan, PI3K, and total Akt immunopreciptitate in this complex.
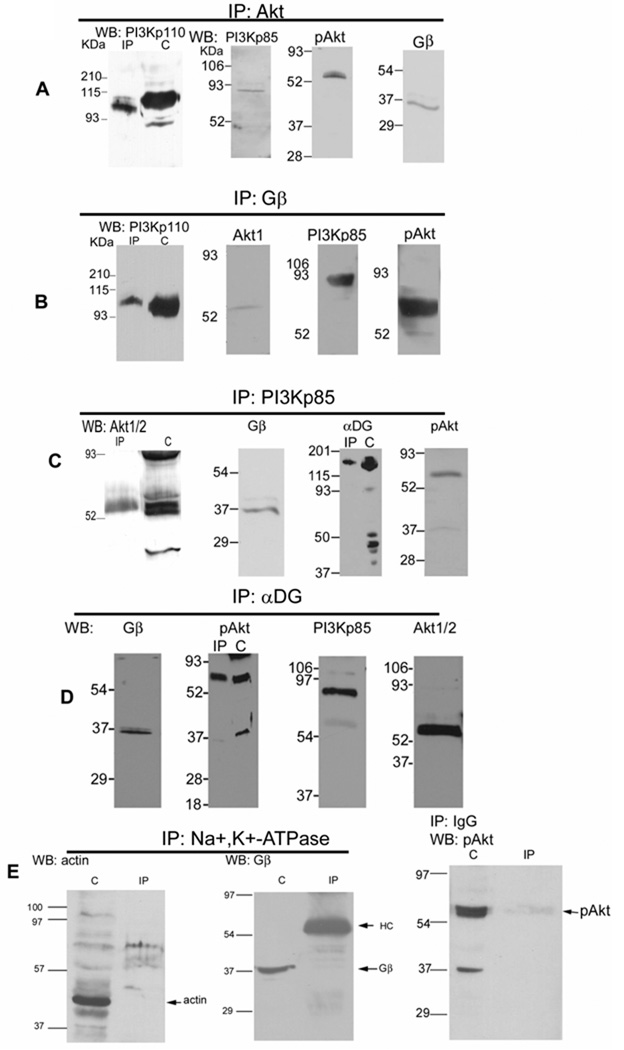
Fig. 1. DGC-Gβγ binds PI3K and Akt in rabbit skeletal muscle.
A, Rabbit muscle microsomes were isolated and the protein concentration (Bradford, 1976) adjusted so that equal amounts were used for all experiments. Microsomes were incubated with antibodies cross-reactive with both Akt 1 and 2 (Akt 1/2), detergent solubilized upon addition of protein G-Sepharose, electrophoresed, electroblotted, and Western blotted for PI3Kp85, pAkt 1/2/3, PI3Kp110, and Gβ. Where shown “C” denotes crude microsome prior to IP, added in equal concentration. IP: Immunoprecipitation, WB: Western blot. B, Microsomes were immunoprecipited with a Gβ antibody, and Western blotted for PI3Kp110, PI3Kp85, Akt 1 and pAkt 1/2/3. C, Microsomes were immunoprecipitated with a PI3Kp85 antibody, and Western blotted for Akt1/2, α-dystroglycan (αDG, VIA4 monoclonal antibody), Gβ, and pAkt1/2/3. D, Microsomes were immunoprecipited with an α-dystroglycan monoclonal antibody (VIA4), and Western blotted for Gβ, PI3Kp85, pAkt and Akt1/2. These show that DGC-Gβγ binds a complex containing PI3K and Akt, as well as the phosphorylated Akt. E, Microsomes (C) or detergent immunoprecipited with Na+/K+ ATPase or pre-immune IgG antibodies, were probed with the antibodies against actin, Gβ and pAkt.
As negative controls, similar experiments were also performed with pre-immune IgG or with the Na+, K+-ATPase antibody since Na+, K+-ATPase is also a membrane protein but with no known association with these cell signaling components. The results in Fig. 1E show that while the detected proteins are present in the crude microsomes, they are not immunoprecipitated.
The role of Gβγ and laminin in the activation of PI3K
Heterotrimeric G-proteins consist of a Gα-subunit and Gβγ-dimer. The Gα-subunit binds guanine nucleotides (GDP or GTP) and possesses an intrinsic low turnover number GTPase activity (Schwindinger and Robishaw, 2001). Gβγ always remains tightly associated as a heterodimer. The Gβγ-dimer does not dissociate from Gα under physiologic conditions except when the Gα-subunits are in the GTP-bound state. Both Gα and Gβγ initiate intracellular signaling through a variety of effector molecules (Maier et al., 2000), but in many cases it is the Gβγ rather than the Gα that transmits signals for proliferation and survival (Schwindinger and Robishaw, 2001). Here we will focus on the role of Gβγ in the activation of PI3K, an important step in G-protein coupled survival signaling. We investigated whether this Akt activation results from Gβγ using two experiments. In Fig. 2A, rabbit skeletal muscle microsomes were immunoprecipitated with an antibody against αDG (VIA4) in the presence of either 1 mM GTP-γS or 1 mM GDP. GDP should force the Gαβγ state and inhibit PI3K while the non-hydrolysable GTP-γS analogue, should have the opposite effect (Klein et al., 2000; Xie et al., 2000). PI3K activity was then monitored by down-stream Akt activation using the phosphorylated pAkt (Ser 473) antibody. The blot was then stripped and re-probed with an antibody to actin (known to be associated with the DGC) or total Akt 1/2 (data not shown), as loading controls. As expected interactions between αDG and pAkt were higher in the presence of GTP-γS compared to GDP, while actin (Fig. 2A was not significantly altered. Based upon densitometry, GTP-γS gave 4.3-fold higher pAkt activation while loading on the two blots assessed by β-actin differed by less than 4% (Fig. 2A). For other panels in the figure, C2C12 myotubes were used to measure the effect of inhibitors and a blocking antibody. Wortmannin (200 nM), an inhibitor of PI3K, prevents the pAkt activation, showing that this activation requires PI3K (Fig. 2B) while the total amount of Akt is unaltered Wortmannin (200 nM) resulted in 6.2-fold lower pAkt (6.2 ± 0.7, n=2), while total Akt differed by less than 3% (Fig. 2B).
To further probe whether laminin-binding to the DGC is required, an additional αDG antibody, IIH6 was included in Fig. 2C. The IIH6 antibody binds αDG and blocks its interaction with laminin while the VIA4 antibody, used in other experiments, does not (Ervasti and Campbell, 1993). When immunoprecipitation is performed with either antibody, βDG is found showing that both immunoprecipitate the DGC, but with the blocking antibody, there is no longer an association of the DGC with PI3K detected. By densitometry, there is 243-fold more PI3K with the non-blocking VIA4 antibody than there is with the blocking IIH6 antibody, while both antibodies precipitate nearly equal amounts of βDG, differing by less than 20%. These results strongly suggests that the laminin-DGC interaction is required for the DGC-Gβγ-PI3K-Akt signaling.
The heterotrimeric G-protein associated with the DGC is Gs (Zhou et al., 2005) and the GTPase activity of Gs is irreversibly inhibited by cholera toxin; thus, both GTP-γS and cholera toxin would increase the dissociation of Gsα from Gβγ. This results in more free Gβγ for activation of PI3K and Akt. As shown in Fig. 2D, incubation for 5–30 min with 100 ng/ml cholera toxin caused an increase in the level of phosphorylated pAkt compared to control (upper panel) while total Akt (lower panel) provides a loading control. By densitometry, at 10 min, pAkt increased 4.9-fold (± 0.4, n=2) relative to no cholera toxin. These results confirm the results with nucleotides that downstream Akt is activated via Gβγ. Interestingly, wortmannin and cholera toxin affect the activation of Akt to pAkt without altering its total amount or association with the DGC or in the C2C12 myotubes, respectively.
