Abstract

Cultivation of an obligate marine Streptomyces strain has furnished the marinopyrroles A and B, densely halogenated, axially chiral metabolites that contain an uncommon bispyrrole structure. X-ray analysis of marinopyrrole B showed that the natural product exists as an atropo-enantiomer with the M-configuration. Though configurationally stable at room temperature, M-(−)-marinopyrrole A can be racemized at elevated temperatures to yield the non-natural P-(+)-atropo-enantiomer. The marinopyrroles possess potent antibiotic activities against methicillin-resistant Staphylococcus aureus.
For over half a century, the cultivation of terrestrial actinomycetes has yielded structurally diverse secondary metabolites with remarkable biological activities.1 Today, studies of these chemically prolific bacteria have been expanded from traditional soil habitats to include strains obtained from marine samples. At a time when a pressing need exists for the discovery of new antibiotics,2 the rationale to sample under-explored habitats in search of new microbial resources is great. For these reasons, we have focused our attention on actinomycetes that inhabit ocean sediments. Chemical studies of these bacteria, which include new species and genera, have yielded a growing number of unique, bioactive natural products.3
Actinomycete strain CNQ-418, obtained from a marine sediment sample collected near La Jolla, CA, at a depth of 51 m, required seawater for growth and shared 98.1% 16S rRNA gene sequence identity with its nearest neighbor (Streptomyces sannurensis). This level of similarity suggests that it may be a new Streptomyces species.4 Strain CNQ-418, the culture extracts of which displayed significant antibiotic activity, may represent the first strain of a new marine lineage to be examined for its ability to produce biologically active natural products.
Strain CNQ-418 was cultivated in a seawater-based medium (20 × 1 L) for 7 days under vigorous shaking. Solid-phase extraction of the broth using Amberlite resin (XAD-16), filtration through cheesecloth, and elution of the resin with acetone afforded, after solvent removal under vacuum, a gummy extract that was subjected to fractionation on silica gel. Two prominent metabolites, the marinopyrroles A (1) and B (2), were isolated from a fraction using C8 reversed-phase HPLC. These compounds were analyzed for the molecular formulas C22H12Cl4N2O4 and C22H11BrCl4N2O4, respectively.
Initial progress toward the structural elucidation of these new metabolites began with 2D NMR studies of marinopyrrole A (1). Two independent benzoyl groups were identified by interpretation of COSY, HSQC, and HMBC spectral data (Table 1). The rather limited NMR spectral data defining the central core of these molecules, however, made unambiguous structural elucidation difficult if not impossible.
Table 1.
1H, 13C, and HMBC NMR Spectral Data for Marinopyrrole A (1) (CDCl3)
| C nos. | δCa | δH, mult. (J, Hz)b | HMBCb |
|---|---|---|---|
| 1-4,1′,2′,4′ c | |||
| 5 | 185.9 | ||
| 6 | 118.9 | ||
| 7 | 161.1 | ||
| 8 | 117.8 | 6.92, md | 5,7 |
| 9 | 136.2 | 7.35, t (7.8) | 7,11 |
| 10 | 118.7 | 6.52, t (7.8) | 6–9,11 |
| 11 | 130.3 | 7.48, d (7.8) | 5,7,9 |
| 3′ | 120.3 | 6.72, s | 5′ |
| 5′ | 186.8 | ||
| 6′ | 118.9 | ||
| 7′ | 162.5 | ||
| 8′ | 118.4 | 7.03, d (7.8) | 5′,7′,10′ |
| 9′ | 136.3 | 7.51, t (7.8) | 7′,11′ |
| 10′ | 119.1 | 6.90, md | 8′ |
| 11′ | 131.9 | 7.58, d (7.8) | 5′,7′,9′ |
| −OH, −NH | 11.18, 10.42, 9.73, br s |
75 MHz.
500 MHz.
δC = 128.8, 124.1, 123.8, 123.1, 120.4, 112.7, 110.7
Overlapping signals.
The structure of marinopyrrole A (1) was ultimately assigned using X-ray crystallographic techniques. At first, several derivatives of 1 were prepared in order to procure material that would be suitable for crystallographic analysis. The phenolic groups were cleanly esterified to produce the corresponding O,O′-diacetyl, di-p-bromobenzoyl, and di-p-nitrobenzoyl esters. Reaction of 1 with ethereal diazomethane provided an N-methyl derivative that was also crystalline. Although these derivatives did not yield X-ray quality crystals, we were able to obtain crystals of marinopyrrole B (2) via slow evaporation from toluene. The structure of marinopyrrole B, including its absolute configuration, was then readily determined (Figure 1). Subsequently, the planar structure of marinopyrrole A (1) was assigned by comparison of its spectral data with those derived from 2.
