Abstract
Human cytomegalovirus (HCMV) produces the following two gH/gL complexes: gH/gL/gO and gH/gL/UL128-131. Entry into epithelial and endothelial cells requires gH/gL/UL128-131, and we have provided evidence that gH/gL/UL128-131 binds saturable epithelial cell receptors to mediate entry. HCMV does not require gH/gL/UL128-131 to enter fibroblasts, and laboratory adaptation to fibroblasts results in mutations in the UL128-131 genes, abolishing infection of epithelial and endothelial cells. HCMV gO-null mutants produce very small plaques on fibroblasts yet can spread on endothelial cells. Thus, one prevailing model suggests that gH/gL/gO mediates infection of fibroblasts, while gH/gL/UL128-131 mediates entry into epithelial/endothelial cells. Most biochemical studies of gO have involved the HCMV lab strain AD169, which does not assemble gH/gL/UL128-131 complexes. We examined gO produced by the low-passage clinical HCMV strain TR. Surprisingly, TR gO was not detected in purified extracellular virus particles. In TR-infected cells, gO remained sensitive to endoglycosidase H, suggesting that the protein was not exported from the endoplasmic reticulum (ER). However, TR gO interacted with gH/gL in the ER and promoted export of gH/gL from the ER to the Golgi apparatus. Pulse-chase experiments showed that a fraction of gO remained bound to gH/gL for relatively long periods, but gO eventually dissociated or was degraded and was not found in extracellular virions or secreted from cells. The accompanying report by P. T. Wille et al. (J. Virol., 84:2585-2596, 2010) showed that a TR gO-null mutant failed to incorporate gH/gL into virions and that the mutant was unable to enter fibroblasts and epithelial and endothelial cells. We concluded that gO acts as a molecular chaperone, increasing gH/gL ER export and incorporation into virions. It appears that gO competes with UL128-131 for binding onto gH/gL but is released from gH/gL, so that gH/gL (lacking UL128-131) is incorporated into virions. Thus, our revised model suggests that both gH/gL and gH/gL/UL128-131 are required for entry into epithelial and endothelial cells.
Human cytomegalovirus (HCMV) infects many different cell types in vivo, including epithelial and endothelial cells, fibroblasts, monocyte-macrophages, smooth muscle cells, dendritic cells, hepatocytes, neurons, glial cells, and leukocytes (reviewed in references 5, 30, 38, and 45). In the laboratory, HCMV is normally propagated in primary human fibroblasts because most other cell types yield low titers of virus. Commonly studied laboratory strains, such as AD169, were propagated extensively in fibroblasts, and this was accompanied by deletions or mutations in a cluster of 22 genes known as ULb′ (6). These mutations were correlated with the inability to infect other cell types, including endothelial and epithelial cells and monocyte-macrophages. Targeted mutagenesis of three of the ULb′ genes, UL128, UL130, and UL131, abolished infection of endothelial cells, transmission to leukocytes, and infection of dendritic cells (13, 15). Restoration of the UL128-131 genes in laboratory strains of HCMV strains restored the capacity to infect endothelial and epithelial cells and other cells (15, 52).
The UL128, UL130, and UL131 proteins assemble onto the extracellular domain of HCMV gH/gL (1, 42, 53). For all herpesviruses, gH/gL complexes mediate entry into cells (12, 33, 39), suggesting that gH/gL/UL128-131 might participate in the entry mechanism. Indeed, we demonstrated that gH/gL/UL128-131 mediates entry into epithelial and endothelial cells by using the fusogenic agent polyethylene glycol to force entry of HCMV UL128-131 mutants into these cell types (41). This was consistent with reports that UL128-, UL130-, and UL131-specific antibodies blocked the capacity of HCMV to infect epithelial and endothelial cells but not fibroblasts (1, 53). Furthermore, expression of gH/gL/UL128-131, but not gH/gL or gB, in epithelial cells interfered with HCMV infection, consistent with saturable gH/gL/UL128-131 receptors (40). Expression of all five proteins was necessary so that the gH/gL/UL128-131 complexes were exported from the endoplasmic reticulum (ER) and could function (40-42, 53). Together, these data suggested that gH/gL/UL128-131 mediates entry into epithelial/endothelial cells but is not required for entry into fibroblasts. By extension, it was reasonable to propose that other forms of gH/gL might facilitate the entry into fibroblasts.