Laminin-binding increases PI3K/Akt signaling
Laminins are multifunctional, performing key roles in development, differentiation, and migration through their ability to interact with cells via cell surface receptors, including α-dystroglycan. In adult skeletal muscle, laminin-2 (α2β1γ1) is the major laminin present endogenously while laminin-1 (α1β1γ1) is known to have many of the same effects. Whether laminins affect G-protein binding and activates PI3K/Akt signaling via the DGC is not well understood but is strongly suggested by the results in Fig. 2C. Previously (Oak and Jarrett, 2003), we showed that laminins can be depleted from skeletal muscle microsomes and here we used laminin-depletion to examine the phosphorylation state of pAkt and the amount of total Akt bound (Fig 3A). When endogenous (endo) laminin is present, Akt is activated (pAkt). When laminin is depleted (−), less active pAkt is found, 50% (±11, n=2) less by densitometry, and activation is restored to 92% of the endogenous laminin amount by the addition of exogenous (exo) laminin-1. Thus, either the endogenous laminin-2 or exogenous laminin-1 have similar effects. The same membrane was stripped and detected for total Akt which is unaffected by these treatment (Fig. 3A), thus Akt is associated in all cases but only activated to pAkt when laminin is present. GDP, which prevents Gβγ release, prevents the laminin-dependent activation of Akt (Fig. 3B), showing this proceeds through a G-protein. Indeed, pAkt is so low, it is difficult to see in the figure while total Akt is clearly observed.
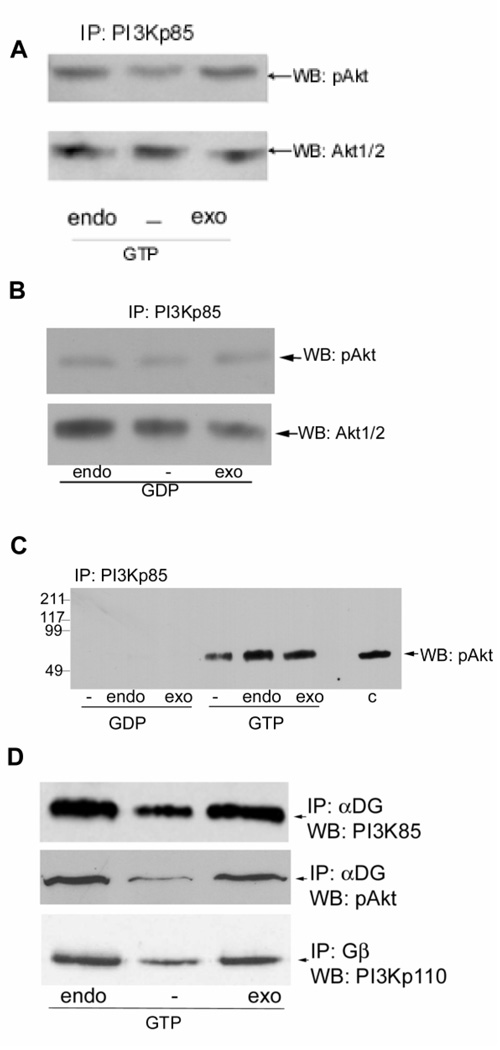
Fig. 3. Laminin binding alters PI3K/Akt signaling.
A and B, rabbit skeletal muscle microsomes were treated with Sepharose 4B (endo) or with heparin-Sepharose to deplete laminin (−) for 1 hour at 4°. The microsomes were then adjusted to equal protein concentration (Bradford, 1976) and were made 1 mM CaCl2, 10 mM MgCl2, 1 mM ATP, and 1 mM GTP-γS (panel A) or 1 mM GDP (panel B). To the laminin-depleted portions of the microsome 3 µg exogenous laminin-1 (exo) was added for 1 hour at 4°, with gentle mixing. The microsomes were immunoprecipitated for PI3Kp85α and Western blotted with antibody against phosphorylated Akt (pAkt). Membrane were then stripped and re-probed with antibody to total Akt to show total (phosphorylated and unphosphorylated) protein levels in equal loads. The level of Akt phosphorylation was reduced in the laminin-depleted immunoprecipitate, and laminin addition induces the phosphorylation of Akt. C, Microsomes were treated, incubated and immuno-precipitated as in panels A and B, except that the GTP-γS and GDP treated samples and an unequal amount of untreated microsomes (C) were applied to the same gel, and Western blotted for pAkt on the same blot. The data show that much greater amounts of pAkt are immunoprecipitated from microsomes incubated with GTP-γS than GDP and that laminin is also affecting the phosphorylation of pAkt.. D, Rabbit skeletal muscle microsomes were treated as in panel A. Immunoprecipitates with the α-dystroglycan (α-DG) VIA4 or Gβ antibody as indicated were probed with antibodies against PI3Kp85, PI3Kp110 or pAkt. Laminin binding increased αDG-Gβ-PI3K-pAkt interactions. Depletion of laminin resulted in decreased DGC-Gβγ-PI3K-Akt interactions, and exogenous laminin-1 increases PI3K binding and activation of pAkt.
To observe this GTP-γS/GDP effect more clearly, in an independent experiment samples were applied to the same gel, blotted, and pAkt detected by Western Blot (Fig. 3C). The results show pAkt is present in crude microsomes in an amount similar to that seen after GTP-γS treatment and PI3K-immunoprecipitation. Thus, most pAkt present in microsomes must be associated with PI3K in a complex. Furthermore, untreated microsomes behave as though they are in a GTP-rich environment, not surprising given the prevalence of GTP over GDP in muscle and essentially all tissues. GDP-treatment results in such low amounts of pAkt immunoprecipitating with PI3K that it is not observed at this exposure (although longer exposures do reveal a small amount). Again, laminin-depletion affects pAkt activation.
We also investigated the effects of laminin on the interactions of DGC-Gβγ-PI3K-pAkt in Fig. 3D. The data show that regardless of whether immunopreciptation is with DGC α-dystroglycan or Gβ, PI3K and pAkt co-precipitate and that these components are greater in the presence of either endogenous or exogenous laminin than in laminin-depleted microsomes. Since total Akt remains associated regardless of laminin, laminin must be required for its activation to pAkt but not its association with the complex. These findings strongly suggest that laminin binding can initiate PI3K/Akt cell signaling. DGC-Gβγ is binding PI3K and pAkt is activated in a laminin-dependent manner. Densitometry results confirm what is seen by visual inspection. In the upper panel of Fig. 3D, PI3Kp85 decreases by 71±31% upon depletion of laminin, while in the middle panel, pAkt decreases 68±33%, and in the lower panel, PI3Kp110 is decreased by 37±9% upon depletion of laminin.
