Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV), the most recently identified member of the herpesvirus family, infects a variety of target cells in vitro and in vivo. This minireview surveys current information on the early events of KSHV infection, including virus-receptor interactions, involved envelope glycoproteins, mode of entry, intracellular trafficking, and initial viral and host gene expression programs. We describe data supporting the hypothesis that KSHV manipulates preexisting host cell signaling pathways to allow successful infection. The various signaling events triggered by infection, and their potential roles in the different stages of infection and disease pathogenesis, are summarized.
Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) is a gamma-2-lymphotropic oncogenic herpesvirus implicated in the pathogenesis of neoplasm of two distinct cell types, the endothelial cell-based Kaposi's sarcoma (KS) and B-cell primary effusion lymphoma (PEL) and the body cavity-based B-cell lymphoma (BCBL) and multicentric Castleman's disease (MCD) (13, 16). KSHV's ∼160-kb genome shows homologies with gamma-1-herpesvirus Epstein-Barr virus (EBV) and gamma-2-herpesvirus saimiri (HVS), and of KSHV's >90 open reading frames (ORFs), ORF4 to ORF75 are so designated by their homology to HVS ORFs (16, 38, 46). The genome contains gene blocks conserved with other herpesviruses, as well as divergent regions encoding more than 20 KSHV unique genes (K genes). Several KSHV-encoded proteins are homologs of host proteins with immunomodulatory, antiapoptotic, signal induction, transcriptional regulation, and other functions (16, 38, 46).
Entry into target cells by herpesviruses is a multistep complex process involving series of temporal interactions between multiple host cell surface molecules (that may vary according to cell type) with multiple viral envelope glycoproteins. Binding to the cell surface receptors is followed by penetration into the cytosol, either by direct fusion of the viral envelope with the plasma membrane or by internalization and transport in the cytoplasm by endocytosis and fusion of the viral envelope with the endosomal membranes. Viral capsid released in the cytoplasm is transported to the nuclear periphery, where disassembly of capsid and release of viral genome via the nuclear pore into the nucleus occurs (Fig. 1). Even though several host receptors have been identified for human and animal herpesviruses, how these interactions with cell surfaces facilitate the various subsequent steps of successful infection is not fully understood. Similarly, though several advances have been made in our understanding of the KSHV genome, gene functions, latency, and potential immune evasion strategies, information regarding early events of KSHV infection of target cells is somewhat limited.
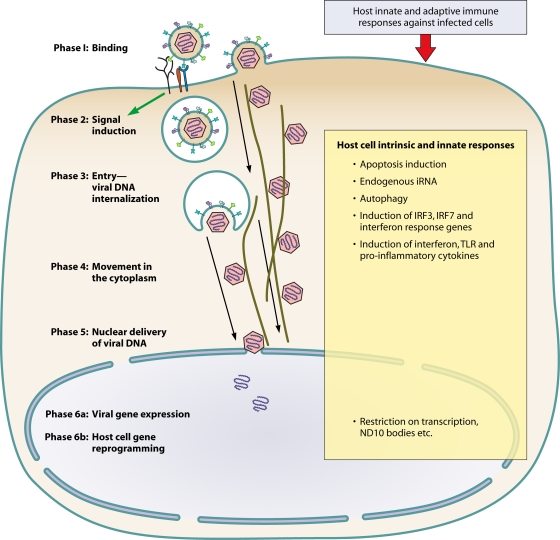
FIG. 1.
Model illustrating the different phases of early events of KSHV infection of target cells and the obstacles encountered by the virus. iRNA, interfering RNA.
OBSTACLES ENCOUNTERED BY KSHV DURING TARGET CELL INFECTION
During infection, similar to other viruses, KSHV needs to overcome several formidable obstacles imposed by the host cells (Fig. 1). The first obstacle is the recognition of and binding to the appropriate surface receptors that are distributed over a large area of the target cell (which is colossal compared to the petite size of the virus) in an environment of rapid movement of extracellular fluids. Second, though virus binding with the receptor(s) could occur at lower temperatures, energy is needed for the various phases of infection shown in Fig. 1. Third, for the tiny viral particles, the crowded, highly packed cytoplasm creates a difficult challenge for trafficking. Additional obstacles include apoptosis triggered by the engagement of multiple receptors during virus binding and entry, induction of various intrinsic, innate and adaptive immune responses (including interfering RNA, toll-like receptors (TLR), inflammasome, interferons [IFNs], etc.), autophagy, and restriction on virus gene transcription in the quiescent cells, including p53, ND10 bodies, etc. (Fig. 1). These obstacles need to be counteracted rapidly in a sustained manner not only during early times of infection but also throughout the duration of infection and during latency. Besides using its gene products, due to its limited genome size, KSHV must have evolved to use host cell molecules to overcome these obstacles. The take-home message from the available evidence discussed here is that KSHV manipulates the host cell's preexisting signal pathways via its interactions with cell surface receptors early during infection as one of the best strategies to overcome several obstacles and to create an environment that is conducive to infection.
For conceptual purposes, early events of KSHV infection are discussed as six overlapping dynamic phases (Fig. 1, 2, and 3). Phase 1 involves the binding of viral envelope glycoproteins to cell surface receptors overlapping with the induction of host cell signal pathways (phase 2). This is followed by virus entry (phase 3), movement of the viral capsid/tegument in the cytoplasm (phase 4), nuclear entry of the viral genome (phase 5), and the overlapping expression of viral genes (phase 6a) and host cell genes (phase 6b). To differentiate among and identify the phase(s) in which KSHV-induced host signal molecules play roles, an assortment of methods have been used (Fig. 2). Phases 1, 3, 4, 5, and 6 are discussed first, followed by signaling events (phase 2) triggered by infection and their potential roles in different phases of KSHV infection.
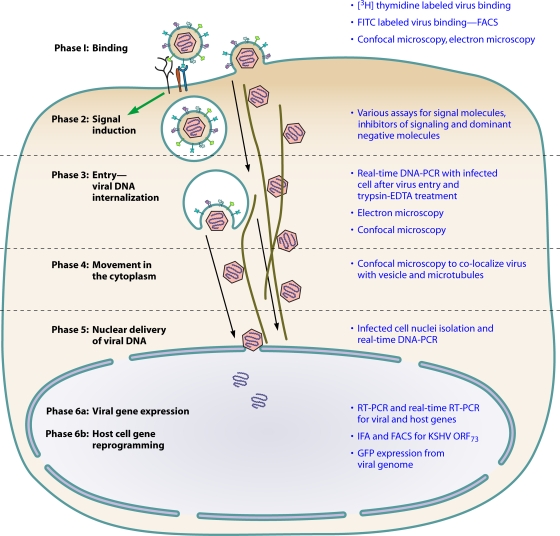
FIG. 2.
Model illustrating the various methods used to analyze the different phases of early events of KSHV infection of in vitro target cells. FITC, fluorescein isothiocyanate; FACS, fluorescence-activated cell sorter; IFA, immunofluorescence assay.
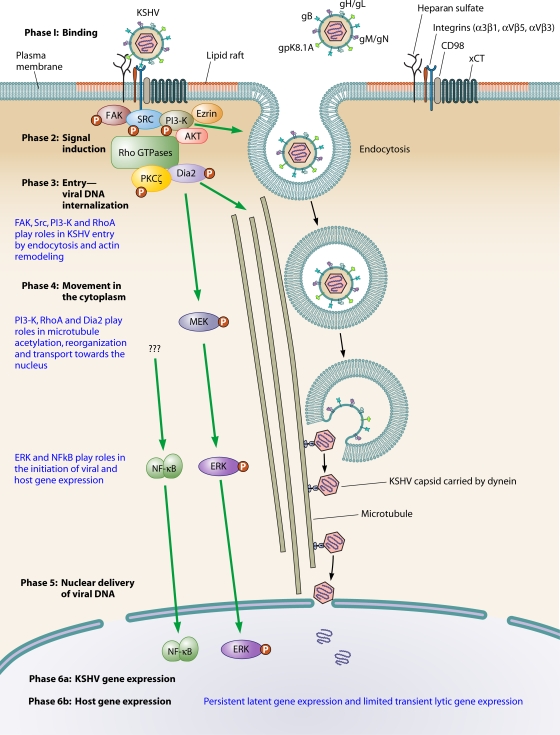
FIG. 3.
