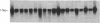Abstract
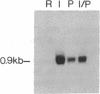
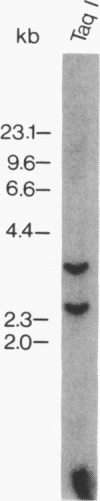
A cDNA clone encoding a human serine esterase gene was isolated from a library constructed from poly(A)+ RNA of allogeneically stimulated, interleukin 2-expanded peripheral blood mononuclear cells. The clone, designated HSE26.1, represents a full-length copy of a 0.9-kilobase mRNA present in human cytotoxic cells but absent from a wide variety of noncytotoxic cell lines. Clone HSE26.1 contains an 892-base-pair sequence, including a single 741-base-pair open reading frame encoding a putative 247-residue polypeptide. The first 20 amino acids of the polypeptide form a leader sequence. The mature protein is predicted to have an unglycosylated Mr of approximately equal to 26,000 and contains a single potential site for N-linked glycosylation. The nucleotide and predicted amino acid sequences of clone HSE26.1 are homologous with all murine and human serine esterases cloned thus far but are most similar to mouse granzyme B (70% nucleotide and 68% amino acid identity). HSE26.1 protein is expressed weakly in unstimulated peripheral blood mononuclear cells but is strongly induced within 6-hr incubation in medium containing phytohemagglutinin. The data suggest that the protein encoded by HSE26.1 plays a role in cell-mediated cytotoxicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Verret C. R., Wolley R. C., Eisen H. N. Calcium ion concentrations and DNA fragmentation in target cell destruction by murine cloned cytotoxic T lymphocytes. J Exp Med. 1988 Feb 1;167(2):514–527. doi: 10.1084/jem.167.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birktoft J. J., Blow D. M., Henderson R., Steitz T. A. I. Serine proteinases. The structure of alpha-chymotrypsin. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):67–76. doi: 10.1098/rstb.1970.0009. [DOI] [PubMed] [Google Scholar]
- Brunet J. F., Dosseto M., Denizot F., Mattei M. G., Clark W. R., Haqqi T. M., Ferrier P., Nabholz M., Schmitt-Verhulst A. M., Luciani M. F. The inducible cytotoxic T-lymphocyte-associated gene transcript CTLA-1 sequence and gene localization to mouse chromosome 14. Nature. 1986 Jul 17;322(6076):268–271. doi: 10.1038/322268a0. [DOI] [PubMed] [Google Scholar]
- Carpen O., Virtanen I., Saksela E. Ultrastructure of human natural killer cells: nature of the cytolytic contacts in relation to cellular secretion. J Immunol. 1982 Jun;128(6):2691–2697. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenfeld H. K., Hershberger R. J., Shows T. B., Weissman I. L. Cloning and chromosomal assignment of a human cDNA encoding a T cell- and natural killer cell-specific trypsin-like serine protease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1184–1188. doi: 10.1073/pnas.85.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenfeld H. K., Weissman I. L. Cloning of a cDNA for a T cell-specific serine protease from a cytotoxic T lymphocyte. Science. 1986 May 16;232(4752):854–858. doi: 10.1126/science.2422755. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L. H., Yang R. C., Wu R. An improved strategy for rapid direct sequencing of both strands of long DNA molecules cloned in a plasmid. Nucleic Acids Res. 1983 Aug 25;11(16):5521–5540. doi: 10.1093/nar/11.16.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley B. S. Homologies in serine proteinases. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):77–87. doi: 10.1098/rstb.1970.0010. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Dennert G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol. 1984 Nov;133(5):2762–2766. [PubMed] [Google Scholar]
- Liu C. C., Steffen M., King F., Young J. D. Identification, isolation, and characterization of a novel cytotoxin in murine cytolytic lymphocytes. Cell. 1987 Nov 6;51(3):393–403. doi: 10.1016/0092-8674(87)90635-0. [DOI] [PubMed] [Google Scholar]
- Lobe C. G., Finlay B. B., Paranchych W., Paetkau V. H., Bleackley R. C. Novel serine proteases encoded by two cytotoxic T lymphocyte-specific genes. Science. 1986 May 16;232(4752):858–861. doi: 10.1126/science.3518058. [DOI] [PubMed] [Google Scholar]
- Lobe C. G., Havele C., Bleackley R. C. Cloning of two genes that are specifically expressed in activated cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1448–1452. doi: 10.1073/pnas.83.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D., Nabholz M., Estrade C., Tschopp J. Granules of cytolytic T-lymphocytes contain two serine esterases. EMBO J. 1986 Jul;5(7):1595–1600. doi: 10.1002/j.1460-2075.1986.tb04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D., Tschopp J. A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell. 1987 Jun 5;49(5):679–685. doi: 10.1016/0092-8674(87)90544-7. [DOI] [PubMed] [Google Scholar]