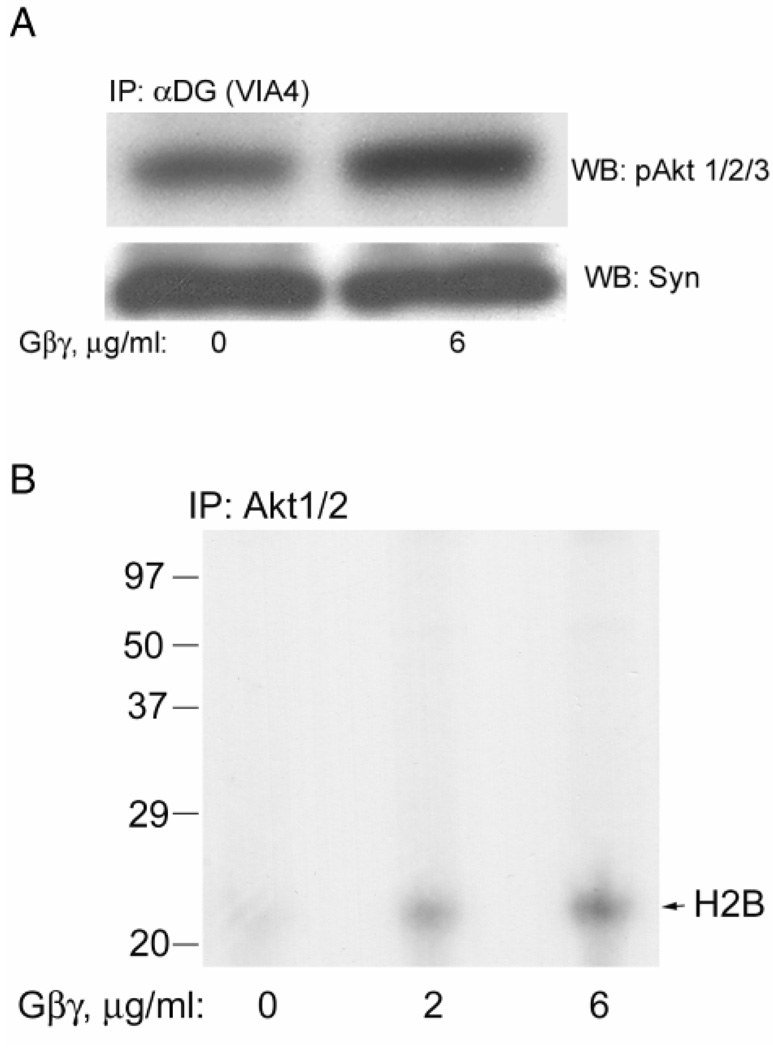
Purified bovine brain Gβγ activates PI3K and pAkt and these are associated with the DGC
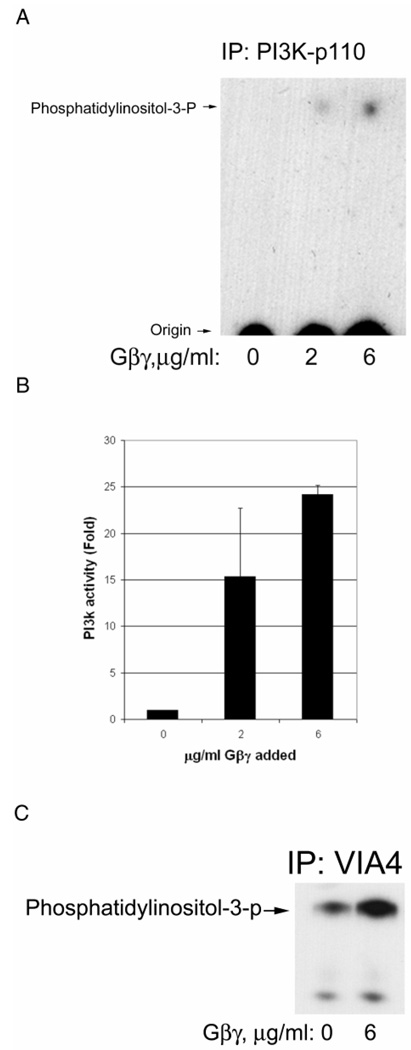
Fig. 4A shows that the addition of purified Gβγ increases the enzymatic activity of PI3K. Densitometry of independent experiments confirms that the activation is increased 24±1-fold when 6 µg/ml Gβγ is used and the activation is dose-dependent (Fig. 4B). The PI3K associated with the DGC α-dystroglycan was also increased by Gβγ as shown in Fig. 4C. In this latter figure, a longer exposure is shown and just the upper region of the chromatogram is shown to emphasize a second, slower migrating phospholipid also observed. Since PI3K can phosphorylate microsomal phosphatidylinositides at the 3-position of inositol, it is likely this additional spot is microsomal lipid which differs from the added substrate by either having different fatty acyl chains or additional phosphorylations on the inositol at other positions (e.g., 4,5). The latter possibility would be consistent with slower migration on the silica thin layer chromatogram.
Fig. 4. Purified Gβγ activates PI3K, which is associated with the DGC.
A, Rabbit skeletal muscle microsomes were incubated with GDP and the concentrations of purified Gβγ shown for 15 min. Then digitonin was added for solubilization followed by immunoprecipitation for PI3Kp110 catalytic subunit. The immunoprecipitate was added to a PI3K assay where phosphatidylinositol is added along with γ-32P-ATP as described in Methods. The reaction mixture is extracted for lipids and analyzed by silica thin layer chromatography and autoradiography. The position of product phosphatidylinositol-3-phosphate, obtained from a standard also applied to the plate, is shown to the left. B, The same as panel A except that densitometry was performed and the data normalized so that the absence of Gβγ was one-fold activation. The bars represent the mean of two independent experiments and the standard deviation is shown. C, The same as panel A except that VIA4 antibody was used to immunoprecipitate the DGC. Only the upper portion of the chromatogram is shown and at a longer exposure to emphasize an additional 3-phospholipid also detected.
Figure 5A shows that purified Gβγ also increases the DGC associated activated pAkt Syntrophin provides a loading control. Based on densitometry of two independent experiments, for Fig. 5A, Gβγ increased pAkt 4.3 ±2.8-fold while syntrophin was unchanged (1.05±0.03-fold). Presumably, this pAkt activation is a result of the increased PI3K activity shown in Fig. 4 since it is inhibited by the PI3K inhibitor wortmannin (Fig. 2B,and see below). That this pAkt is enzymatically activated is shown in Fig. 5B, where Gβγ activates the phosphorylation of histone H2B, a known substrate for pAkt (Dogra et al., 2006). Based on densitometry of two independent experiments, for Fig. 5B, Gβγ increased pAkt activity 3.3 ±1.9-fold and 4.8±3.2-fold at 2 and 6 µg/ml. respectively.
Fig. 5. Purified Gβγ activates Akt, which is associated with the DGC.
A, Microsomes were treated as described in Fig. 4A and immunoprecipitated with VIA4 antibody and Western blotted. The pAkt antibody shows that pAkt was activated. The same blot was stripped and probed with the syntrophin antibody to provide a loading control. B, Immunoprecipitates were prepared as in panel A except with the Akt1/2 antibody and added to an Akt assay using histone as substrate. The autoradiogram is shown and the expected position of histone H2B is shown to the right.
Thus, we conclude that the form of PI3K present in muscle and which associates with the DGC is activated by Gβγ. This is also consistent with the effect of cholera toxin, guanine nucleotides and α-dystroglycan blocking antibodies already shown (Fig. 2 and 3) and that this PI3K is resulting in pAkt activation.
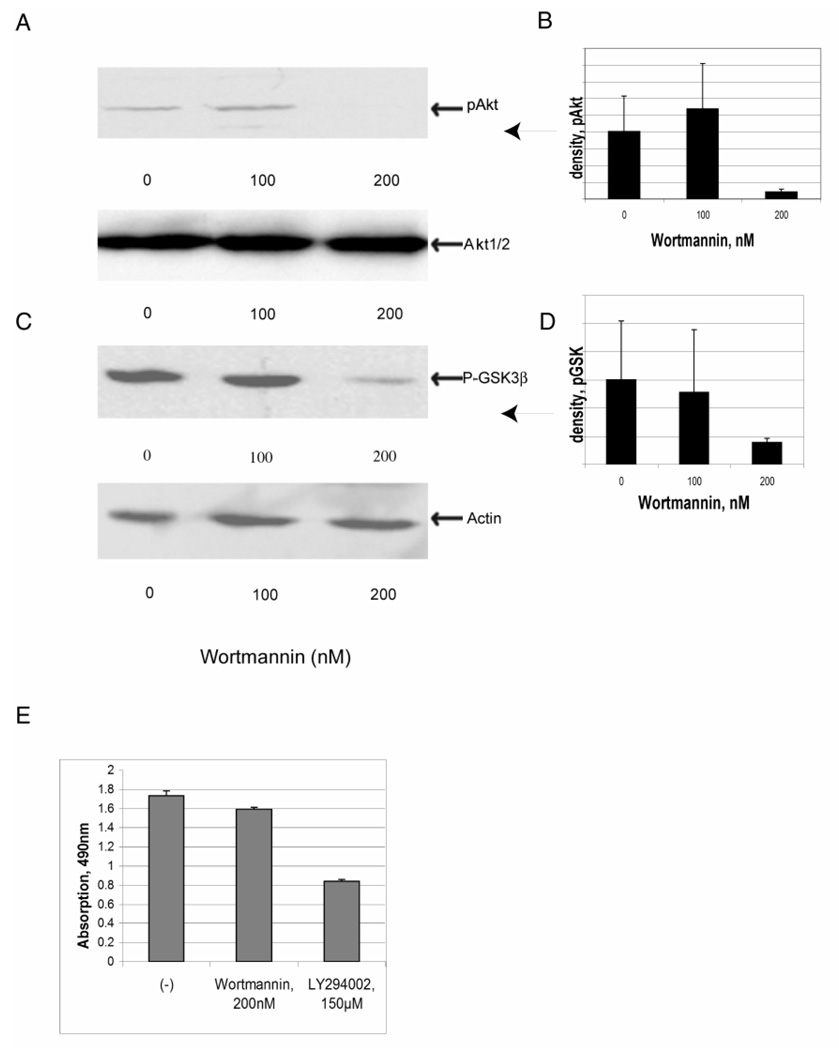
Effect of Wortmannin on PI3K activation
Activation of PI3K and formation of its lipid products in vivo lead to activation of pAkt, the downstream serine-threonine protein kinase involved in cell survival and protein synthesis pathways (Li et al., 2005). Whether this activation of Akt or downstream GSK is occurring through PI3K was next further investigated. Two pharmacological inhibitor of PI3K, LY294002 and Wortmannin, were added to cultured C2C12 myotubes. Cultured myotubes were incubated for 24 h with LY294002 at concentration from 25 µM to 150 µM (data not shown) or Wortmannin at concentration 100 nM or 200 nM (Fig. 6A–D) and the phosphorylation state of Akt (Fig. 6A and B) and GSK-3β (Fig. 6C and D) were examined in treated and untreated myotube cultures. pAkt and pGSK-3β phosphorylation were reduced by either PI3K inhibitor, and the inhibitory effects of Wortmannin were more pronounced than the effects of LY294002 over these concentrations and is shown in Fig. 6. Densitometry results are shown in Fig. 6B and D. The amounts of total Akt and actin were unchanged, providing loading controls. These results strongly suggest that the phosphorylation of pAkt or pGSK is mediated via PI3K.