Model illustrating the dynamic overlapping phases of early events of KSHV infection in endothelial and fibroblast cells and KSHV-induced signal pathways and their role in infection. In phase 1, KSHV infection is initiated by binding to the cell surface via interactions with heparan sulfate (HS) followed by temporal interactions with αVβ5, α3β1, and αVβ3 integrins and xCT (CD98) molecules which overlap with the induction of host cell preexisting signal molecules (phase 2) that play roles in actin remodeling, the formation of endocytic vesicles, virus entry, movement in the cytoplasm, delivery into the nucleus, and viral and host gene expression. KSHV interactions with cell surface integrins leads to autophosphorylation of FAK at tyrosine 397, which creates a binding site for the SH2 domain of Src family kinases and leads to the subsequent phosphorylation of PI3-K and Rho GTPases. FAK, Src, PI-3K, and Rho GTPases play roles in KSHV entry. In the absence of FAK, Pyk2 is induced to compensate for the function of FAK. In phase 3, overlapping with phase 2, the virus enters the cells by endocytosis, and in phase 4, viral capsid/tegument moves in the cytoplasm. RhoA activates Dia2, which in turn augments Src activation, all of which are probably essential for the formation of endocytic vesicles and their movement in the cytoplasm. Capsid is released from the endocytic vesicles via fusion of the viral envelope with the endocytic vesicles. The role of signal pathways in the acidification of endosomes and changes in viral envelope architecture are poorly understood. RhoA GTPase facilitates the transport of capsid toward the nucleus by inducing MT stabilization and regulating MT dynamics via Dia2. The endocytic vesicles with virus or released capsid/tegument complexes bind to dynein motor components, transported along the MT to reach the nuclear vicinity, and deliver the viral DNA into the nucleus. RhoA GTPases facilitate the transport of the capsid toward the nucleus by inducing microtubule stabilization and regulating microtubule dynamics. KSHV-induced ERK and NF-κB play roles in the modulation of both viral and host gene expression and to overcome the host restriction on transcription. Nuclear delivery of the KSHV genome into the infected cell nucleus is followed by simultaneous induction of viral gene expression (phase 6a) and host gene expression (phase 6b). The overlapping viral gene-induced host cell gene expression may exert an influence on subsequent viral and host gene expression. These studies demonstrate that KSHV interactions with host cell receptors play vital roles in manipulating the host cell signaling pathway to create a conducive intracellular environment for the establishment of a successful infection. The arrows indicate the stage of KSHV infection in which the induced signal pathways are shown to play roles.
KSHV INFECTS MULTIPLE TARGET CELLS IN VIVO AND IN VITRO
Detection of KSHV DNA and transcripts in CD19+ peripheral blood B cells, endothelial cells, CD45+/CD68+ monocytes, keratinocytes, and epithelial cells suggests a broad in vivo cellular tropism of KSHV (16). KSHV DNA is present in a latent form in the vascular endothelial and spindle cells of KS tissues and latency-associated LANA-1 (ORF73), vCyclin D (ORF72), vFLIP (K13), and kaposin (K12) genes are expressed in these cells (16). KSHV lytic cycle K5, which downregulates a variety of cell surface molecules, such as major histocompatibility complex (MHC) class IA and IC, ICAM-1, and PE-CAM, has also been detected in KS endothelial/spindle cells. Lytic infection is also detected in <1% of infiltrating inflammatory monocytic cells of KS lesions (16). Unfortunately, the endothelial cell line carrying KSHV has not been established from KS lesions, since KS cells grow poorly in cell culture and viral DNA is lost within a few passages (16).
Human B cells and monocytes appear to be the major reservoir of latent infection, and cell lines with B-cell characteristics, such as BC-1, BC-3, BCBL-1, HBL-6, and JSC, have been established from BCBL tumors (16). BC-1, HBL-6, and JSC cells carry multiple copies of KSHV and EBV genomes in a latent state, and BCBL-1 and BC-3 cells carry only the KSHV genome. Gene expression in PEL cells is limited to the latency locus, and KSHV expresses LANA-1, vCyclin, vFLIP, kaposin, and ORF10.5 (LANA-2) genes as well as 12 microRNAs (9, 16). Similar to EBV-carrying B-cell lines, about 1 to 3% of PEL cells spontaneously enter the lytic cycle, and viruses induced from these cells by chemicals serve as the source of virus for various studies.
In vitro, KSHV has been shown to infect human B, endothelial, epithelial, and fibroblast cells, CD34+ stem cell precursors of dendritic cells, and monocytes (16). KSHV also infects monkey kidney cells, BHK-21 cells, CHO cells, and mouse fibroblast cells (1-4, 7, 8, 12, 16, 17, 23, 25, 27, 29, 30, 31, 35, 39, 43-45, 47, 53, 56, 57). In vitro infection by alpha- and betaherpesviruses results in the rapid initiation of the lytic cycle, virus progeny formation, and cell death. This is not unexpected, since the majority of cells used in these studies do not represent the in vivo target cells of latency. In contrast, in vitro infection of human primary B cells by gamma-1-EBV results in the establishment of latent infection, transformation, and a B-lymphoblastoid cell line. Primary nonstimulated B cells show poor infection with KSHV, and this infection does not lead to immortalization. Instead, a lytic replication is reported for activated B cells (44). Interestingly, in vitro KSHV infection of adherent target cells does not result in a productive lytic cycle (16). Instead, KSHV infection of human endothelial cells (human microvascular dermal cells [HMVEC-d]), human umbilical vein endothelial cells (HUVEC), human foreskin fibroblasts (HFF), human endothelial cells immortalized by telomerase (TIME), and human embryonic kidney epithelial cells (HEK 293 cells) results in the expression of latency-associated genes, thus providing a reasonable model for studying latency in vitro (7, 16, 29, 31, 47, 49, 57). Lytic replication can be induced from these cells by chemicals or by the lytic switch KSHV ORF50 (RTA) protein (7, 16, 29, 31, 57).
KSHV ENVELOPE GLYCOPROTEINS
Besides encoding the gB (ORF8), gH (ORF22), gL (ORF47), gM (ORF39) and gN (ORF53) glycoproteins that are conserved among the herpesviruses (16, 38, 46), KSHV also encodes the unique lytic cycle-associated glycoproteins ORF4, gpK8.1A, gpK8.1B, K1, K14, and K15 (16, 38, 46). Among these, ORF4, gB, gH/gL, gM/gN, and gpK8.1A are associated with KSHV virion envelopes (2, 5, 8, 12, 28, 34, 54, 58). KSHV gB synthesized as a 110-kDa precursor undergoes cleavage and processing, and the envelope-associated form consists of disulfide-linked multimers of 75- and 54-kDa polypeptides with high levels of mannose and complex sugars (2, 5, 55). As in other herpesviruses, the 120-kDa KSHV glycoprotein gH forms a noncovalent complex with the 41- to 42-kDa glycoprotein gL (21, 34), while the N-glycosylated KSHV glycoproteins gM and gN form heterodimers (28). The KSHV gpK8.1 gene codes for 228-aa-long gpK8.1A and for 167-aa-long gpK8.1B, which is generated by an in-frame deletion of 61 amino acids (aa) from gpK8.1A. Both gpK8.1A and gpK8.1B contain N- and O-linked sugars, and the 68- to 72-kDa gpK8.1A is the predominant form detected in the virion envelopes (12, 58).
KSHV BINDING TO THE TARGET CELLS (PHASE 1)
When analyzing herpesvirus interactions with target cells, the common readouts used are viral gene expression or expression of the indicator gene from the viral genome, which occurs after viral entry into the infected cell nuclei. However, when studying agents blocking infection, since the downstream effects of agents blocking the entry or transport or signaling could also block the cellular and viral transcription, it is critical to determine whether the blockage is due to interference at the binding stage or the entry stage, during transport in the cytoplasm or viral DNA delivery into the nucleus, or at the transcription level. Hence, various methods have been designed to evaluate the different phases of KSHV infection (Fig. 2).
Heparan sulfate.