- Masson D., Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T-lymphocytes. J Biol Chem. 1985 Aug 5;260(16):9069–9072. [PubMed] [Google Scholar]
- Millard P. J., Henkart M. P., Reynolds C. W., Henkart P. A. Purification and properties of cytoplasmic granules from cytotoxic rat LGL tumors. J Immunol. 1984 Jun;132(6):3197–3204. [PubMed] [Google Scholar]
- Ostergaard H. L., Kane K. P., Mescher M. F., Clark W. R. Cytotoxic T lymphocyte mediated lysis without release of serine esterase. Nature. 1987 Nov 5;330(6143):71–72. doi: 10.1038/330071a0. [DOI] [PubMed] [Google Scholar]
- Pasternack M. S., Eisen H. N. A novel serine esterase expressed by cytotoxic T lymphocytes. 1985 Apr 25-May 1Nature. 314(6013):743–745. doi: 10.1038/314743a0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Konigsberg P. J. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984 Sep 1;160(3):695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. H., Masakowski V., Rucinsky T., Phillips G. Mechanisms of immune lysis. III. Characterization of the nature and kinetics of the cytotoxic T lymphocyte-induced nuclear lesion in the target. J Immunol. 1982 May;128(5):2087–2094. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D. S., Tite J. P., Ruddle N. H. DNA fragmentation: manifestation of target cell destruction mediated by cytotoxic T-cell lines, lymphotoxin-secreting helper T-cell clones, and cell-free lymphotoxin-containing supernatant. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1881–1885. doi: 10.1073/pnas.83.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J., Weissmann C. Induction of mRNA for a serine protease and a beta-thromboglobulin-like protein in mitogen-stimulated human leukocytes. J Immunol. 1987 Jul 1;139(1):250–256. [PubMed] [Google Scholar]
- Simon M. M., Hoschützky H., Fruth U., Simon H. G., Kramer M. D. Purification and characterization of a T cell specific serine proteinase (TSP-1) from cloned cytolytic T lymphocytes. EMBO J. 1986 Dec 1;5(12):3267–3274. doi: 10.1002/j.1460-2075.1986.tb04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenn G., Takayama H., Sitkovsky M. V. Exocytosis of cytolytic granules may not be required for target cell lysis by cytotoxic T-lymphocytes. Nature. 1987 Nov 5;330(6143):72–74. doi: 10.1038/330072a0. [DOI] [PubMed] [Google Scholar]
- Ucker D. S. Cytotoxic T lymphocytes and glucocorticoids activate an endogenous suicide process in target cells. Nature. 1987 May 7;327(6117):62–64. doi: 10.1038/327062a0. [DOI] [PubMed] [Google Scholar]
- Wright S. C., Bonavida B. Studies on the mechanism of natural killer (NK) cell-mediated cytotoxicity (CMC). I. Release of cytotoxic factors specific for NK-sensitive target cells (NKCF) during co-culture of NK effector cells with NK target cells. J Immunol. 1982 Jul;129(1):433–439. [PubMed] [Google Scholar]
- Yannelli J. R., Sullivan J. A., Mandell G. L., Engelhard V. H. Reorientation and fusion of cytotoxic T lymphocyte granules after interaction with target cells as determined by high resolution cinemicrography. J Immunol. 1986 Jan;136(2):377–382. [PubMed] [Google Scholar]
- Young J. D., Clark W. R., Liu C. C., Cohn Z. A. A calcium- and perforin-independent pathway of killing mediated by murine cytolytic lymphocytes. J Exp Med. 1987 Dec 1;166(6):1894–1899. doi: 10.1084/jem.166.6.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A. Role of granule proteins in lymphocyte-mediated killing. J Cell Biochem. 1986;32(2):151–167. doi: 10.1002/jcb.240320207. [DOI] [PubMed] [Google Scholar]
- Young J. D., Hengartner H., Podack E. R., Cohn Z. A. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer activity. Cell. 1986 Mar 28;44(6):849–859. doi: 10.1016/0092-8674(86)90007-3. [DOI] [PubMed] [Google Scholar]
- Young J. D., Leong L. G., Liu C. C., Damiano A., Cohn Z. A. Extracellular release of lymphocyte cytolytic pore-forming protein (perforin) after ionophore stimulation. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5668–5672. doi: 10.1073/pnas.83.15.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Leong L. G., Liu C. C., Damiano A., Wall D. A., Cohn Z. A. Isolation and characterization of a serine esterase from cytolytic T cell granules. Cell. 1986 Oct 24;47(2):183–194. doi: 10.1016/0092-8674(86)90441-1. [DOI] [PubMed] [Google Scholar]
- Young J. D., Nathan C. F., Podack E. R., Palladino M. A., Cohn Z. A. Functional channel formation associated with cytotoxic T-cell granules. Proc Natl Acad Sci U S A. 1986 Jan;83(1):150–154. doi: 10.1073/pnas.83.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Podack E. R., Cohn Z. A. Properties of a purified pore-forming protein (perforin 1) isolated from H-2-restricted cytotoxic T cell granules. J Exp Med. 1986 Jul 1;164(1):144–155. doi: 10.1084/jem.164.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]