The laboratory HCMV strain AD169 is known to express a second gH/gL complex containing glycoprotein O (gO) (21-23, 53). In cells infected with a recombinant AD169 in which the UL131 mutation was repaired, gH/gL/gO complexes were separate from gH/gL/UL128-131 complexes, i.e., gO was not detected following immunoprecipitation (IP) with UL128- and UL130-specifc antibodies, and gO-specific antibodies did not precipitate UL128 and UL130 (53). AD169 and Towne gO− mutants produce small plaques on fibroblast monolayers and low titers of virus, supporting an important, although not essential, role for gH/gL/gO in virus replication in fibroblasts (11, 19). AD169 does not infect endothelial and epithelial cells, so AD169 gO− mutants were not tested on these cells. Jiang et al. described a gO-null mutant derived from an endotheliotropic HCMV strain, TB40/E (27). The TB40/E gO-null mutant spread normally on endothelial cells, suggesting that gO or gH/gL/gO is less important for infection and spread in these cells. Given that the role of gH/gL in entry is highly conserved among the herpesviruses, it seemed likely that gH/gL/gO might be involved in entry into fibroblasts. Consistent with this notion, Paterson et al. showed that anti-gO antibodies decreased fusion from without caused by infection of cells with HCMV AD169 (37). These observations supported our working model in which gH/gL/UL128-131 mediates entry into epithelial and endothelial cells, while gH/gL/gO mediates entry into fibroblasts. There is also the possibility that gH/gL (lacking gO and UL128-131) might be incorporated into the virion envelope, although there is presently no direct evidence for this. Any gH/gL detected biochemically might result from dissociation of gO or UL128-131 during sample preparation and analysis. gH/gL expressed without other HCMV proteins was retained in the ER (42), arguing against incorporation into the virion.
Other herpesviruses, e.g., Epstein-Barr virus, human herpesvirus 6 (HHV-6), and HHV-7, use different forms of gH/gL to enter different cell types via different pathways (25, 34, 43). Similarly, HCMV entry into fibroblasts occurs by fusion at the plasma membrane at a neutral pH and does not require gH/gL/UL128-131 (7), whereas entry into epithelial and endothelial cells involves endocytosis and low pH-dependent fusion and requires gH/gL/UL128-131 (41).
All of the biochemical analyses of gO in terms of binding to gH/gL and intracellular transport have involved fibroblast-adapted strain AD169 (21-23, 31, 53). These studies indicated that gO is a 110- to 125-kDa glycoprotein encoded by the UL74 gene (22). Glycosidase digestion experiments demonstrated that the gO polypeptide chain is ∼62 to 65 kDa (21-23, 53). Pulse-chase studies showed that gH/gL assembles in the ER as a disulfide-linked heterodimer (28) that subsequently binds to, and establishes disulfides with, gO (22, 23). The 220-kDa immature gH/gL/gO trimer is initially sensitive to endoglycosidase H (endo H), which removes immature N-linked oligosaccharides from glycoproteins present in the ER (22, 23). Transport of gH/gL/gO to the Golgi apparatus is associated with processing of N-linked oligosaccharides to mature forms that resist endo H. Also associated with transport to the Golgi apparatus is the addition of O-linked oligosaccharides and phosphorylation, increasing the molecular weight of gO (after reduction) to 125 to 130 kDa and that of the gH/gL/gO complex to 240 to 260 kDa (22, 23, 29). It is the mature glycoprotein complex, previously known as gCIII, that is trafficked to HCMV assembly compartments for incorporation into the virion envelope (22, 23, 29).
In addressing the function of gO, it is important to recognize that AD169 has adapted to replication in fibroblasts, losing expression of UL131 and failing to assemble gH/gL/UL128-131 complexes (6) (15). There seems to be strong pressure to mutate UL128-131, because clinical strain Merlin acquired a UL128 mutation within 5 passages on fibroblasts (2). It is also reasonable to suggest that fibroblast adaptation includes changes in gO. The gO genes (UL74) of several laboratory and clinical strains and clinical isolates are highly variable (up to 25% of amino acids) (10, 35, 37, 47). However, it is important to note that AD169-derived UL131-repair virus can infect epithelial and endothelial cells (52). Thus, if AD169 gO is important for infection of these cells, then gO must be functionally normal in this regard. These differences in laboratory versus clinical HCMV prompted us to characterize the gO molecule expressed by the HCMV strain TR. HCMV TR is a clinical isolate that was stabilized in the form of a bacterial artificial chromosome (BAC) after very limited passage in fibroblasts (35, 41). HCMV TR expresses gH/gL/UL128-131 (42) and infects epithelial and endothelial cells (41) and monocyte-macrophages well (D. Streblow and J. Nelson, unpublished results).
Here, we report our biochemical and cell trafficking analyses of the TR gO protein. We were surprised to find that TR gO was not present in extracellular virus particles. In contrast, gO was detected in extracellular AD169 particles, consistent with previous findings (22). TR gO expressed either in HCMV-infected cells or by using nonreplicating Ad vectors (expressed without other HCMV proteins) was largely retained in the ER. Coexpression of TR gO with gH/gL promoted transport of gH/gL beyond the ER, and gO was slowly lost from gH/gL complexes but not secreted from cells and not observed in extracellular virus particles. Thus, TR gO acts as a chaperone. Consistent with this, in the accompanying paper by Wille et al. (54), a TR gO-null mutant was described that secreted extracellular particles containing markedly reduced quantities of gH and gL. The gO− mutant failed to enter fibroblasts and also epithelial and endothelial cells. Together, these results suggest that it is gH/gL, not gH/gL/gO, which is incorporated into HCMV TR virions. It appears that gH/gL is required for entry into fibroblasts, and both gH/gL and gH/gL/UL128-131 are required for entry into epithelial and endothelial cells.