KSHV's broad in vitro cellular tropism may be in part due to its interactions with the ubiquitous cell surface heparan sulfate (HS) proteoglycan (3, 8), and this interaction with HS is similar to that of several other herpesviruses (Table 1). KSHV infection is inhibited by soluble heparin but not by chondroitin sulfates A and C (3). Due to the low level of expression of Ext1, a key glycosyltransferase enzyme in HS biosynthesis, HS is not expressed in appreciable levels in many B-cell lines and in primary B cells (25). The refraction of primary B cells for KSHV infection may be due to the lack of HS as the expression of HS in BJAB cells (an EBV-negative B-cell line) increased the binding of KSHV to the cell surface (25). Interestingly, BJAB cells from one laboratory did not express any HS, while cells from another laboratory expressed abundant HS, which could explain some discrepancies reported for the infection of BJAB cells (25). KSHV gB, gpK8.1A, ORF4, and gH bind to cell surface HS molecules (2, 8, 21, 33, 54, 55). Binding of soluble forms of gB and gpK8.1A is saturable and can be blocked by soluble heparin (8, 54, 55). KSHV gpK8.1A binds to heparin with an affinity comparable to that of gB and gC of herpes simplex virus (8). KSHV's possession of four HS binding proteins reemphasizes the importance of cell surface HS for attachment of many herpesviruses. KSHV interactions with HS may be the first set of ligand-receptor interactions that concentrate virus on the adherent human endothelial, epithelial, and fibroblast cells. This is not surprising, as HS with its highly branched sugar chains is probably one of the longest molecules in the cell surfaces, and binding by charge interactions with HS probably provides the initial first foothold (surfing) for KSHV in the rapid fluid movement prevalent in the extracellular environment, thus overcoming the first obstacle.
TABLE 1.
KSHV binding and entry in the various in vitro target cells
| Cell type | Binding receptor(s) | Entry receptor(s) | Mode of entry |
|---|---|---|---|
| HFF | HS | α3β1, αVβ3, αVβ5, xCT/CD98 | Endocytosis (major pathway; clathrin mediated) |
| HMVEC-d | HS | α3β1, αVβ3, αVβ5, xCT/CD98 | Endocytosis (major pathway; actin-dependent macropinocytosis) |
| HUVEC | HS | α3β1, αVβ3, αVβ5, xCT/CD98 | Endocytosis (major pathway; actin-dependent macropinocytosis and/or clathrin mediated) |
| HEK 293 | HS | α3β1, αVβ3, αVβ5, xCT/CD98 | Endocytosis |
| Monocytesa | HS, DC-SIGN | Unknown | Endocytosis |
| B cells | HS, DC-SIGN | Unknown | Endocytosis |
| Keratinocytes | Unknown | Unknown | Unknown |
Activated macrophages.
DC-SIGN.
Dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN; CD209) is reported to be used by many viruses to adhere to the target cells. Similarly, DC-SIGN appears to be utilized by KSHV during infection of human myeloid dendritic cells (DCs), macrophages, and activated B cells (43, 44). KSHV binding and infection were blocked by anti-DC-SIGN monoclonal antibody, by mannan (a natural ligand for DC-SIGN), and by soluble DC-SIGN (43, 44). Pretreatment of cells with anti-DC-SIGN antibodies did not completely block KSHV binding and infection, a result which could be due to binding to HS and/or other receptors that was not tested in these studies. Whether activation of B cells also leads to increased expression of HS and other KSHV receptors is not known. The identity of the KSHV envelope glycoprotein(s) interacting with DC-SIGN is not known, and KSHV gB, with its high mannose level, is a potential candidate.
Integrins.
Among all the gB homologs of human herpesviruses sequenced to date, only KSHV-gB possesses an integrin-binding RGD (Arg-Gly-Asp) motif at amino acids 27 to 29. The RGD motif is the minimal peptide region of many extracellular matrix (ECM) proteins known to interact with subsets of host cell surface integrins. Several studies demonstrate that α3β1, αVβ3, and αVβ5 integrins play important roles in KSHV infection. A reduction (about 50%) in KSHV infection of HMVEC-d and HFF cells by RGD peptides, antibodies against RGD-gB peptide (RGDTFQTSSSPTPPGSSS) and fibronectin demonstrated a role for integrins in KSHV infection (4) The suggestion that α3β1 integrin is utilized by KSHV for HMVEC-d and HFF cell infection came from evidence such as the 30 to 50% reduction in infection by pretreating cells with function-blocking anti-α3 and -β1 antibodies and by mixing virus with soluble α3β1 integrin before infection, and the immunoprecipitation of virus-α3 and -β1 complexes by anti-KSHV gB antibodies (4). Though KSHV entry and infection increased in CHO cells expressing human α3 integrin (which forms a complex with hamster β1), this increase did not reach the level of infection seen with endothelial and fibroblast cells, suggesting that there are other receptors that are critical for entry (4, 35).
Subsequent studies demonstrate that αVβ3 and αVβ5 integrins also play roles in KSHV entry of adherent target cells (53) (Table 1; Fig. 3). KSHV-integrin interactions was also demonstrated by KSHV gB-mediated adhesion of HMVEC-d, HFF cells, CV-1 cells, and human fibrosarcoma cell line HT-1080, which was inhibited by anti-αV and -β1 integrin antibodies (53). Variable levels of neutralization of HMVEC-d and HFF cell infection were observed with anti-αVβ3 and -αVβ5 antibodies (53) Similarly, variable levels of inhibition of KSHV entry into adherent human (HMVEC-d, HFF, 293) and monkey kidney (Vero) cells was observed by soluble α3β1, αVβ3, and αVβ5 integrins. Though a cumulative inhibition was observed with combinations of integrins, a complete block in infection was not achieved, which may be due to KSHV's first interaction with HS. The role of integrin in KSHV infection of B cells, monocytes, keratinocytes, and other cells has not been studied (Table 1).
xCT.
The 12-transmembrane glutamate/cysteine exchange transporter protein xCT is reported to be a fusion entry receptor in adherent cells (27). The xCT molecule is part of the cell surface 125-kDa disulfide-linked heterodimeric membrane glycoprotein CD98 (4F2 antigen) complex containing a common glycosylated heavy chain (80 kDa) and a group of 45-kDa light chains; the xCT molecule is one of the light chains (14, 15, 26, 53). Nonsusceptible adherent target cells become susceptible by the expression of recombinant xCT (27). As yet, the identities of KSHV envelope glycoprotein(s) interacting with xCT molecule are not characterized (Table 1). Similarly, the role of xCT in KSHV infection of other target cells and whether xCT is also involved in fusion of the KSHV envelope with endocytic vesicles need to be elucidated.
CD98, a multifunctional protein involved in amino acid transport, cell adhesion, fusion, proliferation, and integrin activation, was initially identified as a molecule associated with integrin α3. It is interesting to note that CD98 and integrin α3 were named fusion regulation protein 1 (FRP-1) and FRP-2, respectively, as this interaction was shown to play important roles in cell-cell fusion and virus-induced cell fusion (14, 15, 26, 53). CD98 has also been shown to be involved in membrane clustering, β1 integrin-mediated signaling events, and stimulation of α3β1-dependent adhesion of cells and the signal transduction cascade of αVβ3 integrin (14, 15, 26, 53).
Incubation of virus with HMVEC-d cells followed by immunoprecipitation with anti-integrin antibodies and Western blots for CD98 and xCT revealed that αVβ5 interaction with CD98/xCT occurred predominantly within 1 min postinfection (p.i.) and dissociated by 10 min p.i. (53). The α3β1-CD98/xCT interaction was maximal at 10 min p.i. and dissociated by 30 min p.i., while the αVβ3-CD98/xCT interaction was maximal at 10 min p.i. and remained essentially unchanged at 30 min p.i. Immunofluorescence colocalization studies with purified KSHV also confirmed these data (53). Preincubation of KSHV with soluble heparin and α3β1 significantly inhibited the association with CD98/xCT, thus suggesting that KSHV's first contact occurs with HS and that integrins are essential elements in subsequent CD98-xCT interactions. These studies demonstrated temporal interactions of KSHV with a family of functionally related proteins, such as HS, integrins, and CD98-xCT molecules, in endothelial, epithelial, and fibroblast cells (53). The biological relevance of such temporal interactions and the involved KSHV glycoproteins needs to be assessed further (Table 1; Fig. 3).
Although the role of α3β1 in KSHV infection was uncertain in two studies (17, 23), methodological differences could explain these discrepancies. For example, using an RTA-dependent reporter 293 T-cell line, Inoue et al. (23) reported the inability of soluble α3β1 integrin and RGD peptides to block KSHV infectivity. However, the validity of this observation is questionable, since when cells pretreated with RGD peptide were infected, or when cells were infected with a virus-integrin mixture, centrifugation and Polybrene were used in these studies (23). Both centrifugation and Polybrene enhance virus infection without the need to interact with specific receptors. It is a well-known fact that Polybrene forming a complex with the viral envelope is used for gene delivery into various target cells to bypass the need for specific receptors.