Fig. 6. Effects of Wortmannin on PI3K activation in C2C12 myotube cultures.
Myotube cultures were maintained in 1% FBS DMEM medium for 6 days before addition of inhibitors. Myotubes were incubated in the absence or presence of different concentrations of Wortmannin as indicated in the figure. After 24 h, cells were lysed and subjected to Western blot assay. A, Western blots for pAkt are shown above with total Akt (on the same blot after stripping) below providing a loading control. B, Densitometry of the pAkt staining. C, Western blots for pGSK3β are shown above with actin shown below providing a loading control. D, Densitometry of the pGSK staining. E, The effect of Wortmannin and LY294002 on C2C12 myotube viability was measured using the MTS assay. The error bars throughout are standard deviation with n=2 for panels B and D, and n=3 for panel E.
One concern is the effect of such inhibitors on cell viability. Shown in Fig. 6E is a measure of cell viability for the two inhibitors, using the MTS assay. While Wortmannin at 200 nM has only minimal effect on viability and cannot account for the changes seen in Fig. 6A–D, LY294002 decreases the number of viable cells by over 50%. Thus, the Wortmannin results on cell signaling are not caused by an effect on cell viability while the results with LY294002 are more problematic and are not shown.
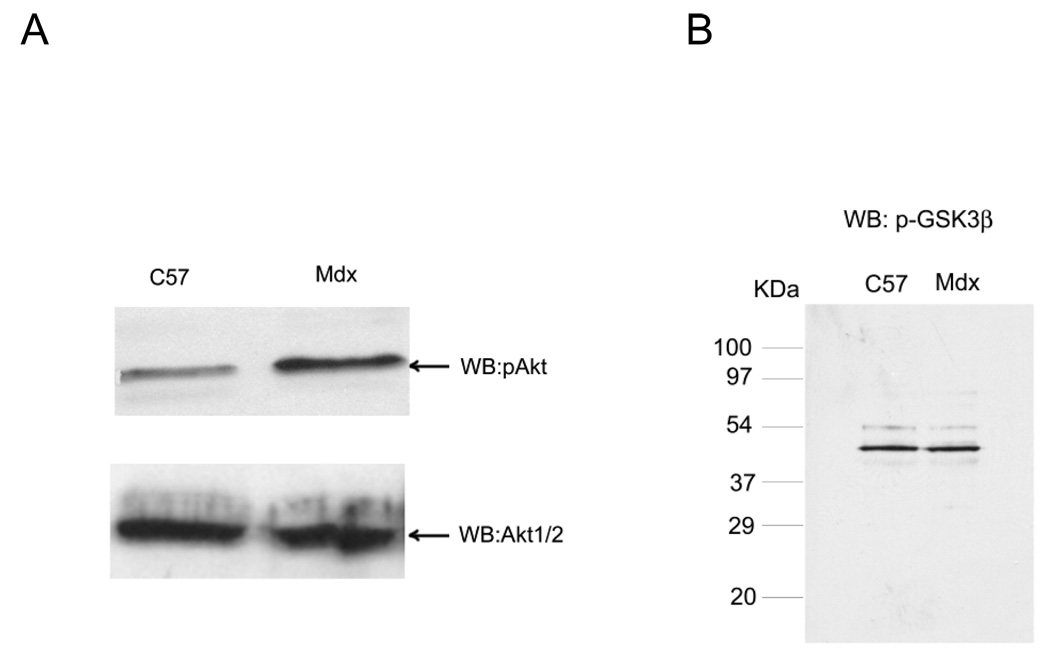
PI3K/Akt signaling is upregulated in skeletal muscle of mdx mice
The mdx mouse has a dystrophin mutation which makes it severely deficient in dystrophin and in most of the components of the DGC (Ohlendieck and Campbell, 1991). Others have previously reported that total Akt is elevated in mdx mouse at 12 weeks of age (Dogra et al., 2006). In order to investigate how the loss of the DGC impacts PI3K/Akt signaling in the mdx mouse model of Duchenne muscular dystrophy, we examined pAkt phosphorylation and total Akt levels in the gastrocnemius muscle from mdx mice and their normal control (C57bl/6) in Fig. 7. Mdx mice exhibit a significant increase (p < 0.05, n =2) in pAkt compared to age-matched normal mice, but total Akt (Akt 1 and 2) is not affected (Fig. 7A). By densitometry, mdx mouse at this age have 2.1±0.9-fold greater pAkt (two independent experiments) while total Akt is insignificantly decreased by 7%. Similar results were also obtained in 6 month and older adult mice for the gastrocnemius (data not shown). pGSK was not changed between normal and mdx mice (Fig. 7B). One possible explanation for why pAkt could become more active, while its downstream substrate GSK could remain constant would be if GSK is completely phosphorylated even at the lower activity of pAkt found in normal mice. Thus, mdx displays elevated pAkt but similar amounts of total Akt and pGSK compared to normal tissue.
Fig. 7. Activation (phosphorylation) of Akt is increased in dystrophin-deficient skeletal muscles.
A, Skeletal muscle microsomes from 4-week-old normal and mdx mice were prepared from the same wet weight of gastocnemius muscle, separated on 12% SDS-PAGE and transferred to nitrocellulose. The blots were probed with antibody against pAkt, and membranes were then stripped and reprobed with the Akt 1/2 antibody to detect total Akt levels. pAkt (Ser 473) was elevated in 4-week-old muscle from mdx mice compared to muscle isolate from age-matched normal mice (C57) while total Akt was not. B, Skeletal muscle (gastrocnemius) microsomes from adult (6 month old) normal and mdx mice were probed for the phosphorylated, p-GSK3β, as indicated. pGSK was not different between normal and mdx mice.