MATERIALS AND METHODS
Cell lines.
Neonatal normal human dermal fibroblasts (NHDF) were obtained from Invitrogen, and U373-MG microglial cells were obtained from American Type Culture Collection (ATCC) (Manassas, VA) and grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 12% fetal bovine serum (FBS; HyClone) or 10% bovine growth serum (BGS; HyClone). 293 and 293IQ (Microbix) cells were grown in minimum essential medium (MEM; Invitrogen) plus 10% FBS.
Antibodies.
Monoclonal antibodies (MAb) specific for gH (14-4b) and gB (27-156) were generously provided by Bill Britt (University of Alabama, Birmingham, AL) (3, 26). Anti-calnexin MAb was obtained from BD Biosciences. Anti-AD169 gO antibodies produced in rabbits using AD169 gO residues 32 to 466 expressed in bacteria (22) were previously described (22) and kindly provided by Teresa Compton (Novartis, Boston, MA). Rabbit polyclonal anti-peptide antisera directed against HCMV gH, gL, and UL130 have been described previously (42). Two rabbit polyclonal antisera specific to TR gO were produced using synthetic peptides, according to standard protocols (16). TR gO254 antibodies were made using TR gO residues 254 to 271 (KRKQAPVKEQSEKKSKKS), and TR gO110 antibodies were made using TR gO residues 110 to 127 (WFDFYSTQLRKPAKYVFS). Both peptides were synthesized (EZ BioLabs, Carmel, IN) with an additional C-terminal cysteine residue and then coupled via this cysteine to keyhole limpet hemocyanin (KLH) or bovine serum albumin (BSA) using m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS; Sigma, St. Louis, MO). The peptide/protein conjugates were mixed with Freund's adjuvant and used to immunize New Zealand White rabbits. Subsequently, rabbits were boosted with KLH- or BSA-conjugated peptides in TiterMax Gold adjuvant (Sigma; St. Louis, MO).
HCMV.
TR is a clinical HCMV strain that was derived from a ocular vitreous fluid sample from a patient with HIV disease (46) and was cloned into a BAC after limited passage in fibroblasts (36). Clinical strain Merlin was provided as a BAC clone by Gavin Wilkinson (University of Wales, Cardiff, United Kingdom) (10). Clinical strain FIX and low-passage laboratory strain Toledo were provided as a BAC clones by Tom Shenk (Princeton University, Princeton, NJ) (14). Infectious virus was recovered from each of these BAC clones by electroporation of BAC DNA into MRC-5 fibroblasts, as described in the accompanying report by Wille et al. (54). Laboratory strains AD169 and Towne (not derived from BAC clones) that have been passaged extensively on fibroblasts were obtained from Jay Nelson and ATCC (VR-977), respectively. HCMV stocks were produced by infecting NHDF using 0.1 PFU per cell for 10 to 16 days. Viral particles were purified and concentrated from culture supernatants by centrifugation through a cushion of 20% sorbitol in PBS at 50,000 × g for 1 h. Pellets were resuspended in DMEM plus 10% FBS and frozen at −70°C. The number of plaque-forming units was determined by plaque assay on replicate NHDF cultures. The number of infectious units (IU) was determined by plating serial dilutions on replicate NHDF cultures. After 2 days, the number of infected cells was determined by immunofluorescence detection of HCMV IE-86 antigens (41), and the numbers of IU/ml were defined as the numbers of IE-86-positive cells per ml.
Replication-defective Ad vectors.
Nonreplicating (E1−) Ad vectors that express HCMV TR gH and gL have been described (42). Ad vectors expressing TR gO, AdTRgO and AdTRgO(co), were generating using a commercial (Microbix, Toronto, Canada) modification of the method of Matthews et al. (32). Briefly, the gO gene (UL74) was PCR amplified from the TR genome (in the case of AdTRgO) or synthesized by GeneArt (Regensburg, Germany) as a codon-optimized gene [in the case of AdTRgO(co)], and then these gO genes were ligated into shuttle plasmid pDC316(io) (Microbix). Shuttle plasmids containing the TR gO or TR gO(co) genes were then transfected into 293IQ cells (Microbix, Toronto, Canada), along with the Ad genomic plasmid pBHGloxΔE1,3Cre (Microbix). Cre-Lox recombination resulted in Ad vectors that were subsequently propagated on 293IQ cells that express the Lac repressor protein. The Lac repressor protein binds to sequences between the promoter and gO gene, reducing gO expression in 293 cells. The number of Ad vector plaque-forming units was determined using 293IQ cells. Multiplicities of infection of U373 cells were determined empirically for each Ad vector to give appropriate expression and ranged from 10 to 100 PFU/cell. Radiolabeling and analysis of HCMV proteins expressed by Ad vectors were performed at 18 to 24 h postinfection. There was little production of Ad proteins and little or no cytopathic effects under these conditions.
Immunoblot analysis of HCMV-infected cells and extracellular virus particles.