In another study, a 15-mer AHSRGDTFQTSSGCG peptide of KSHV gB-mediated HT1080 adhesion, which was blocked by αVβ3 and αVβ5 antibodies and peptide-bound beads, detected only αVβ3 integrin (17). The GCG amino acids in the peptide used in this study are not present in KSHV gB sequence and may potentially give rise to dimers and multimers due to the cysteine residue (17). Though HT1080 cell infection was inhibited by anti-αVβ3 antibodies, anti-α3β1 and -αVβ5 antibodies were not tested (17). Mouse keratinocytes lacking α3β1 were infectible with KSHV, and expression of human α3 resulted in only a 55% level of infection in these cells. Even though the level of αVβ3 in these cells and the ability of anti-αVβ3 to block KSHV infection were not tested, it was concluded that α3β1 expression must have a dominant negative effect on αVβ3 integrin. Since the dominant effect of α3β1 has been demonstrated only after specific ligand binding to α3β1, it is not clear whether virus binding to α3β1 generates such an effect, which itself is an interesting area to study. The discrepancies of the usage of different integrins were expected, since it is common for different cells to express different combinations of integrins, and a specific integrin could be one of the receptors in some and not in all target cells of KSHV. Since herpesvirus-cell receptor interactions are temporarily coordinated events mediated by interactions of viral glycoproteins with one receptor leading to conformational changes of viral glycoproteins and allowing for interaction with the next receptor(s), the detection of α3 and β1 in HFF or HMVEC-d incubated with virus or purified gB (4, 53) could represent the event occurring during virus-host cell interactions under physiological conditions.
Further studies are required to determine the role of the additional receptor(s) in different target cells of KSHV (Table 1; Fig. 3).
KSHV ENTRY INTO THE TARGET CELLS (PHASE 3)
KSHV binds and enters a variety of human (BCBL-1, BJAB, Raji, 293, HFF, HeLa, endothelial), monkey (Vero, CV-1), hamster (BHK-21, CHO), and mouse (Du17) cells, as shown by the detection of viral DNA, limited KSHV gene expression, and green fluorescent protein (GFP) expression (Fig. 2) (1-4, 7, 18, 23, 25, 27, 29-31, 34, 35, 41-45). KSHV DNA is internalized rapidly in adherent cells, reaching a peak by 60 min p.i. (29). Various evidence demonstrates that KSHV utilizes endocytosis as the predominant method of entry into B cells (3, 42), monocytes (43), fibroblast cells (HFF) (1), epithelial cells (293 cells) (23), and endothelial cells (HMVEC-d and HUVEC) (18, 41). Electron microscopy revealed KSHV virions in large endocytic vesicles within 5 min of HMVEC-d and HFF cell infection, and fusion of the virion envelope with endocytic vesicles was also observed (1, 41). Viral capsids were detected near the vicinity of the nuclear membrane by 15 min p.i., and anti-KSHV envelope gB and gpK8.1A antibodies colocalized with virus-containing endocytic vesicles (1, 18, 41) (Fig. 2).
It is reasonable to assume that KSHV uses endocytosis for its entry, as endosomes offer a convenient and often rapid system of transit across the plasma membrane and through the obstacles of crowded cytoplasm.
Use of various chemical inhibitors suggests that KSHV utilizes different modes of endocytic entry in fibroblast and endothelial cells. In HFF cells, KSHV DNA internalization (entry) is significantly inhibited by preincubating cells with chlorpromazine blocking clathrin-coated pits, but not by nystatin and cholera toxin B blocking endocytosis via caveolae and by dissociation of lipid rafts, respectively. KSHV gene expression (infection) is also inhibited by blocking the acidification of endosomes by NH4Cl and bafilomycin A in HFF and 293 cells without affecting virus entry (1, 23). In contrast, chlorpromazine and filipin (caveolar endocytosis inhibitor) did not have any effect on KSHV binding, entry, or gene expression in HMVEC-d and HUVEC (41). KSHV entry and gene expression in both types of endothelial cells were significantly blocked by macropinocytosis inhibitors EIPA and rottlerin and by cytochalasin D affecting actin polymerization (41). KSHV induces the actin polymerization and formation of lamellipodial extensions that are essential for endocytosis (18, 35, 41). Inhibition of lipid rafts blocked the viral gene expression in HMVEC-d but not in HUVEC or HFF cells (41, 42). Internalized KSHV in HMVEC-d and HUVEC colocalized with the macropinocytosis marker dextran and not with the clathrin pathway marker transferrin or with caveolin (41). Dynasore, an inhibitor of dynamin, did not block viral entry into endothelial cells but did inhibit entry into HFF cells (41) (Fig. 2). KSHV was not associated with the early endosome marker EEA-1 in HMVEC-d but was associated with the late endosome marker LAMP1, as well as with Rab34 GTPase, a known regulator of macropinocytosis (41). Silencing Rab34 with small interfering RNA dramatically inhibited KSHV gene expression, as did bafilomycin-mediated disruption of endosomal acidification (41). These studies suggested that KSHV utilizes the actin polymerization-dependent, dynamin-independent macropinocytic pathway involving Rab34 GTPase-dependent late endosome and low-pH environment for its infectious entry into HMVEC-d and HUVEC (Table 1).
Anti-gB, -gH, -gL, and -gpK8.1A antibodies neutralize KSHV infection without affecting virus binding to the target cells (2, 34, 54, 55). This suggests that these glycoproteins play critical roles in the entry process, and it seems likely that the minimal fusion machinery of KSHV comprises gB, gH, and gL. Further work is required to determine the role of KSHV glycoproteins in the entry and fusion steps of infection.
KSHV MOVEMENT IN THE CYTOPLASM AND DELIVERY OF DNA INTO THE NUCLEUS (PHASES 4 AND 5)
KSHV-DNA accumulates rapidly in the infected HFF cell, HMVEC-d, and HUVEC nuclei, reaching a peak by about 90 min p.i. (29, 35, 37, 41, 52). Microtubules (MT) play important roles in KSHV infection, since depolymerization of MT, even though it did not affect KSHV binding and internalization, inhibited the nuclear delivery of viral DNA and infection in HFF cells, HMVEC-d, and HUVEC (37, 41, 42). Similar to HSV-1, KSHV also utilizes dynein motors to reach the vicinity of the nucleus (37). Overexpression of p50/dynamitin and sodium orthovanadate (Na3VO4), a well-described inhibitor of dynein activity, significantly reduced the infected cell nuclei-associated KSHV DNA (37). KSHV proteins involved in the interaction of dynein motors need to be evaluated further.
KSHV GENE EXPRESSION DURING PRIMARY INFECTION OF ADHERENT TARGET CELLS (PHASE 6A)
Quantitative real-time RT-PCR and a whole-genome array detected high levels of KSHV early lytic ORF50 (RTA) transcripts within 2 h p.i. of HMVEC-d and HFF cells, which declined sharply by 24 h p.i. (29). In contrast, comparatively low levels of latent ORF73 expression were detected within 2 h p.i., increased subsequently, maintained at a steady state and declined slowly by 5 days p.i. It is interesting that subsets of lytic transcripts with antiapoptotic and immune-modulation functions were also expressed soon after infection, and many of these transcripts could not be detected at later time points. Bechtel et al. (6) showed that 10 of the 29 RNAs detected in the above studies (29) encoding ORFs such as K8.1, K12, ORF58/59, and ORF54 were present in the purified virion particles. However, other detected transcripts (29) were absent, suggesting de novo transcription of the remaining lytic genes during the first hours of infection. Further examination revealed a steady quantitative increase in early lytic K5, K8, and v-IRF2 gene expression in the infected HMVEC-d and HFF cells (29, 47, 50).