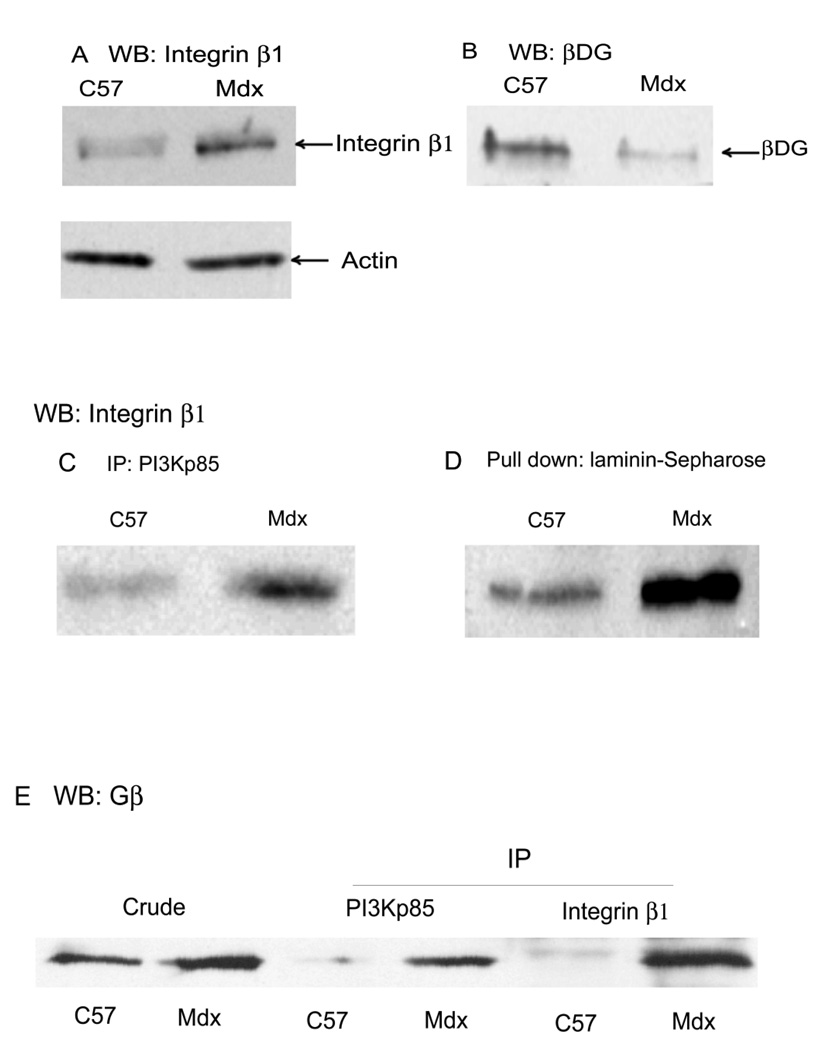
Enhanced expression of integrin β1 and Gβ in mdx skeletal muscle induces activation PI3K/Akt
Muscle fibers attach to basal lamina laminin using either the dystrophin glycoprotein complex or integrins (Burkin et al., 2001). Thus, both the integrin- and dystrophin-mediated transmembrane linkage contribute to the functional integrity of skeletal muscle (Guo et al., 2006), and defects in either linkage system results in muscular dystrophy (Burkin et al., 2001). Our data and those of others (Dogra et al., 2006) show the PI3K/Akt pathway was upregulated in mdx mice compared to normal tissue. This has led us to hypothesize that regulation of pAkt is defective in mdx mice and may proceed through an integrin-mediated process rather than a DGC-mediated one. To test this hypothesis we analyzed the expression of integrins and DGC β-dystroglycan in the gastrocnemius muscle. Since all muscle integrins contain the integrin β1 subunit, this was analysed to reflect total integrin expression. The results in Fig. 8 show that integrin β1 is expressed at a much higher level in mdx muscle relative to control (Fig. 8A) while β-dystroglycan was greatly reduced (Fig. 8B) as expected (Ohlendieck and Campbell, 1991). Based on densitometry, integrin increased 4.0±0.2-fold (n=2) while β-dystroglycan decreased 4.8-fold in mdx mouse relative to control (n=2). In Fig. 8C and D, we demonstrate a higher interaction between integrin β1 with both laminin and PI3K in mdx mouse microsomes relative to control in vitro. By densitometry, 7.2±5.1-fold (n=2) as much integrin was co-immunoprecipitated with PI3Kp85 in mdx mouse (Fig. 8C) while at least 3-fold greater amounts of integrin were pulled down by laminin-Sepharose in mdx mouse gastrocnemius (n=2). Blots were stripped and probed for actin to provide a control for equal loading (data not shown). In Fig. 8E, the results show that Gβ was increased in mdx muscle microsome compared with control (by densitometry, 2.2±0.1-fold, n=2) and interactions of PI3K-Gβ (11.1±1.3-fold, n=2) and integrin β1-Gβ (7.9±1.4-fold, n=2) were also enhanced in mdx mice. These results suggest that PI3K signaling was increased due to the increased expression of integrins and Gβ as well as their increased interaction with laminin and PI3K in mdx mice (Fig. 8C and D), which may partially compensate for the absence of the dystrophin and diminished amounts of other DGC components (Farias et al., 2005; Wang et al., 2007). These results further suggest that disruption of the DGC in mdx mouse is causing dis-regulation of the laminin-DGC-Gβγ-PI3K-Akt signaling, which is likely being replaced by integrin-Gβγ-PI3K-Akt signaling.
Fig. 8. Enhanced expression of integrin β1 and Gβ in mdx muscle induces PI3K recruitment.
A, Skeletal muscle (gastrocnemius) crude microsomes from adult (6 month old) normal (C57bl) and mdx mice, prepared from the same wet weight of muscle, were Western blotted with anti-integrin β1 or (after stripping the same blot) anti-actin antibodies. Integrin β1 protein expression was found to be higher in mdx mouse muscle compared with control while actin was not. B, Same as panel A except Western blotting with anti-βDG (shown) or anti-actin antibodies (data not shown). βDG was decreased in mdx muscle compared to muscle isolate from age-matched C57bl mice (shown) while actin was not significantly changed (data not shown). C, microsomes from 6 mo. old C57bl and mdx mice were immunoprecipitated with antibodies against PI3Kp85 and analyzed by Western blotting with antibody to integrin β1. Interaction of PI3K-integrin β1 was enhanced in mdx muscle compared with normal muscle. D, Same as panel C except laminin-Sepharose was used instead of immunoprecipitation and again Western blotting with integrin β1 antibody. Laminin-bound integrin β1 increases in skeletal muscle of mdx compared to normal mice. E, Skeletal muscle microsomes (crude) from adult C57 and mdx mice and its immune precipitates (IP) with antibodies against PI3Kp85 and integrin β1 were analyzed by Western blotting with antibody against Gβ. The results show that Gβ was increased in mdx muscle microsome compared with normal, and interactions of PI3K-Gβ and integrin β1-Gβ were enhanced in mdx mice.
DISCUSSION
The results show that the DGC, Gβγ, PI3K, and Akt exist as a complex in skeletal muscle microsomes (Fig. 1). Our previous work had shown that the Gs heterotrimeric G-protein bound to the DGC syntrophin via syntrophin's PDZ domain and that the activation state of Gs was laminin-dependent. The Gsα apparently interacts with the L-type Ca2+-channels to lessen intracellular Ca2+ when laminin is bound (Zhou et al., 2005). Possible Gβγ effects were not then explored and are the subject of the current investigation. Laminin-binding to the DGC also causes activation of pAkt and downstream signaling which prevents apoptosis (Langenbach and Rando, 2002). Upstream of pAkt is PI3K. Here, we have presented convincing evidence that this DGC-associated Gβγ associates with PI3K, activates it, and causes the pAkt activation.
PI3Ks in mammals are the products of at least eight different genes divided into three different classes (Hirsch et al., 2007). The combination of antibodies used here would be consistent with the PI3K type 1A while most of the literature on Gβγ activation of PI3K involves members of the type 1B PI3Ks. However, there are clear reports of PI3K type 1A being activated by Gβγ (Hirsch et al., 2007; Murga et al., 2000). Type 1A PI3Ks are typically recruited to the membrane by interactions between PI3Kp85 SH2 domains with phosphotyrosine sequences on a target protein and are also activated by Ras (Hirsch et al., 2007). Since syntrophin is phosphorylated by Src family tyrosine kinases (Zhou et al., 2007; Zhou et al., 2006) as has also been reported for β-dystroglycan (Sotgia et al., 2003), recruitment to phosphotyrosine sequences may indeed be involved here, although we did no experiments to directly address this. However, this would not explain the direct effects of purified Gβγ on PI3K (Fig. 4) and Akt (Fig. 5). However, the PI3K class 1A p110β subunit is also activated by Gβγ (Kurosu et al., 1997). It is most likely that it is this PI3K which accounts for our results.