NHDF cultures (150 cm2; approximately 7 × 106 cells) were infected with 2 to 3 IU per cell of HCMV and incubated in 20 ml of DMEM plus 5% horse serum for 4 to 7 days. More than 95% of cells remained attached to culture dishes under these conditions, and cells were not broken. Culture supernatants were removed and clarified by centrifugation at 1,000 × g for 10 min and again at 6,000 × g for 10 min. Viral particles were then partially purified by centrifugation at 50,000 × g for 1 h through a 20% sorbitol cushion. HCMV-infected cells were scraped into TBS (50 mM Tris, pH 7.5; 100 mM NaCl; 1.5 mM MgCl2) and collected by centrifugation at 1,000 × g for 10 min. The cells and pelleted extracellular virus particles derived from one culture dish were each resuspended in 375 μl of TBS plus 1% Triton X-100. Intact cells and nuclei were removed from the cell extracts by centrifugation at 1,500 × g for 10 min. Insoluble material was removed from cell and viral particle extracts by centrifugation at 100,000 × g for 30 min. Triton X-100 extracts of cells or partially purified extracellular virus particles were adjusted to 2% sodium dodecyl sulfate (SDS) and 2% β-mercaptoethanol and boiled for 10 min before Western blot analysis. For experiments involving endoglycosidase H (endo H) or peptide-N-glycosidase F (PNGase F), cell and virus Triton X-100 extracts were adjusted to 1× G5 (endo H) or G7 (PNGase F) buffer and 1× glycoprotein denaturation buffer (New England Biolabs), boiled for 10 min, cooled, and then incubated for 2 h at 37 C with 20 U endo H or 20 U PNGase F (New England Biolabs) per μl of extract. Treated extracts were then adjusted to 2% SDS and 2% β-mercaptoethanol, boiled, and analyzed by Western blotting. Extracts corresponding to ∼5% of the original culture (3.5 × 105 cells or cell culture supernatants from the same quantities of cells) were loaded into a single lane of 8% or 12% SDS-polyacrylamide gel and separated by electrophoresis (55). Proteins were electrophoretically transferred to Immobilon membranes (Millipore) in a buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol. Transferred proteins were probed with rabbit polyclonal antibodies or MAb specific for HCMV proteins or calnexin, followed by horseradish peroxidase-conjugated secondary antibodies, and detected by chemiluminescence as described previously (24, 55).
Radiolabeling of cells and immunoprecipitation.
Cells were washed extensively in labeling medium (DMEM lacking methionine and cysteine) and then incubated in this medium for 30 min at 37 C. Cells were then incubated for the indicated times in labeling medium supplemented with [35S]methionine/cysteine (100 to 500 μCi/ml; Amersham). In some experiments, radiolabeling was chased by incubation of cells in DMEM containing a 10-fold excess of nonradioactive methionine and cysteine. Extracts of cells or virus particles (purified from culture supernatants as described above) were made using 1% Triton X-100 in TBS supplemented with 1 mg/ml bovine serum albumin and 1 mM phenylmethylsulfonyl fluoride. Extracts were clarified by centrifugation at 1,500 × g for 10 min and then at 100,000 × g for 30 min. Extracts were then precleared by incubation with protein A-agarose beads for 1 to 2 h, and then the protein A-agarose removed by centrifugation. Proteins were immunoprecipitated by the addition of MAb (1 to 5 μl of ascitic fluids) or rabbit polyclonal antiserum (5 to 10 μl) to 1 ml of cell extract for 2 h. In some cases, the peptides used to produce antipeptide sera were present at 10 μg/ml in order to compete with antibodies. Protein A-agarose (20 to 50 μl) was added for an additional 2 h, and then agarose beads were collected and washed three times in 1% Triton X-100 in TBS. For endo H and PNGase F treatment, immunoprecipitated proteins bound to protein A-agarose were incubated with 50 U of endo H or PNGase F (New England Biolabs) for 1 h at 37°C in the buffer specified by the manufacturer. Proteins were eluted from protein A-agarose by boiling in 2% SDS and 2% β-mercaptoethanol and separated by electrophoresis using 8% or 12% SDS-polyacrylamide gel.
RESULTS
HCMV gO was not present in extracellular particles of the clinical HCMV strain TR.
Given the differences between lab and clinical HCMV in terms of gH/gL/UL128-131 expression, it was important to compare a gO molecule expressed by a low-passage HCMV to that of a lab strain. TR and AD169 extracellular virus particles were prepared by centrifugation of cell culture supernatants through a 20% sorbitol cushion and compared by immunoblotting to TR- and AD169-infected cell extracts. Calnexin, a component of ER membranes, was analyzed as a control and was not detected in these extracellular virus particles (Fig. 1). Previous studies involving virus purification by these methods found that only a small subset of cellular proteins were present in extracellular virus particles, and it was concluded that these proteins were specifically incorporated into virions (51). In those studies and here, virus particles were harvested from cultures of intact cells. Our preparations likely contained dense bodies, but these contain a normal complement of HCMV membrane proteins.