When one examines the functions of the expressed KSHV lytic cycle genes, an interesting picture emerges. Since KSHV ORF50 (RTA) can activate ORF73 promoter, the initiation of latent gene expression must be mediated by ORF50. Since K5 has been shown to downregulate cell surface MHC class IA and IC, ICAM-1, B7-2, PE-CAM, and Cd1d molecules, infected cells may remain in a stealth state and could escape from the host CTL and NK cells (16). KSHV lytic vIRF-2 has profound inhibitory effects on alpha IFN (IFN-α) and IFN-β gene transcription (16). KSHV lytic genes K4 (vMIP-II), K6 (vMIP-I), and K2 (v-IL-6) were expressed briefly (29, 41, 50), and these proteins have been shown to possess antiapoptotic and immunomodulatory functions and to protect cells from IFN and NK cell action (16). KSHV lytic gene K7 and K6 proteins were also expressed briefly (29), and these proteins possess antiapoptotic functions (16). Thus, the expression of limited lytic proteins early during infection of HMVEC-d and HFF cells must serve to overcome host cell restriction on transcription as well as to provide a survival advantage for KSHV-infected cells and the necessary factors and time to establish and/or maintain latency during the initial phases of infection.
As is the case in cultures of KS lesion endothelial cells, in vitro KSHV latent infection in primary fibroblasts and endothelial cells is unstable. KSHV viral DNA is not efficiently maintained in the infected cells, and the proportion of infected cells decreases over time (7, 16, 20).
HOST GENE EXPRESSION IN THE INFECTED TARGET CELLS (PHASE 6B)
Use of oligonucleotide arrays to examine the modulation of HMVEC-d and HFF cell gene expression at 2 h and 4 h p.i. (36) demonstrated that KSHV reprogrammed host cell transcriptional machinery involved in regulating apoptosis, cell cycle regulation, signaling, inflammatory response, and angiogenesis (36). A subsequent cytokine array analysis showed that KSHV infection induced in an NF-κB-dependent manner significant secretion of several endothelial cell angiogenic molecules (angiogenin, vascular endothelial growth factor [VEGF], angiopoietin, and SDF-1), growth factors (platelet-derived growth factor [PDGF], fibroblast growth factor [FGF], and granulocyte-macrophage colony-stimulating factor [GMCSF]), chemokines (MCP-2, macrophage inflammatory protein [MIP], and eotaxin), and proinflammatory COX-2/PGE2, interleukin-2 (IL-2), IL-3, IL-8, GRO, and IL-16 and anti-inflammatory IL-4, IL-5, and IL-15 cytokines (47). The cytokines and growth factors seen during KSHV primary infection of endothelial cells are strikingly similar to the factors detected in the KS lesions (16, 36, 47).
KSHV INDUCES HOST CELL PREEXISTING SIGNAL MOLECULES EARLY DURING INFECTION THAT PLAY ROLES IN ENTRY AND ESTABLISHMENT OF INFECTION (PHASE 2)
Integrin interactions with extracellular matrix proteins (ligand) activate a variety of signaling molecules, such as focal adhesion kinase (FAK), c-Src, p130CAS, phospholipase C-γ, phosphatidylinositol 3-kinase (PI3-K), PKC, and cytosolic proteins, such as talin, vinculin, paxillin, and α-actinin, and assemble into focal adhesions (FA), thereby linking integrins to ECM proteins on the outside and the cytoplasmic actin cytoskeleton on the inside (10, 19, 48). These signaling events mediate several functions, such as activation of the cytoskeleton, endocytosis, gene expression, cell motility, attachment, the cell cycle, cell growth, apoptosis, and differentiation (10, 11, 19, 32, 48). During early times of infection, KSHV induces integrin-mediated FAK phosphorylation that is followed by the activation of a variety of focal adhesion-associated signal molecules, such as Src, PI-3K, Rho GTPases (RhoA, Rac, and Cdc42), and Diaphanous 2 (Dia2), as well as several downstream effector molecules, such as AKT, Ezrin, PKC-ζ, MEK, ERK1/2, NF-κB, and p38MAPK (4, 18, 30, 35, 37, 39, 41, 42, 47, 49, 50-53, 55-57) (Fig. 3).
When one asks why KSHV induces host cell preexisting signal pathways during binding and entry stages of infection, the probable answer is that these signaling pathways are most likely required in the different energy-dependent stages of viral infection (Fig. 3). When the available evidence linking signal induction with endocytosis and other cellular functions is examined, the above answer appears to be correct. After ligand interactions with receptors, associated kinases have been shown to be activated by autophosphorylation on tyrosine residues, leading to the recruitment of signal complexes to the plasma membrane, which then rapidly translocates to clathrin, caveolae, and other vesicles (10, 11, 19, 32, 48). Src-mediated tyrosine phosphorylation of clathrin is believed to regulate clathrin translocation to the plasma membrane, which is critical for its interactions with a number of other essential proteins, such as AP2, Eps15, and dynamin (10, 11, 19, 32, 48). Src-dependent phosphorylation also regulates dynamin self-assembly and ligand-induced endocytosis by releasing the internalized endocytic vesicles and also initiates the assembly of the plasma membrane-associated Ras activation complex. By interactions with Grb2, mSOS is recruited to the plasma membrane to activate Ras. Rho and Rab GTPases activated by PI-3K and Ras are critical for the formation of various types of endocytic vesicles and their movements as well as for microtubule and microfilament reorganization (10, 19, 22, 24, 40). Activated ERK1/2 has also been shown to be associated with endocytic vesicles. The interlink between signal pathways with endocytosis (11, 32, 48) together with the observation that anti-integrin antibodies and soluble integrins neutralize KSHV infection without affecting virus binding clearly indicate that KSHV could be manipulating the host cell signaling pathways for its advantage, and the studies discussed below support this suggestion.
KSHV-INDUCED FAK PLAYS ROLES IN VIRUS ENTRY INTO THE TARGET CELLS
Within minutes of infection of HMVEC-d, HFF, 293, and FAK+/+ mouse Du17 fibroblasts, KSHV induces integrin-mediated tyrosine phosphorylation of pp125 FAK (4, 30, 35, 42, 50, 51, 52, 55). Soluble gB also induces FAK autophosphorylation (51, 55), and KSHV-induced FAK colocalizes with vinculin, paxillin, Src, and RhoA in the infected cells (4, 51, 52, 55) (Fig. 3). Though KSHV binding to Du3 (FAK−/−) and Du17 (FAK+/+) was equal and could be inhibited by soluble heparin, the efficiency of DU3 cells was much lower (30, 35). Du3 cells showed an approximately 70% reduction in KSHV DNA internalization and expression of FAK in these cells via augmented viral DNA internalization, thus suggesting that FAK plays a critical role in KSHV entry. FAK dominant negative mutant FAK-related nonkinase (FRNK) expression in Du17 cells significantly reduced KSHV entry (30). Reduced virus entry in Du3 cells, delivery of viral DNA to the infected cell nuclei, and expression of KSHV genes suggested that in the absence of FAK, another molecule(s) may partially compensate for FAK function. KSHV indeed induced the phosphorylation of the Pyk2 molecule that compensates for some of the functions of FAK (30). Expression of an autophosphorylation mutant of Pyk2 reduced viral entry into Du3 cells (30).
Since FAK activation is central to many paradigms of outside-in signaling by integrins, actin assembly, and endocytosis, KSHV must have evolved to take advantage of these signaling pathways to promote entry and the subsequent steps of infection.
KSHV-INDUCED Src AND PI-3K PLAY ROLES IN VIRUS ENTRY INTO THE TARGET CELLS
The converging point of FAK and Pyk2 induction is the activation of Src kinases, which leads to the activation of PI-3K and Rho GTPases. The Src family of tyrosine kinases, including Src, Lyn, Fyn, Yes, Lck, Blk, and Hck, play critical roles in various signal transduction pathways. After the Src-SH2 domain binds to FAK-Tyr397, the activated Src kinases then phosphorylate a number of FA components. KSHV infection induces a robust Src response within minutes and colocalizes with FAK (52), and KSHV-gB also induced FAK-dependent Src phosphorylation in adherent target cells (51).
PI-3K consists of a p85 regulatory subunit and p110 catalytic subunit, and phosphorylation of specific tyrosine residues on the p85 subunit is an indication of PI-3K activation as it recruits substrates to the dimer, where they are phosphorylated by the p110 catalytic subunit. PI-3K is one of the important downstream effector molecules of FAK and Src activation, and KSHV infection induced PI-3K early during infection (35, 50). Src is an upstream mediator of PI-3K, since phosphorylation of Src by KSHV-gB was not affected when cells were incubated with nontoxic doses of the PI-3K inhibitors LY294002 and wortmannin. Src kinase-specific inhibitor SU6656 completely blocked KSHV gB-induced p85-PI-3K phosphorylation (51), thus demonstrating that PI-3K is downstream of Src. In FAK-null Du3 cells, no significant PI-3K p85 phosphorylation was stimulated by KSHV-gB, and in contrast, p85 phosphorylation was observed for FAK-wt Du17 cells (51).