Figure 1.

The marinopyrroles A (1) and B (2).
The marinopyrroles were isolated as single atropo-enantiomers, suggesting that the pyrrole–pyrrole coupling is a genuine enzyme-catalyzed process. Similar optical rotations of −69 (c = 0.39, MeOH) and −72 (c = 0.20, MeOH) were observed for 1 and 2, respectively. An X-ray Flack parameter, refined to 0.03(1), allowed us to assign an M-configuration to 2. By comparison of CD spectra, marinopyrrole A (1) was also assigned an M-configuration (Figure 2). Both spectra showed negative first and positive second Cotton effects [1: Δ∊ = −6.2 (372 nm) and Δ∊ = +6.8 (315 nm); 2: Δ∊ = −2.9 (373 nm) and Δ∊ = +2.0 (314 nm)], in agreement with the negative exciton chirality between the two salicyloyl substituents.5
Figure 2.
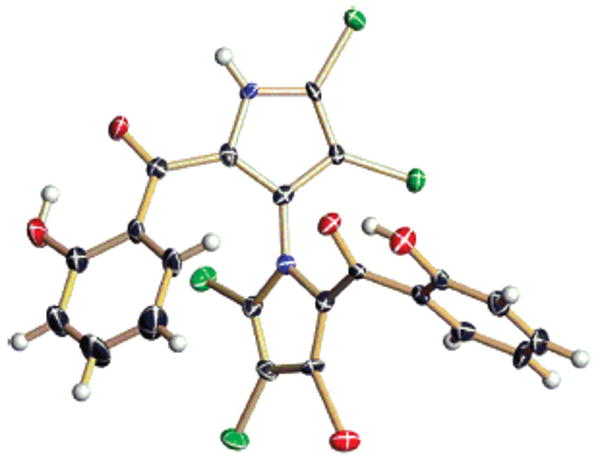
X-ray drawing of 2, depicting its absolute configuration. The hydrogen bond between the perihydroxyl group and the carbonyl is apparent.
Though these metabolites are configurationally stable at room temperature, we sought to determine whether the molecules could be racemized at elevated temperatures. Accordingly, marinopyrrole A (1) was heated in toluene at 120 °C. The resulting atropo-enantiomers could be separated on an analytical chiral HPLC column (Chiracel OD-H). Acylation of the racemic mixture with R-Mosher's chloride produced a mixture of diastereomers (S-M-S and S-P-S) that was separated on silica gel. The non-natural atropo-enantiomer of 1, [α]D = +110 (c = 0.12, MeOH), was then isolated upon saponification of the appropriate bisester. The CD spectrum of P-1 displayed split Cotton effects of opposite sign, as expected [Δ∊ = +6.5 (370 nm) and Δ∊ = −7.0 (310 nm)] (see Figure 3).
Figure 3.

CD spectra of 1, 2, and non-natural P-1 in acetonitrile.
The marinopyrroles, a component of which is reminiscent of the Pseudomonas metabolite pyoluteorin,6 have several novel structural features. Foremost, the N,C2-linked bispyrrole motif has not been previously described in the natural products literature.7 Though their synthesis via the Paal-Knorr reaction was first detailed in 1957,8 bispyrroles of this sort have received little attention in the synthetic community.9 The unusual biaryl bond, flanked by four ortho substituents, establishes an axis of chirality that further distinguishes these interesting natural products. The biosynthesis of the marinopyrroles would appear to involve a critical pyrrole coupling event that has not been observed in the past.
The compounds display noteworthy activity in antimicrobial bioassays (Table 2). Against methicillin-resistant Staphylococcus aureus, 1 and 2 showed minimum inhibitory concentrations (MIC90) of less than 2 μM. For each of the compounds, cytotoxicity against a human cancer cell line (HCT-116) was less pronounced. Interestingly, in these assays, the configuration about the biaryl axis of 1 appeared to be of little consequence, as the non-natural atropo-enantiomer showed potency similar to that of the natural product.
Table 2.