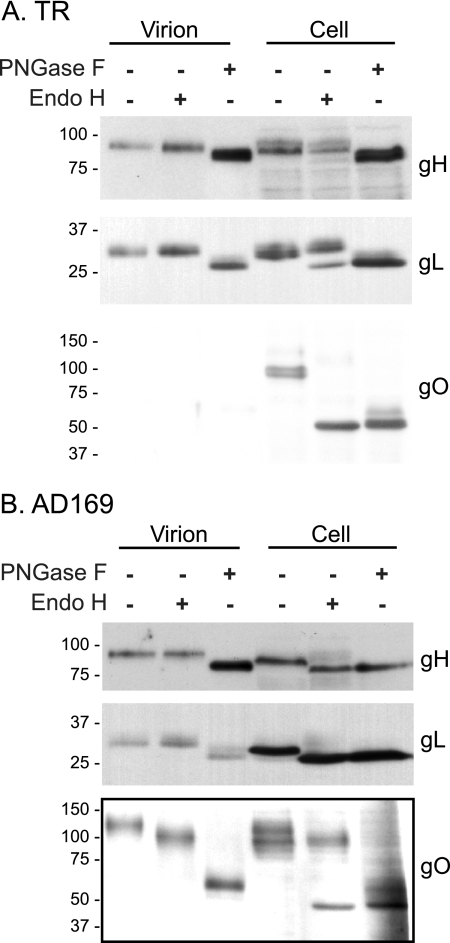
FIG. 1.
Immunoblot analysis of viral glycoproteins in HCMV TR- and AD169-infected cells and extracellular virus particles. Human fibroblasts (NHDF) were infected with 2 IU per cell of TR or AD169 for 7 days. Extracellular viral particles were purified from clarified culture supernatants by centrifugation though 20% sorbitol cushions (V). Virus particles and infected cells (C) were extracted with 1% Triton X-100 and analyzed by Western blotting using mouse MAb specific for gB (27-156) or calnexin or rabbit polyclonal antibodies specific for gH, gL, TR gO (TR gO254 produced against gO residues 254 to 271), AD169 gO, or UL130. Note that the TR gO254 antibodies do not recognize AD169 gO, and AD169 gO antibodies do not recognize TR gO; thus, separate blots are shown. A longer exposure of the TR gO blot was provided to show the very small quantity of TR gO in virus particles. Numbers below each lane indicate the relative abundance of each protein. Shown are representative results from three independent experiments. Molecular weight markers are shown along the left side of each panel.
HCMV gB, gH, and gL were each detected in TR- and AD169-infected cells and extracellular virus particles (Fig. 1). The relative abundances, comparing virions with cells, ranged between 38 and 67%. TR virions contained UL130, whereas AD169 virions did not (Fig. 1). The absence of UL130 from AD169 virions reflects the inability to assemble gH/gL/UL128-131 complexes and incorporate them into the virion envelope because AD169 does not express functional UL131 (42) (52). There were also several differences between TR and AD169 in the banding patterns of gB, gH, and gL, e.g., gH present in TR-infected cells exhibited two distinct species, whereas gH present in AD169-infected cells exhibited a single, faster-migrating species, and AD169 gH in virus particles was slower migrating. In TR-infected cells, a strong band was readily detected. In contrast, gO was not readily detected in HCMV TR extracellular virions (Fig. 1). A long film exposure revealed a very small amount of gO present in TR virus particles, amounting to less than 1% of that present in infected cells, and was similar to the virion abundance of calnexin in long film exposures (not shown). In contrast, ∼26% of the total AD169 gO was present in extracellular virions, consistent with previous studies (22). In AD169-infected cells, gO migrated as a diffuse band ranging from about 100 to 130 kDa, whereas AD169 extracellular particles contained predominately the 130 kDa form (Fig. 1). TR gO present in cells was predominately observed as a tighter band of ∼100 kDa.
One caveat associated with these data is that two different antibodies were used to detect TR versus AD169 gO. This was due to the amino acid variation between the gO proteins of different HCMV strains. AD169 gO-specific antibodies produced by expressing residues 32 to 466 in bacteria (22) did not recognize TR gO (not shown), and TR gO-specific anti-peptide antibodies that denoted TR gO254 (involving TR gO amino acids 254 to 271) did not recognize AD169 gO (Fig. 2A). It was also conceivable that epitopes for the TR gO254 antibodies might be lost or modified during assembly of HCMV virions, so that the antibodies did not recognize virion-associated gO. To address these issues, we raised a second rabbit anti-peptide antiserum involving TR gO residues 110 to 127, a region predicted to be highly conserved (17 of 18 residues), comparing TR gO to AD169 gO and that in all other HCMV strains that have been sequenced. In immunoblot experiments involving TR- and AD169-infected cells and supernatant particles, the TR gO110 antibodies detected a protein of ∼100 kDa in TR-infected cells, similar to that observed with TR gO254 antibodies (Fig. 2A). The less intense signal suggested that the TR gO110 antibodies were of lower affinity than TR gO254 antibodies. In spite of primary sequence conservation, TR gO110 antibodies did not detect AD169 gO. Importantly, TR gO110 antibodies detected gO in cell extracts but not in extracellular TR virions (Fig. 2A). Much darker exposures of TR gO110 blots confirmed these results, and gO in cells, but not in virions, involving anti-TR gO110 sera were observed in three other experiments (not shown). We recognize that it is difficult to formally prove that gO is completely absent from TR virions, and from these data alone, it remains possible that an undetected form of gO is present in TR virions. Nevertheless, the use of two separate antipeptide sera (which recognize continuous protein epitopes) that recognize different regions of gO in Western blots strongly argues against the possibility that there was mature gO present in the virion envelope of TR. For mature gO to remain undetected by anti-peptide antibodies after denaturation in SDS buffer, posttranslational covalent modifications would be required. It seems highly improbable that covalent modification occurred in two different sites in gO, i.e., from amino acids 110 to 127 and 254 to 275, and that both modifications masked recognition anti-peptide antibodies.