These studies suggest a critical role for FAK and Src in the induction of PI-3K during KSHV infection (Fig. 3).
Involvement of Src in the KSHV entry process is shown by evidence such as the failure of KSHV to enter Src-negative mouse fibroblast cells (52), the increase in Src activity by lipid raft disruption, resulting in enhanced virus entry (42), and the RhoA GTPase-facilitated KSHV entry into adherent target cells in a Src-dependent manner (52). Similarly, the role of PI-3K in entry was shown by reduced viral entry by PI-3K inhibition (50).
KSHV-induced PI-3K was also essential for the induction of Rho GTPases (51, 52), suggesting that PI-3K must play an active role in KSHV entry via its role in the activation of Rac, Rho, Cdc42, and Rab5 GTPases, which are essential for actin reorganization, providing the mechanical force required for endosome formation and propulsion of endocytic vesicles and acting as a structural platform to stabilize the half-life of signaling molecules.
Induction of PI-3K involved in AKT activation very early during infection also indicates that this may also help in blocking apoptosis induced by viral binding and entry stages (Fig. 3).
KSHV-INDUCED RhoA GTPases PLAY ROLES IN VIRUS ENTRY, MICROTUBULE ACETYLATION, THICKENING OF MICROTUBULE BUNDLES, AND NUCLEAR DELIVERY OF VIRAL DNA
RhoA, Rac, and Cdc42 Rho GTPases are master regulators of several biological processes via a variety of signaling pathways, including cytoskeleton rearrangement and morphological changes (19, 22, 24, 40, 48). Immediately following infection, KSHV induces PI-3K Rho GTPase-dependent cytoskeletal rearrangements and the formation of structures such as filopodia (CDC42), lamellipodia (Rac), and stress fibers (RhoA) in the target cells (18, 35, 41, 53). Soluble gB induced the FAK-Src-PI-3K Rho GTPase signaling pathway and extensive cytoskeletal rearrangement in target cells (51). KSHV-induced RhoA colocalized with Src in the infected cells (51). Ezrin, which is required to cross-link the actin cytoskeleton with the plasma membrane and to induce the morphological changes, was also induced by KSHV via Rho GTPases (51) (Fig. 3).
The dominant negative form of RhoA GTPase and treatment of target cells with Clostridium difficile toxin B (CdTxB), a specific inactivator of Rho GTPases, significantly blocked KSHV entry. Inhibition of Src activation by CdTxB and reduction of RhoA activation by Src inhibitors suggest that KSHV-induced Src is involved in RhoA activation, which in turn is involved in a feedback-sustained activation of Src (52) (Fig. 3). Noticeable aggregation and thickening of microtubules (MT) in the infected cells was also observed (37, 42). KSHV capsids colocalized with the microtubules; this colocalization was abolished by the destabilization of microtubules with nocodazole and PI-3K inhibitor affecting the Rho GTPases (37, 42). The inactivation of Rho GTPases by CdTxB significantly reduced microtubular acetylation and, subsequently, the delivery of viral DNA to the nucleus (37, 52). Nuclear delivery of viral DNA was increased in cells expressing a constitutively active RhoA mutant and decreased in cells expressing a dominant negative mutant of RhoA (37, 52). Similarly, Escherichia coli cytotoxic necrotizing factor activating the Rho GTPases significantly augmented the nuclear delivery of KSHV DNA. Collectively, these studies suggest that KSHV induces Rho GTPases, modulates stabilization of microtubules, and promotes the rapid trafficking of viral capsids toward the nucleus (37, 42).
Rho GTPases activate formin family Diaphanous 1 and 2 molecules that form part of the signal transduction cascade, leading to rearrangement of the cytoskeleton (22, 24, 40). KSHV induces the activation of RhoA GTP-dependent Diaphanous 2 with no significant activation in Rac- and Cdc42-dependent PAK1/2 and stathmin molecules (37, 42). Dia2 coimmunoprecipitated and colocalized with activated Src in the infected cells, which were inhibited by Src inhibitors (52). Together with the reduced virus entry in RhoA dominant negative cells, these results suggest that activated RhoA-dependent Dia2 probably functions as a link between RhoA and Src in KSHV-infected cells and mediates the sustained Src activation and that KSHV-induced Src and RhoA play roles in facilitating entry and nuclear delivery of viral DNA.
These studies also demonstrated for the first time the modulation of the MT dynamics by virus-induced host cell signaling pathways to aid in the trafficking of viral DNA (Fig. 3).
KSHV-INDUCED ERK1/2 AND NF-κB PLAY ROLES IN VIRAL GENE EXPRESSION
KSHV induced a robust ERK1/2 induction as early as 5 min p.i.; this induction was observed even with a low multiplicity of infection of live and UV-inactivated KSHV in serum-starved cells and in the presence of serum (35, 37, 50, 56). PI-3K and PKC-ζ were recruited as upstream mediators of the KSHV-induced ERK pathway. A biphasic ERK1/2 induction was observed with high levels at earlier time points that returned to the basal level at 4 h p.i., and a second phase of a moderate level of activation from 12 to 72 h p.i. was observed (47). Studies with purified soluble proteins suggest that gpK8.1A and, to a lesser extent, gB are involved in ERK activation (50). Soluble gpK8.1A induced the MEK1/2-dependent ERK1/2 but not ERK5 and p38 MAPK in HMVEC-d and HFF cells (50). KSHV also induced NF-κB as early as 5 to 15 min p.i. of HMVEC-d and HFF cells, and translocation of p65-NF-κB into nuclei was detected. IκB phosphorylation inhibitor Bay11-7082 reduced this activation significantly (47). A sustained moderate level of NF-κB induction was seen during the observed 72 h p.i.; in contrast, p38 MAPK was activated only at later time points (39, 47, 56), and AKT was activated in a cyclic manner. Studies with UV-inactivated KSHV suggested a role for virus entry stages in ERK1/2 and NF-κB induction and a requirement for KSHV viral gene expression in sustained induction (47, 50).
Pretreatment of cells with MEK inhibitor U0126 significantly inhibited the expression of KSHV ORF73, ORF50, K8, and v-IRF2 genes (35, 50). Several MAPK-regulated host transcription factors, such as c-Jun, STAT1, MEF2, c-Myc, ATF-2, and c-Fos, were induced early during KSHV infection, and ERK inhibition significantly blocked c-Fos, c-Jun, c-Myc, and STAT1 activation in the infected cells. AP1 transcription factors binding to the RTA promoter were readily detected in the infected cell nuclear extracts, which were significantly reduced by ERK inhibition (50, 56). Though inhibition of NF-κB did not have any effect on KSHV entry into cells, the expression of viral latent ORF73 and lytic ORF50 genes was significantly reduced (47). Inhibition of NF-κB significantly affected Jun D, Jun B, phospho-c-Jun, cFos, and FosB activation (47).
Together, these studies suggest that very early during infection, KSHV induces ERK1/2 and NF-κB to modulate the initiation of viral gene expression and host cell genes (Fig. 3), a perfect way to overcome restriction on viral gene transcription.
LIPID RAFTS PLAY ROLES IN KSHV INFECTION OF ENDOTHELIAL (HMVEC-d) CELLS
Pretreatment of HMVEC-d cells with the LR-disrupting agent MβCD or nystatin significantly inhibited the expression of KSHV ORF73 and ORF50 genes without affecting virus binding (42). Though increased viral DNA internalization was observed, the association of internalized viral capsids with MT and the infected nucleus-associated viral DNA were significantly reduced (42). Disorganized and disrupted MT and thick, rounded plasma membranes in MβCD-treated cells were observed. LR disruption did not affect KSHV-induced FAK and ERK1/2 phosphorylation; in contrast, it increased the phosphorylation of Src, significantly reduced KSHV-induced PI3-K, RhoA GTPase, and NF-κB activation, and reduced the colocalization of PI3-K and RhoA GTPase with LRs (42). KSHV RhoA GTPase-mediated acetylation and aggregation of MT were also reduced, thus suggesting that LRs of HMVEC-d cells play critical roles in KSHV infection and gene expression, probably due to their roles in modulating KSHV-induced PI3-K, RhoA GTPase, and Dia2 molecules essential for post-binding and entry stages of infection, such as modulation of microtubular dynamics, movement of virus in the cytoplasm, and nuclear delivery (42). Increased Src activation and increased KSHV entry by lipid raft disruption suggests that Src activation is probably tightly regulated by an LR-associated factor(s), probably to control the quantity of KSHV entry into HMVEC-d cells. Disruption of LR did not affect infection of HUVEC or HFF cells, and the reason for the cell type variations is not clear (41, 42).