Other G-proteins, including the small G-proteins, also activate PI3K. Activation by Ras can probably be excluded. Laminin-Sepharose pulls down a complex containing DGC proteins and Rac1 (Oak and Jarrett, 2003; Zhou et al., 2007; Zhou et al., 2005) but not Ras or other p21 G-proteins (Oak and Jarrett, 2003). Laminin-binding is required for recruiting Gs to the DGC (Zhou et al., 2005) and here we show that a specific antibody (IIH6), which blocks laminin-binding by DGC α-dystroglycan, blocks the recruitment of PI3K to the DGC (Fig. 2C). This same antibody has also been shown to block Gβγ- binding (Zhou et al., 2005) and Akt activation and to induce apoptosis (Langenbach and Rando, 2002) in muscle cells. Thus, the data are most consistent with either Rac1 or Gβγ being the G-protein responsible. However, cholera toxin should specifically affect only Gs in a way that would increase the availability of Gβγ as was shown in Fig. 2E by its effect on the activation of pAkt, and the direct effect of purified Gβγ confirms Gβγ. Thus, the results are consistent with a model in which laminin-binding to the DGC increases the availability of Gβγ and this is in turn activating Type 1A PI3K, probably containing the p110β catalytic subunit.
Laminins interact with cell surface receptors, leading to intracellular signaling and cytoskeletal reorganization, and thus have important regulatory roles. Laminin binding to α-dystroglycan or integrins can initiate cell signaling (Hayashi et al., 2001; Miyagoe et al., 1997). However, the role of laminin in the activation of PI3K is unclear and has been a focus of this investigation. The result demonstrates that phosphorylated pAkt was reduced as a result of laminin depletion using heparin-Sepharose, but adding exogenous laminin to the depleted microsomes reconstitutes pAkt activation (Fig 3A). To elucidate further the mechanism of the laminin induce signaling we investigated the effects of laminin on interaction of DGC-Gβγ-PI3K-Akt (Fig 3B). The data shows laminin increases binding activity, and laminin-deficiency decreases the interactions of DGC-Gβγ-PI3K-pAkt. Either endogenous laminin (predominantly laminin-2) or exogenous laminin-1 can enhance signaling. These findings suggest that DGC-Gβγ is binding PI3K and activating pAkt in a laminin-dependent manner. Taken together with previous results, which show that the same IIH6 antibody that blocks the laminin-dystroglycan interaction, inhibits Akt signaling and initiate apoptosis (Langenbach and Rando, 2002), leaves little doubt that this DGC-Gβγ-PI3K-Akt signaling is an important pathway for muscle cell survival.
To determine whether this phosphorylation of Akt or downstream GSK is occurring through PI3K, we have investigated the effects of two pharmacological inhibitor of PI3K on Akt and GSK phosphorylation in cultured C2C12 myotubes. The results show that 150–200 nM wortmannin can inhibit activation of Akt and GSK with little decrease in cell viability (Fig 4), and confirm the PI3K/Akt pathway play an important role mediating cell–survival signaling. LY294002 was also an effective inhibitor but its effects on myotube viability makes the meaning of these results much less clear.
How this may function in intact muscle, where laminin is stably bound, was not investigated. A recent paper (Zhou et al., 2007), however, suggests how this may occur. Laminin-binding to the DGC also results in signaling through a DGC/Rac1/JNK pathway. In this case, stretching or contracting intact muscle also resulted in signaling and it was proposed that it is stressing the laminin-DGC linkage during muscle activity is what initiates signaling in vivo. The same may apply here.
In order to investigate how the loss of the DGC impacts PI3K/Akt signaling during mdx dystrophy we investigated Akt activation in normal and dystrophic muscle. The results show that pAkt levels were significantly elevated in mdx skeletal muscle relative to control in agreement with a previous report (Dogra et al., 2006). These data showing the activation of PI3K/Akt signaling in dystrophin-deficient muscle (Fig. 7 and 8) suggesting that the response of mdx muscle is initiated early in the pathogenic process (Peter and Crosbie, 2006). Akt activation was detected at 4 weeks, at a very early stage of disease pathogenesis as well as at later stages (data not shown). DMD patients exhibit a similar pattern of Akt activation (Dogra et al., 2006; Peter and Crosbie, 2006). Recent investigation have demonstrated the importance of the PI3K/Akt pathway in regulation of muscle apoptosis (Rommel et al., 2001). In our data 4-week-old mdx mice display elevated Akt signaling suggesting a compensatory cell survival response to the disease.
Recently, studies demonstrate that there is an increase in the amount of integrin in DMD patients and mdx mice, which could ameliorate the development of muscle diseases (Burkin et al., 2001; Li et al., 2003), but the mechanism is not currently understood. It remains possible that compensatory mechanisms may be activated via alternative receptor systems such as integrins and may compensate for defective DGC/PI3K/Akt signaling mechanisms (Burkin et al., 2005). Our results demonstrate not only increased integrin β1 expression, but also significantly increased the interaction between integrin β1, laminin, Gβγ and PI3K occurs in mdx mice (Fig. 8). These results point to a mechanism by which enhanced levels of integrin β1 activate the Gβγ/PI3K/Akt pathway in muscle (Farias et al., 2005). Integrin β1 may compensate for the defects of the DGC in mdx mice (Burkin et al., 2005; Burkin et al., 2001; Deconinck et al., 1997) by reinforcing the signal transduction pathways which normally link the DGC to cell survival signaling.
Most G-protein coupled receptors which interact with the heterotrimeric G-proteins are of the seven transmembrane spanning receptor motif. Clearly, while the DGC is a G-protein coupled receptor, it is of a different type. In this case, the DGC is a multiprotein complex, binding laminin via the dystroglycan proteins, spanning the sarcolemma with the β-dystroglycan subunit and the sarcoglycans, and interacting with the cytoskeleton via dystrophin and G-proteins via syntrophin. The DGC is a G-protein coupled laminin-receptor. When laminin-binds, Gβγ apparently activates PI3K, which in turn causes pAkt activation. We have previously shown that the particular region of laminin, the laminin globular (LG) domains 4–5, which binds to DGC α-dystroglycan (Talts et al., 1999), causes proliferation in myoblasts (Zhou et al., 2007) and others have shown that laminin is necessary for normal muscle differentiation and growth. Thus, if Gβγ becomes available, either because of laminin-binding or by other means (e.g., muscle activity), a hypertrophic response mediated by the Akt pathway would be expected. This has been observed with pharmacological agents.
Gβγ can also be made available at the sarcolemma by other means. Of the more typical type of G-protein receptors are the β-adrenergic receptors, of which β2-adrenoceptor is the most abundant muscle sub-type. This receptor also couples through heterotrimeric G-proteins (Lynch et al., 2007). β2- Adrenoceptor agonists, such as clenbuterol, which would also increase available sarcolemma Gβγ, cause muscle hypertrophy and increase muscle mass. Furthermore, clenbuterol also causes increased pAkt activation and downstream signaling through mTOR and S6 kinase. The authors of these studies hypothesized that it could be the Gβγ released by the β2-adrenoceptor in response to clenbuterol that was activating PI3K/Akt to cause muscle hypertrophy (Kline et al., 2007). Here, we provide evidence this occurs for the laminin-DGC-Gβγ-PI3K-pAkt signaling.
There is no perfect model for studying muscle cell signaling and therefore, our study encompassed rabbit and mouse muscle microsomes as well as a cultured mouse muscle cell line. Most of what is known about DGC proteins is known from studies in rabbit muscle microsomes and this makes this an ideal system for the study of DGC protein interactions. However, cultured cells are most easily adapted to inhibitor studies and the mdx mouse is the best characterized animal model for Duchenne muscular dystrophy. Despite the number of model systems used, the results all show a high degree of internal consistency and give some confidence that the results reported are accurate for many skeletal muscle models.