FIG. 2.
Immunoblot analysis of glycoproteins expressed by HCMV clinical and laboratory strains. Human fibroblasts (NHDF) were infected for 7 days with 2 IU per cell of the following various HCMV strains: TR, AD169, Merlin (ME), FIX (FX), Toledo (TL), or Towne (TN). Extracellular viral particles purified from culture supernatants by centrifugation through 20% sorbitol (V) and infected cells (C) were analyzed by immunoblotting as shown in Fig. 1. (A) Blots were probed with either of the two anti-peptide antibodies, TR gO254 antibodies or a second TR gO antipeptide serum, denoted TR gO110 (produced against gO residues 110 to 127). (B) Replicate blots of virions and cells derived from TR-, Merlin-, Fix-, Toledo-, or Towne-infected cells were probed with anti-gH, anti-gL, anti-TR gO254, anti-TR gO110, or anti-AD169 gO antibodies. Each lane in each panel was exposed from the same membrane (i.e., the same experiment), although some lanes were removed digitally. Molecular weight markers are shown along the left side of each panel.
As noted in Materials and Methods, extracellular virions were pelleted from cell culture supernatants and then extracted with 1% Triton X-100. These extracts were centrifuged to remove insoluble proteins before immunoblot analysis. Thus, it was possible that gO was present in the insoluble fraction (pellet) from TR virions. However, this was ruled out by performing immunoblot analyses on the insoluble, pelleted material and by analyzing the total pelleted virion preparation from cell culture supernatants. In all cases, we did not detect TR gO in any fraction from these extracellular virions (results not shown).
Analyses of gO expressed by other HCMV strains.
We attempted to extend these results to other HCMV strains by characterizing infected cells and extracellular viral particles for the clinical strain Merlin (10), strain FIX, which infects endothelial cells (15), and lab strains Toledo and Towne. The TR gO254 antibodies again detected gO in TR-infected cells but not in TR virions (Fig. 2B). However, TR gO254 antibodies did not detect gO in cells infected with any other HCMV strain. In the experiment shown in Fig. 2B, unlike that shown in Fig. 2A, the TR gO110 antibody did not detect measurable amounts of TR gO in infected cells. This was likely due to a combination of the poor affinity of the TR gO110 and reduced amounts of gO in these samples. The AD169-specific antibodies faintly detected gO in Toledo-infected cells and particles but not in other strains (Fig. 2B). The weak signal associated with the Toledo virions detected with AD169 gO antibodies made it difficult to interpret these observations. However, the results were consistent with Toledo gO incorporation into extracellular virions. A third antiserum produced using TR gO residues 332 to 345 did not detect TR gO or molecules produced by other strains (data not shown). Computer sequence analysis suggests that all sequenced forms of HCMV gO contain strongly antigenic domains centered on a region corresponding to residues 254 to 271 of TR gO. However, there are amino acid differences in this region among the strains. Therefore, it may be necessary to generate other antibodies that can recognize the gO molecules expressed by these other strains to extend these observations.
TR gO is largely retained in the endoplasmic reticulum (ER).
The HCMV envelope is acquired by budding into cytoplasmic membranes containing trans-Golgi network (TGN) markers (20, 44). Thus, transport of viral glycoproteins from the ER to the Golgi apparatus and to the TGN-like assembly compartments is necessary for glycoproteins to be incorporated into progeny virions. Since gO was not detected in extracellular TR virions, it was of interest to examine the intracellular transport of gO from the ER to the Golgi apparatus and to compare this with the trafficking of gH/gL. HCMV TR- and AD169-infected fibroblasts were labeled with [35S]methionine/cysteine using a pulse-chase format, then gO or gH/gL were immunoprecipitated, and glycoproteins were treated with endo H or peptide-N-glycosidase F (PNGase F). Endo H removes immature, high-mannose N-linked oligosaccharides from glycoproteins, structures present on glycoproteins found in the ER. However, after transport to the Golgi apparatus, N-linked oligosaccharides become endo H resistant. PNGase F removes both immature and mature N-linked glycans. TR gO migrated as a 100-kDa protein following a 45-min chase, and this protein was entirely or largely sensitive to endo H, which produced a 55- to 60-kDa deglycosylated protein similar to that produced by PNGase F (Fig. 3A). Note that the 45-min chase sample was identical to the pulse sample, and for brevity, the pulse samples were not shown. Over the course of 120- and 240-min chases, TR gO remained at 100 kDa and sensitive to endo H (Fig. 3A).