SUMMARY AND PERSPECTIVES
The interactions of eukaryotic cells with extracellular environments are largely mediated by ligand-induced signaling molecules, and the ensuing multitudes of biological processes are mediated by highly interlinked networks of signal pathways. KSHV induction of FAK, Src, PI3-K, Rho GTPases, and Dia2, required for the formation of endocytic vesicles and their movement and microtubular stabilization, and activation of ERK1/2 and NF-κB to modulate viral and host genes revealed a novel paradigm: by interacting with integrin and a family of functionally related molecules at the cell surfaces early during infection, KSHV utilizes the ligand mimicry as an opportunistic mechanism to subvert host signal molecules for its entry and successful infection, and KSHV interactions with host cell receptors have not evolved to act as a mere conduit for viral DNA entry but have evolved to overcome obstacles in the host cells and to create an appropriate intracellular environment that is conducive to successful infection (Fig. 3).
However, what is currently known is just the tip of the iceberg, and there are many important gaps in the early events of KSHV infection of target cells that remain to be filled. The determinants of KSHV tropism in different target cells and modes of entry remain incompletely defined. Further extensive studies are also required to comprehend fully the utilization of host machinery by KSHV. These studies include but are not limited to the biochemical and proteomic approaches to decipher the interaction of KSHV viral glycoproteins with receptors, their association with signal molecules and lipid rafts, and their roles in infection. Such studies would provide a better understanding of KSHV biology and an insight into whether signal molecules could be targeted to block KSHV infection of target cells. KSHV modulation of host genes that govern vital cellular processes, such as apoptosis, transcription, host defense, inflammation, extracellular matrix remodeling, and angiogenesis during the early time of infection is exciting, since transcriptional reprogramming probably serves vital roles in overcoming obstacles to establish a successful infection. Further understanding of signal induction and host cell molecules modulated by KSHV will provide information in generating designer drugs that can successfully block the potential entry routes hijacked by KSHV.
Acknowledgments
This study was supported in part by grants from the Public Health Service (AI057349 and CA 075911) and the Rosalind Franklin University of Medicine and Science—H.M. Bligh Cancer Research Fund to B.C.
I gratefully acknowledge the efforts and contributions of the following present and past members of my laboratory: Neelam Sharma-Walia, Pramod P. Naranatt, Mohanan Valiya Veettil, Sathish Sadagopan, Harinivas H. Krishnan, Hari Raghu, Clark Bloomer, Shaw M. Akula, Fu-Zhang Wang, Ling Zeng, Szeman Ruby Chan, Liangjin Zhu, Veena Puri, Virginie Bottero, Nagaraj Kerur, Arun G. Paul, Sayan Chakraborty, Nitika Paudel, and Laszlo Varga.
Biography
 Bala Chandran received his M.S. from Jawaharlal Institute of Post-Graduate Medical Education and Research in Pondicherry, India, and his Ph.D. degree in vaccinia virus immunobiology from All-India Institute of Medical Sciences in New Delhi, India. His postdoctoral experiences include research on HSV-2 monoclonal antibodies and glycoproteins at the Cancer Research Group, McMaster University, Hamilton, Canada, and on EBV glycoproteins and monoclonal antibodies at the Department of Comparative and Experimental Pathology at the University of Florida, Gainesville. He was appointed Assistant Professor in the Department of Microbiology, Molecular Genetics and Immunology at the University of Kansas Medical Center in 1986 and then Professor in 1996; there, he studied HHV-6 glycoproteins. From 1995 to present, he has been studying the biology of KSHV (HHV-8). On 1 July 2005, he joined the Department of Microbiology and Immunology, Chicago Medical School at Rosalind Franklin University of Medicine and Science, as Professor and Chairman and as Director of the H.M. Bligh cancer research laboratories. Currently, his laboratory is working on defining the molecular events of target cell infection by KSHV and their role in pathogenesis. Studies in progress include (i) defining the cell surface molecules recognized by KSHV, (ii) determining the signaling pathways induced by KSHV and their role in infection, and (iii) defining the KSHV modulation of host cell genes, including angiogenic and inflammatory molecules and their role in pathogenesis.
Bala Chandran received his M.S. from Jawaharlal Institute of Post-Graduate Medical Education and Research in Pondicherry, India, and his Ph.D. degree in vaccinia virus immunobiology from All-India Institute of Medical Sciences in New Delhi, India. His postdoctoral experiences include research on HSV-2 monoclonal antibodies and glycoproteins at the Cancer Research Group, McMaster University, Hamilton, Canada, and on EBV glycoproteins and monoclonal antibodies at the Department of Comparative and Experimental Pathology at the University of Florida, Gainesville. He was appointed Assistant Professor in the Department of Microbiology, Molecular Genetics and Immunology at the University of Kansas Medical Center in 1986 and then Professor in 1996; there, he studied HHV-6 glycoproteins. From 1995 to present, he has been studying the biology of KSHV (HHV-8). On 1 July 2005, he joined the Department of Microbiology and Immunology, Chicago Medical School at Rosalind Franklin University of Medicine and Science, as Professor and Chairman and as Director of the H.M. Bligh cancer research laboratories. Currently, his laboratory is working on defining the molecular events of target cell infection by KSHV and their role in pathogenesis. Studies in progress include (i) defining the cell surface molecules recognized by KSHV, (ii) determining the signaling pathways induced by KSHV and their role in infection, and (iii) defining the KSHV modulation of host cell genes, including angiogenic and inflammatory molecules and their role in pathogenesis.
Footnotes
Published ahead of print on 18 November 2009.