Taken together, our results demonstrate that the DGC is associated with PI3K/Akt signaling and that DGC-Gβγ is binding PI3K in a laminin-dependent manner and activating pAkt. These results are consistent across a variety of model systems for studying muscle signaling. Further downstream, this leads to GSK-3β phosphorylation. Mdx mouse displays elevated integrin β1 and increased interaction between integrin β1 and laminin, Gβγ and PI3K (Fig. 8) and increased Akt signaling compared to normal tissue. Mdx mice might compensate for the loss of dystrophin and associated DGC proteins by this enhance laminin-integrin β1 interaction. Moreover, disruption of the DGC in mdx mouse is causing dis-regulation of the laminin-DGC-Gβγ-PI3K-Akt signaling and replaces it with laminin-integrin-Gβγ-PI3K-Akt signaling. This further substantiates the hypothesis that this laminin-DGC-Gβγ-PI3K-Akt-GSK signaling is likely to be important to the pathogenesis of muscular dystrophies, and modulation of this pathway may be of therapeutic value for DMD. Most β2-adrenergic agonists have the undesirable side-effect of inducing unwanted cardiac hypertrophy. However, some of the newer agonist, such as formoterol and salmeterol, also induce skeletal muscle hypertrophy with greater selectivity and by stimulating Gβγ-induced pAkt activation may be of therapeutic value for DMD and other muscle wasting diseases (Lynch et al., 2007; Ryall et al., 2006).
Acknowledgements
We appreciate the generous gifts of antibodies from Drs. Tamara Petrucci and Kevin P. Campbell. The excellent technical assistance of Maria Macias and Magda Loranc is greatly appreciated. Yongmin Xiong is a visiting professor from Xi’an Jiaotong University School of Medicine; this work is in partial fulfillment of the requirements for her Ph.D.
Grants: This study was supported by National Institutes of Health grant AR051440, the Muscular Dystrophy Association grant #3789 and the China Scholarship Council.
REFERENCES
- Ananthanarayanan B, Ni Q, Zhang J. Signal propagation from membrane messengers to nuclear effectors revealed by reporters of phosphoinositide dynamics and Akt activity. Proc Natl Acad Sci U S A. 2005;102(42):15081–15086. doi: 10.1073/pnas.0502889102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276(1 Pt 1):C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, Schultz G, Nurnberg B. Roles of G beta gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma. J Cell Biol. 2003;160(1):89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SC, Fassati A, Popplewell L, Page AM, Henry MD, Campbell KP, Dickson G. Dystrophic phenotype induced in vitro by antibody blockade of muscle alpha-dystroglycan-laminin interaction. J Cell Sci. 1999;112(Pt 2):209–216. doi: 10.1242/jcs.112.2.209. [DOI] [PubMed] [Google Scholar]
- Burkin DJ, Wallace GQ, Milner DJ, Chaney EJ, Mulligan JA, Kaufman SJ. Transgenic expression of {alpha}7{beta}1 integrin maintains muscle integrity, increases regenerative capacity, promotes hypertrophy, and reduces cardiomyopathy in dystrophic mice. Am J Pathol. 2005;166(1):253–263. doi: 10.1016/s0002-9440(10)62249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkin DJ, Wallace GQ, Nicol KJ, Kaufman DJ, Kaufman SJ. Enhanced expression of the alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J Cell Biol. 2001;152(6):1207–1218. doi: 10.1083/jcb.152.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80(5):675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Cohn RD, Mayer U, Saher G, Herrmann R, van der Flier A, Sonnenberg A, Sorokin L, Voit T. Secondary reduction of alpha7B integrin in laminin alpha2 deficient congenital muscular dystrophy supports an additional transmembrane link in skeletal muscle. J Neurol Sci. 1999;163(2):140–152. doi: 10.1016/s0022-510x(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90(4):717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- Dittmer JC, Lester RL. A simple, specific spray for the detection of phospholipids on thin-layer chromatograms. J Lipid Res. 1964;5:126–127. [PubMed] [Google Scholar]
- Dogra C, Changotra H, Wergedal JE, Kumar A. Regulation of phosphatidylinositol 3-kinase (PI3K)/Akt and nuclear factor-kappa B signaling pathways in dystrophin-deficient skeletal muscle in response to mechanical stretch. J Cell Physiol. 2006;208:575–585. doi: 10.1002/jcp.20696. [DOI] [PubMed] [Google Scholar]
- Ekstrom PA, Mayer U, Panjwani A, Pountney D, Pizzey J, Tonge DA. Involvement of alpha7beta1 integrin in the conditioning-lesion effect on sensory axon regeneration. Mol Cell Neurosci. 2003;22(3):383–395. doi: 10.1016/s1044-7431(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122(4):809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias E, Lu M, Li X, Schnapp LM. Integrin alpha8beta1-fibronectin interactions promote cell survival via PI3 kinase pathway. Biochem Biophys Res Commun. 2005;329(1):305–311. doi: 10.1016/j.bbrc.2005.01.125. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Kikkawa Y, Sanzen N, Sekiguchi K. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins. J Biol Chem. 2001;276(20):17550–17558. doi: 10.1074/jbc.M010155200. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37(10):1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Tiger C-F, Velling T. Laminins during muscle development and in muscular dystrophies. Cell Mol Life Sci. 1999;56:442–460. doi: 10.1007/PL00000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Willem M, Werner A, Raivich G, Emerson M, Neyses L, Mayer U. Absence of alpha7 integrin in dystrophin-deficient mice causes a myopathy similar to Duchenne muscular dystrophy. Hum Mol Genet. 2006;15(6):989–998. doi: 10.1093/hmg/ddl018. [DOI] [PubMed] [Google Scholar]
- Hayashi YK, Tezak Z, Momoi T, Nonaka I, Garcia CA, Hoffman EP, Arahata K. Massive muscle cell degeneration in the early stage of merosin-deficient congenital muscular dystrophy. Neuromuscul Disord. 2001;11(4):350–359. doi: 10.1016/s0960-8966(00)00203-0. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Costa C, Ciraolo E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J Endocrinol. 2007;194:243–256. doi: 10.1677/JOE-07-0097. [DOI] [PubMed] [Google Scholar]
- Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci. 1997;110(Pt 22):2873–2881. doi: 10.1242/jcs.110.22.2873. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kanagawa M, Toda T. The genetic and molecular basis of muscular dystrophy: roles of cell-matrix linkage in the pathogenesis. J Hum Genet. 2006;51(11):915–926. doi: 10.1007/s10038-006-0056-7. [DOI] [PubMed] [Google Scholar]
- Katada T, Oinuma M, Ui M. Two guanine nucleotide-binding proteins in rat brain serving as the specific substrate of islet-activating protein, pertussis toxin. Interaction of the alpha-subunits with beta gamma-subunits in development of their biological activities. J Biol Chem. 1986;261:8182–8191. [PubMed] [Google Scholar]
- Kikkawa Y, Sanzen N, Fujiwara H, Sonnenberg A, Sekiguchi K. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by alpha 3 beta 1, alpha 6 beta 1 and alpha 6 beta 4 integrins. J Cell Sci. 2000;113:869–876. doi: 10.1242/jcs.113.5.869. [DOI] [PubMed] [Google Scholar]
- Klein S, Reuveni H, Levitzki A. Signal transduction by a nondissociable heterotrimeric yeast G protein. Proc Natl Acad Sci U S A. 2000;97(7):3219–3223. doi: 10.1073/pnas.050015797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline WO, Panaro FJ, Yang H, Bodine SC. Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J Appl Physiol. 2007;102:740–747. doi: 10.1152/japplphysiol.00873.2006. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, Ui M, Hazeki O, Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. J Biol Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol. 2004;24(21):9295–9304. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbach KJ, Rando TA. Inhibition of dystroglycan binding to laminin disrupts the PI3K/AKT pathway and survival signaling in muscle cells. Muscle & Nerve. 2002;26:644–653. doi: 10.1002/mus.10258. [DOI] [PubMed] [Google Scholar]