FIG. 3.
Pulse-chase analysis of the maturation of N-linked oligosaccharides associated with gO and gH in TR-infected cells versus AD169-infected cells. Human fibroblasts (NHDF) were infected for 4 days with 3 IU/cell of TR (A and C) or AD169 (B and D). Cells were labeled for 15 min with [35S]methionine/cysteine and then label chased for 45, 120, or 240 min. Cell extracts were analyzed by immunoprecipitation (IP) with anti-TR gO254 antibodies (A), anti-AD169 gO antibodies (B), or anti-gH MAb 14-4b (C and D). Immunoprecipitated proteins were either left untreated or treated with endo H or PNGase F. Arrows indicate bands corresponding to gO, gH, and gL, and faster-migrating forms of these proteins after oligosaccharides were removed by endo H or PNGase F (H, F). Molecular weight markers are shown along the left side of each panel.
Analysis of AD169-infected cells was more difficult because there were higher backgrounds associated with the AD169-specific anti-gO antibodies. After the 45-min chase, most gO appeared as a diffuse band between 100 and 130 kDa, as with TR gO. It was also clear that in the 120- and 240-min chase samples, most of the AD169 gO remained endo H sensitive (Fig. 3B). However, there were also faint bands in the 90- to 100-kDa region of the gel in both the 120- and 240-min chase samples that resisted endo H but not PNGase. These minor bands likely represented a smaller fraction of gO that was exported to the Golgi apparatus, consistent with Western blot data described below (Fig. 4) showing steady-state, endo H-resistant AD169 gO. The small shifts in the electrophoretic migration of gO, comparing untreated (100- to 130-kDa) and endo H-treated (90- to 100-kDa) samples likely represent removal of a single N-linked glycan (that remained high in mannose) from AD169 gO that was exported from the ER, as previously reported (23).
FIG. 4.
Immunoblot analysis of the maturation of HCMV glycoproteins in virions and cells. TR- and AD169-infected fibroblast extracts and extracts of extracellular particles were prepared using 1% Triton X-100. Extracts were either left untreated or treated with endo H or PNGase F, and then proteins were subjected to gel electrophoresis and immunoblot analysis using rabbit polyclonal antibodies specific for gH, gL, or gO. (A) TR gO254 were used to detect gO; (B) AD169 gO-specific antibodies were used. Molecular weight markers are shown along the left side of each panel.
The intracellular transport of TR and AD169 gH/gL was also analyzed in these pulse-chase experiments (Fig. 3C and D). In TR-infected cells, gH was entirely endo H sensitive after a 45-min chase (Fig. 3C). After 240 min, approximately half of TR gH became endo H resistant. gL was coprecipitated with gH at all time points, and ∼30% of the gL became endo H resistant by 240 min. (Fig. 3C). The 55-kDa form of TR gO produced after endo H digestion was coprecipitated with gH/gL at all chase times, suggesting a relatively stable interaction between gH/gL and an immature form of gO. It appears that endo H-sensitive gO was bound to that fraction of gH/gL that was endo H sensitive. In AD169-infected cells, the majority of the gH and associated gL remained endo H sensitive throughout the duration of the chase, although a small fraction of endo H-resistant gH was observed (Fig. 3D). Steady-state analyses of AD169 gO using Western blots (Fig. 4) showed that much higher quantities of gO were ER exported. The inefficiency of the maturation of AD169 gH/gL might also be explained by the fact that AD169 harbors a frameshift mutation in UL131 (6, 15). We showed that gH/gL/UL128/UL130 (missing UL131) was retained in the ER (42). The majority of AD169 gO that was coprecipitated with gH/gL, even after the 240-min chase, was also endo H sensitive (Fig. 3D), although the caveat here was that endo H-resistant gO has an electrophoretic mobility similar to that of gH and would be difficult to discern.
As an alternative approach to characterizing radiolabeled proteins in HCMV-infected cells, the maturation of gO and gH/gL was analyzed by immunoblotting. Extracellular TR and AD169 particles and virus-infected cells were extracted with detergent, treated with endo H or PNGase F, and then analyzed in immunoblot experiments with gH-, gL-, or gO-specific antibodies. By this method, steady-state glycoproteins present in cells and mature virions were analyzed. In extracellular TR particles, gH and gL were entirely endo H resistant, and gO was not detected (Fig. 4A). In TR-infected cells, the majority of the gH and about half of the gL was endo H resistant, whereas all of the gO was endo H sensitive. In extracellular AD169 particles, gH, gL, and gO were all endo H resistant (Fig. 4B). As mentioned above, the minor shift in electrophoretic mobility of AD169 virion gO produced by endo H treatment likely represents one or two N-linked glycans that are not converted to complex-type glycans. A comparison of that shift to the shift due to PNGase F treatment suggested that the majority of N-linked glycans associated with virion AD169 gO were endo H resistant, indicative of ER export and maturation. In AD169-infected cells, a large fraction of steady-state gH and gL remained endo H sensitive (Fig. 4B). This was consistent with the pulse-chase results shown in Fig. 3D and might reflect the lack of UL131. However, a substantial fraction of AD169 gO in cells was endo H resistant, likely reflecting the population of gO that is ER exported and incorporated into the AD169 envelope. Observations that there were only endo H-resistant forms of TR and AD169 gH, gL, and gO (in case of AD169) in extracellular particles was consistent with ER export, transport through the Golgi apparatus to TGN compartments where viral envelopment occurs. Together, these pulse-chase and Western blotting results demonstrated that TR gO remains largely endo H sensitive, is thus retained in the ER, and either does not reach assembly compartments or is not stably incorporated into extracellular virions. An important implication of this hypothesis is that TR gO must dissociate from gH/gL in either the ER or the Golgi apparatus, at least prior to release of the mature particle.