Bioassay Results (μM)
MRSA = methicillin-resistant Staphylococcus aureus. Positive control: vancomycin (MIC90 = 0.14–0.27 μM) and penicillin G (MIC90 = 18–34 μM).
HCT-116 is a human colon cancer cell line. Positive control: etoposide (IC50 = 0.49–4.9 μM).
Derived from what appears to represent a new, obligate marine Streptomyces sp., the marinopyrroles are the first examples of natural products containing a N,C2-linked bispyrrole structure. These bioactive molecules may encompass a new pharmacophore that could prove useful in the treatment of drug-resistant bacterial pathogens. Studies are now in progress to examine the mechanism of action of these novel antibiotics.
Supplementary Material
Acknowledgments
Financial support was provided by the National Cancer Institute, NIH (under grant CA44848). We thank Drs. Arnold L. Rheingold and Antonio G. D'Pasquale (UCSD) for assistance with X-ray diffraction experiments. We also acknowledge Sara Kelly (SIO) for performing the bioassays.
Footnotes
Supporting Information Available: Isolation procedure for 1 and 2. Procedure for the synthesis of P-1. HRMS data, proton and carbon NMR spectra for 1, 2, and P-1. Crystallographic data for 2 (CCDC 645271) in CIF format. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Newman DJ, Cragg GM. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 2.von Nussbaum F, Brands M, Hinzen B, Weigand S, Haebich D. Angew Chem, Int Ed. 2006;45:5072–5129. doi: 10.1002/anie.200600350. [DOI] [PubMed] [Google Scholar]
- 3.(a) Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Angew Chem, Int Ed. 2003;42:355–357. doi: 10.1002/anie.200390115. [DOI] [PubMed] [Google Scholar]; (b) Fenical W, Jensen PR. Nat Chem Biol. 2006;2:666–673. doi: 10.1038/nchembio841. and references therein. [DOI] [PubMed] [Google Scholar]
- 4.Based on comparisons with DNA–DNA reassociation data, it has been shown that 98% 16S rRNA gene sequence identity is highly conservative in terms of delineating species-level relationships among actinomycetes; see: Stach JEM, Maldonado LA, Masson DG, WARD AC, Goodfellow M, Bull AT. Appl Environ Microbiol. 2003;69:6189–6200. doi: 10.1128/AEM.69.10.6189-6200.2003.
- 5.Harada N, Nakanishi K. Circular Dichroic Spectroscopy: Exciton Coupling in Organic Stereochemistry. University Science Books; Mill Valley, CA: 1983. [Google Scholar]
- 6.(a) Takeda RJ. J Am Chem Soc. 1958;80:4749–4750. [Google Scholar]; (b) Takeda RJ. Bull Agr Chem Soc Jpn. 1959;23:126–130. [Google Scholar]; See, also, pyrrolomycin: Kaneda M, Nakamura S, Ezaki N, Iitaka Y. J Antibiot. 1981;34:1366–1368. doi: 10.7164/antibiotics.34.1366.Ezaki N, Koyama M, Shamura T, Tsuruoka T, Inouye S. J Antibiot. 1983;36:1263–1267. doi: 10.7164/antibiotics.36.1263.
- 7.For related bisindole, biscarbazole, and N,C1-linked bispyrrole natural products, see: Norton RS, Wells RJ. J Am Chem Soc. 1982;104:3628–3625.Ito C, Thoyama Y, Omura M, Kajiura I, Furukawa H. Chem Pharm Bull. 1993;41:2096–2100.Wu J, Vetter W, Gribble GW, Schneekloth JS, Jr, Blank DH, Gorls H. Angew Chem, Int Ed. 2002;41:1740–1743. doi: 10.1002/1521-3773(20020517)41:10<1740::aid-anie1740>3.0.co;2-7.
- 8.Treibs A, Hitzler O. Chem Ber. 1957;90:787–788. [Google Scholar]
- 9.The bispyrrole structure can be recognized in intermediates of dipyrroloisoquinoline and pyrrolopyrrolizinone syntheses, as well as certain analogues of the antifungal compound bifonazole. See: Mingoia F. Tetrahedron. 2001;57:10147–10153.Rochais C, Lisowski V, Dallemagne P, Rault S. Tetrahedron Lett. 2004;45:6353–6355.Santo RD, Massa S, Costi R, Simonetti G, Retico A. Il Farmaco. 1994;49:229–236.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