REFERENCES
- 1.Akula, S. M., P. P. Naranatt, N. S. Walia, F. Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 77:7978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akula, S. M., P. P. Naranatt, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. [DOI] [PubMed] [Google Scholar]
- 3.Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282:245-255. [DOI] [PubMed] [Google Scholar]
- 4.Akula, S. M., P. P. Naranatt, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV8) entry into target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 5.Baghian, A., M. Luftig, J. B. Black, Y. X. Meng, C. P. Pau, T. Voss, P. E. Pellett, and K. G. Kousoulas. 2000. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology 269:18-25. [DOI] [PubMed] [Google Scholar]
- 6.Bechtel, J. T., A. Grundhoff, and D. Ganem. 2005. RNAs in the virion of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:10138-10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechtel, J. T., Y. Liang, J. Hvidding, and D. Ganem. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 77:6474-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birkmann, A., K. Mahr, A. Ensser, S. Yağuboğlu, F. Titgemeyer, B. Fleckenstein, and F. Neipel. 2001. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J. Virol. 75:11583-11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boss, I. W., K. B. Plaisance, and R. Renne. 2009. Role of virus-encoded microRNAs in herpesvirus biology. Trends Microbiol. 17:544-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calderwood, D. A., S. J. Shattil, and M. H. Ginsberg. 2000. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J. Biol. Chem. 275:22607-22610. [DOI] [PubMed] [Google Scholar]
- 11.Cavalli, V., M. Corti, and J. Gruenberg. 2001. Endocytosis and signaling cascades: a close encounter. FEBS Lett. 498:190-196. [DOI] [PubMed] [Google Scholar]
- 12.Chandran, B., C. Bloomer, S. R. Chan, L. Zhu, E. Goldstein, and R. Horvat. 1998. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology 249:140-149. [DOI] [PubMed] [Google Scholar]
- 13.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 14.Fenczik, C. A., R. Zent, M. Dellos, D. A. Calderwood, J. Satriano, C. Kelly, and M. H. Ginsberg. 2001. Distinct domains of CD98hc regulate integrins and amino acid transport. J. Biol. Chem. 276:8746-8752. [DOI] [PubMed] [Google Scholar]
- 15.Feral, C. C., N. Nishiya, C. A. Fenczik, H. Stuhlmann, M. Slepak, and M. H. Ginsberg. 2005. CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. U. S. A. 102:355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganem, D. 2007. Kaposi's sarcoma-associated herpesvirus, p. 2875-2888. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 17.Garrigues, H. J., Y. E. Rubinchikova, C. M. Dipersio, and T. M. Rose. 2008. Integrin αVβ3 binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J. Virol. 82:1570-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene, W., and S. J. Gao. 2009. Actin dynamics regulate multiple endosomal steps during Kaposi's sarcoma-associated herpesvirus entry and trafficking in endothelial cells. PLoS Pathog. 5:e1000512. doi: 10.1371/journal.ppat.1000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giancotti, F. G. 2000. Complexity and specificity of integrin signaling. Nat. Cell Biol. 2:E13-E14. [DOI] [PubMed] [Google Scholar]
- 20.Grundhoff, A., and D. Ganem. 2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Invest. 113:124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn, A., A. Birkmann, E. Wies, D. Dorer, K. Mahr, M. Stürzl, F. Titgemeyer, and F. Neipel. 2009. Kaposi's sarcoma-associated herpesvirus gH/gL: glycoprotein export and interaction with cellular receptors. J. Virol. 83:396-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall, A., and C. D. Nobes. 2000. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 355:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue, N., J. Winter, R. B. Lal, M. K. Offermann, and S. Koyano. 2003. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J. Virol. 77:8143-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishizaki, T., Y. Morishima, M. Okamoto, T. Furuyashiki, T. Kato, and S. Narumiya. 2001. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat. Cell Biol. 3:8-14. [DOI] [PubMed] [Google Scholar]
- 25.Jarousse, N., B. Chandran, and L. Coscoy. 2008. Lack of heparan sulfate expression in B-cell lines: implications for Kaposi's sarcoma-associated herpesvirus and murine gammaherpesvirus 68 infections. J. Virol. 82:12591-12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabir-Salmani, M., M. N. Fukuda, M. Kanai-Azuma, N. Ahmed, S. Shiokawa, Y. Akimoto, K. Sakai, S. Nagamori, Y. Kanai, K. Sugihara, and M. Iwashita. 2008. The membrane-spanning domain of CD98 heavy chain promotes αvβ3 integrin signals in human extravillous trophoblasts. Mol. Endocrinol. 22:707-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaleeba, J. A., and E. A. Berger. 2006. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 311:1921-1924. [DOI] [PubMed] [Google Scholar]
- 28.Koyano, S., E. C. Mar, F. R. Stamey, and N. Inoue. 2003. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J. Gen. Virol. 84:1485-1491. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan, H. H., P. P. Naranatt, M. S. Smith, L. Zeng, C. Bloomer, and B. Chandran. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 78:3601-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan, H. H., N. Sharma-Walia, D. N. Streblow, P. P. Naranatt, and B. Chandran. 2006. Focal adhesion kinase is critical for entry of Kaposi's sarcoma-associated herpesvirus into target cells. J. Virol. 80:1167-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McPherson, P. S., B. K. Kay, and N. K. Hussain. 2001. Signaling on the endocytic pathway. Traffic 2:375-384. [DOI] [PubMed] [Google Scholar]
- 33.Mark, L., W. H. Lee, O. B. Spiller, B. O. Villoutreix, and A. M. Blom. 2006. The Kaposi's sarcoma-associated herpesvirus complement control protein (KCP) binds to heparin and cell surfaces via positively charged amino acids in CCP1-2. Mol. Immunol. 43:1665-16675. [DOI] [PubMed] [Google Scholar]
- 34.Naranatt, P. P., S. M. Akula, and B. Chandran. 2002. Characterization of gamma2-human herpesvirus-8 glycoproteins gH and gL. Arch. Virol. 147:1349-1370. [DOI] [PubMed] [Google Scholar]
- 35.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 77:1524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naranatt, P. P., H. H. Krishnan, S. R. Svojanovsky, C. Bloomer, S. Mathur, and B. Chandran. 2004. Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 64:72-84. [DOI] [PubMed] [Google Scholar]
- 37.Naranatt, P. P., H. H. Krishnan, M. S. Smith, and B. Chandran. 2005. Kaposi's sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J. Virol. 79:1191-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1997. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 71:4187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan, H., J. Xie, F. Ye, and S. J. Gao. 2006. Modulation of Kaposi's sarcoma-associated herpesvirus infection and replication by MEK/ERK, JNK, and p38 multiple mitogen-activated protein kinase pathways during primary infection. J. Virol. 80:5371-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palazzo, A. F., T. A. Cook, A. S. Alberts, and G. G. Gundersen. 2001. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat. Cell Biol. 3:723-729. [DOI] [PubMed] [Google Scholar]
- 41.Raghu, H., N. Sharma-Walia, M. Valiya Veettil, S. Sadagopan, and B. Chandran. 2009. Kaposi's sarcoma associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. J. Virol. 83:4895-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raghu, H., N. Sharma-Walia, M. Valiya Veettil, S. Sadagopan, A. Caballero, R. Sivakumar, L. Varga, V. Bottero, and B. Chandran. 2007. Lipid rafts of primary endothelial cells are essential for Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry. J. Virol. 81:7941-7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rappocciolo, G., F. J. Jenkins, H. R. Hensler, P. Piazza, M. Jais, L. S. Borowski, C. Watkins, and C. R. Rinaldo. 2006. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J. Immunol. 176:1741-1749. [DOI] [PubMed] [Google Scholar]
- 44.Rappocciolo, G., H. R. Hensler, M. Jais, T. A. Reinhart, A. Pegu, F. J. Jenkins, and C. R. Rinaldo. 2008. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J. Virol. 82:4793-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. U. S. A. 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadagopan, S., N. S. Walia, M. V. Veettil, H. Raghu, R. Sivakumar, and B. Chandran. 2007. Kaposi's sarcoma-associated herpesvirus induces a sustained NF-κB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J. Virol. 81:3949-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sastry, S. K., and K. Burridge. 2000. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 261:25-36. [DOI] [PubMed] [Google Scholar]
- 49.Sharma-Walia, N., H. Raghu, S. Sadagopan, R. Sivakumar, M. V. Veettil, P. P. Naranatt, M. M. Smith, and B. Chandran. 2006. Cyclooxygenase 2 induced by Kaposi's sarcoma-associated herpesvirus early during in vitro infection of target cells plays a role in the maintenance of latent viral gene expression. J. Virol. 80:6534-6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma-Walia, N., H. H. Krishnan, P. P. Naranatt, L. Zeng, M. S. Smith, and B. Chandran. 2005. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 79:10308-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma-Walia, N., P. P. Naranatt, H. H. Krishnan, L. Zeng, and B. Chandran. 2004. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase rho GTPase signal pathways and cytoskeletal rearrangements. J. Virol. 78:4207-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veettil, M. V., N. Sharma-Walia, S. Sadagopan, H. Raghu, R. Sivakumar, P. P. Naranatt, and B. Chandran. 2006. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner. J. Virol. 80:11432-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veettil, M. V., S. Sadagopan, N. Sharma-Walia, F.-Z. Wang, H. Raghu, L. Varga, and B. Chandran. 2008. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (αvβ5, αvβ3, and α3β1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J. Virol. 82:12126-12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, F. Z., S. M. Akula, N. P. Pramod, L. Zeng, and B. Chandran. 2001. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J. Virol. 75:7517-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, F. Z., S. M. Akula, N. Sharma-Walia, L. Zeng, and B. Chandran. 2003. Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. J. Virol. 77:3131-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie, J., H. Pan, S. Yoo, and S. J. Gao. 2005. Kaposi's sarcoma-associated herpesvirus induction of AP-1 and interleukin 6 during primary infection mediated by multiple mitogen-activated protein kinase pathways. J. Virol. 79:15027-15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoo, S. M., F. C. Zhou, F. C. Ye, H. Y. Pan, and S. J. Gao. 2005. Early and sustained expression of latent and host modulating genes in coordinated transcriptional program of KSHV productive primary infection of human primary endothelial cells. Virology 343:47-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu, L., V. Puri, and B. Chandran. 1999. Characterization of human herpesvirus-8 K8.1A/B glycoproteins by monoclonal antibodies. Virology 262:237-249. [DOI] [PubMed] [Google Scholar]