- Lansman JB, Franco-Obregon A. Mechanosensitive ion channels in skeletal muscle: a link in the membrane pathology of muscular dystrophy. Clin Exp Pharmacol Physiol. 2006;33(7):649–656. doi: 10.1111/j.1440-1681.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res. 2004;94(8):1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- Li BG, Hasselgren PO, Fang CH. Insulin-like growth factor-I inhibits dexamethasone-induced proteolysis in cultured L6 myotubes through PI3K/Akt/GSK-3beta and PI3K/Akt/mTOR-dependent mechanisms. Int J Biochem Cell Biol. 2005;37(10):2207–2216. doi: 10.1016/j.biocel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Li J, Rao H, Burkin D, Kaufman SJ, Wu C. The muscle integrin binding protein (MIBP) interacts with alpha7beta1 integrin and regulates cell adhesion and laminin matrix deposition. Dev Biol. 2003;261(1):209–219. doi: 10.1016/s0012-1606(03)00304-x. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Schertzer JD, Ryall JG. Therapeutic approaches for muscle wasting disorders. Pharmacol Ther. 2007;113:461–487. doi: 10.1016/j.pharmthera.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Maier U, Babich A, Macrez N, Leopoldt D, Gierschik P, Illenberger D, Nurnberg B. Gbeta 5gamma 2 is a highly selective activator of phospholipid-dependent enzymes. J Biol Chem. 2000;275(18):13746–13754. doi: 10.1074/jbc.275.18.13746. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Yamada H, Saito F, Sunada Y, Shimizu T. The role of dystroglycan, a novel receptor of laminin and agrin, in cell differentiation. Histol Histopathol. 1997;12(1):195–203. [PubMed] [Google Scholar]
- Mayer U. Integrins, redundant or important players in skeletal muscle? J Biol Chem. 2003;278:14587–14590. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- Michele DE, Campbell KP. Dystrophin-glycoprotein complex: post-translational processing and dystroglycan function. J Biol Chem. 2003;278(18):15457–15460. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Nabeshima Y, Takeda S. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415:33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- Murga C, Fukuhara S, Gutkind JS. A novel role for phosphatidylinositol 3-kinase beta in signaling from G protein-coupled receptors to Akt. J Biol Chem 2000. 2000 Apr 21;275(16):12069–12073. doi: 10.1074/jbc.275.16.12069. 275:12069–12073. [DOI] [PubMed] [Google Scholar]
- Oak SA, Jarrett HW. Skeletal Muscle Signaling Pathway Through the Dystrophin glycoprotein Complex and Rac1. Biochemistry. 2003;278(41):39287–39295. doi: 10.1074/jbc.M305551200. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K, Campbell KP. Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J Cell Biol. 1991;115:1685–1694. doi: 10.1083/jcb.115.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998;273(32):19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Peter AK, Crosbie RH. Hypertrophic response of Duchenne and limb-girdle muscular dystrophies is associated with activation of Akt pathway. Exp Cell Res. 2006;312(13):2580–2591. doi: 10.1016/j.yexcr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3(11):1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Rooney JE, Welser JV, Dechert MA, Flintoff-Dye NL, Kaufman SJ, Burkin DJ. Severe muscular dystrophy in mice that lack dystrophin and alpha7 integrin. J Cell Sci. 2006;119(Pt 11):2185–2195. doi: 10.1242/jcs.02952. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Sillence MN, Lynch GS. Systemic administration of beta2-adrenoceptor agonists, formoterol and salmeterol, elicit skeletal muscle hypertrophy in rats at micromolar doses. Br J Pharmacol. 2006;147:587–595. doi: 10.1038/sj.bjp.0706669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakova IN, Humston JL, Sonnemann KJ, Ervasti JM. Dystrophin and utrophin bind actin through distinct modes of contact. J Biol Chem. 2006;281(15):9996–10001. doi: 10.1074/jbc.M513121200. [DOI] [PubMed] [Google Scholar]
- Schnitzler AC, Burke JM, Wetzler LM. Induction of cell signaling events by cholera toxin B-subunit in antigen presenting cells. Infect Immun. 2007;75:3150–3159. doi: 10.1128/IAI.00581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindinger WF, Robishaw JD. Heterotrimeric G-protein betagamma-dimers in growth and differentiation. Oncogene. 2001;20:1653–1660. doi: 10.1038/sj.onc.1204181. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Bonucceli G, Bedford MT, Brancaccio A, Mayer U, Wilson MT, Campos-Gonzales R, Brooks JW, Sudol M, Lisanti MP. Localization of phospho-beta-dystroglycan (pY892) to an intracellular vesicular compartment in cultured cells and skeletal muscle fibers in vivo. Biochemistry. 2003;42:7110–7123. doi: 10.1021/bi0271289. [DOI] [PubMed] [Google Scholar]
- Stawowy P, Margeta C, Blaschke F, Lindschau C, Spencer-Hansch C, Leitges M, Biagini G, Fleck E, Graf K. Protein kinase C epsilon mediates angiotensin II-induced activation of beta1-integrins in cardiac fibroblasts. Cardiovasc Res. 2005;67(1):50–59. doi: 10.1016/j.cardiores.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Kureishi Y, Yang J, Luo Z, Guo K, Mukhopadhyay D, Ivashchenko Y, Branellec D, Walsh K. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol. 2002;22(13):4803–4814. doi: 10.1128/MCB.22.13.4803-4814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R. Binding of the G domains of laminin alpha1 and alpha2 chains and perlecan to heparin, sulfatides, alpha-dystroglycan and several extracellular matrix proteins. EMBO J. 1999;18:863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Lessan K, Kahm J, Kleidon J, Henke C. beta 1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J Biol Chem. 2002;277(27):24667–24675. doi: 10.1074/jbc.M203565200. [DOI] [PubMed] [Google Scholar]
- Urbich C, Walter DH, Zeiher AM, Dimmeler S. Laminar shear stress upregulates integrin expression: role in endothelial cell adhesion and apoptosis. Circ Res. 2000;87(8):683–689. doi: 10.1161/01.res.87.8.683. [DOI] [PubMed] [Google Scholar]
- Vachon PH, Xu H, Liu L, Loechel F, Hayashi Y, Arahata K, Reed JC, Wewer UM, Engvall E. Integrins (alpha7beta1) in muscle function and survival. Disrupted expression in merosindeficient congenital muscular dystrophy. J Clin Invest. 1997;100(7):1870–1881. doi: 10.1172/JCI119716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HQ, Bai L, Shen BR, Yan ZQ, Jiang ZL. Coculture with endothelial cells enhances vascular smooth muscle cell adhesion and spreading via activation of beta1-integrin and phosphatidylinositol 3-kinase/Akt. Eur J Cell Biol. 2007;86(1):51–62. doi: 10.1016/j.ejcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Watchko JF, O'Day TL, Hoffman EP. Functional characteristics of dystrophic skeletal muscle: insights from animal models. J Appl Physiol. 2002;93(2):407–417. doi: 10.1152/japplphysiol.01242.2001. [DOI] [PubMed] [Google Scholar]
- Xie P, Browning DD, Hay N, Mackman N, Ye RD. Activation of NF-kappa B by bradykinin through a Galpha(q)- and Gbeta gamma-dependent pathway that involves phosphoinositide 3-kinase and Akt. J Biol Chem. 2000;275(32):24907–24914. doi: 10.1074/jbc.M001051200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jiang D, Thomason DB, Jarrett HW. Laminin-Induced Activation of Rac1 and JNKp46 Is Initiated by Src Family Kinases and Mimics the Effects of Skeletal Muscle Contraction. Biochemistry. 2007;46:14907–14916. doi: 10.1021/bi701384k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YW, Oak SA, Senogles SE, Jarrett HW. Laminin-alpha1 globular domains 3 and 4 induce heterotrimeric G protein binding to alpha-syntrophin's PDZ domain and alter intracellular Ca2+ in muscle. Am J Physiol Cell Physiol. 2005;288(2):C377–C388. doi: 10.1152/ajpcell.00279.2004. [DOI] [PubMed] [Google Scholar]
- Zhou YW, Thomason DB, Gullberg D, Jarrett HW. Binding of laminin alpha1-chain LG4-5 domain to alpha-dystroglycan causes tyrosine phosphorylation of syntrophin to initiate Rac1 signaling. Biochemistry. 2006;45:2042–2052. doi: 10.1021/bi0519957. [DOI] [PubMed] [Google Scholar]