Expression of TR gO using replication-defective Ad vectors.
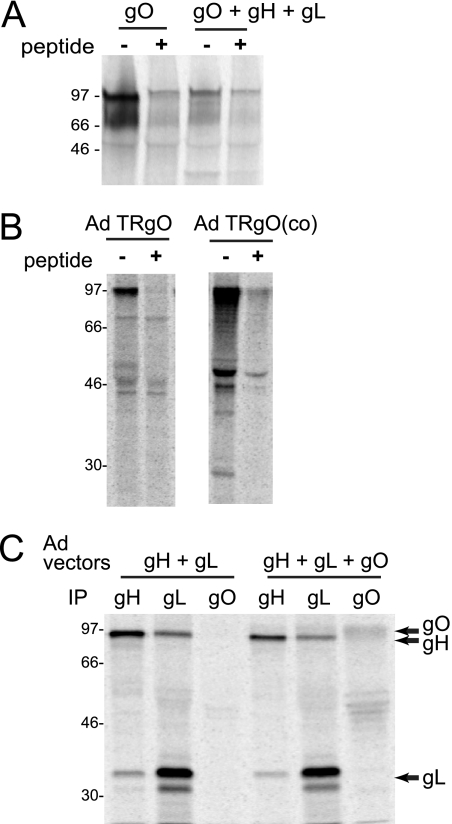
We previously studied the assembly and function of TR gH/gL/UL128-131 complexes by using nonreplicating Ad vectors (40, 42). This approach allowed expression of individual proteins or combinations of proteins so that their contribution to assembly and intracellular transport could be examined. Note that these E1− Ad vectors do not substantially express Ad proteins. However, it is often necessary to regulate the expression of glycoproteins that poison the 293 cells used to propagate Ad vector stocks. Initial efforts to construct an Ad vector expressing TR gO involved coupling the gO gene to a promoter that is unregulated by a tetracycline transactivator expressed by AdtetTrans (42, 49). However, this vector did not stably express gO. Thus, a second Ad vector was constructed in which the TR gO gene was coupled to a promoter element containing a lac operator sequence that is repressed in 293 cells expressing the lac repressor (32). AdTRgO stably expressed gO through numerous passages. However, gO expression by AdTRgO was markedly reduced when cells were coinfected with Ad vectors expressing gH and gL (Fig. 5A). This was unrelated to the gH/gL proteins because a vector expressing green fluorescent protein (GFP), AdGFP, also dramatically reduced gO expression (data not shown). To increase expression of TR gO, we constructed a third Ad vector using a synthesized, codon-optimized gO gene. By introducing silent mutations, the synthetic gene was engineered to remove potential negative, cis-acting RNA elements and to avoid the use of rare codons, without changing the coded amino acids. The resulting Ad vector, denoted AdTRgO(co), expressed at least 10-fold more gO per infectious unit compared with AdTRgO (Fig. 5B) and allowed us to coexpress gH/gL with gO in the cells (Fig. 5C).
FIG. 5.
Expression of TR gO by nonreplicating Ad vectors. U373 cells were infected with various Ad vectors for 16 to 24 h and labeled for 2 h with [35S]methionine/cysteine, and then expressed HCMV proteins were immunoprecipitated and analyzed by gel electrophoresis. (A) Cells were infected with 50 PFU/cell of an Ad vector expressing TR gO or with Ad vectors expressing gH, gL, and gO (50 PFU/cell each). gO was immunoprecipitated using rabbit TR gO254 antibodies in the presence or absence of gO 254-271 peptide as a competitive inhibitor. (B) Cells were infected with 50 PFU/cell of either Ad TR gO or an Ad vector containing a codon-optimized TR gO gene, AdTRgO(co). gO was immunoprecipitated using anti-TR gO254 antibodies with or without peptide. The two panels represent samples analyzed on the same gel. (C) Cells were infected with Ad vectors expressing gH and gL or gH, gL, and AdTRgO(co). gH was precipitated using anti-gH MAb 14-4b, gL with rabbit anti-gL antibodies, or gO precipitated with rabbit anti-TR gO254 antibodies. Molecular weight markers are shown along the left side of each panel.
TR gO promotes intracellular transport of gH/gL from the ER to the Golgi apparatus.
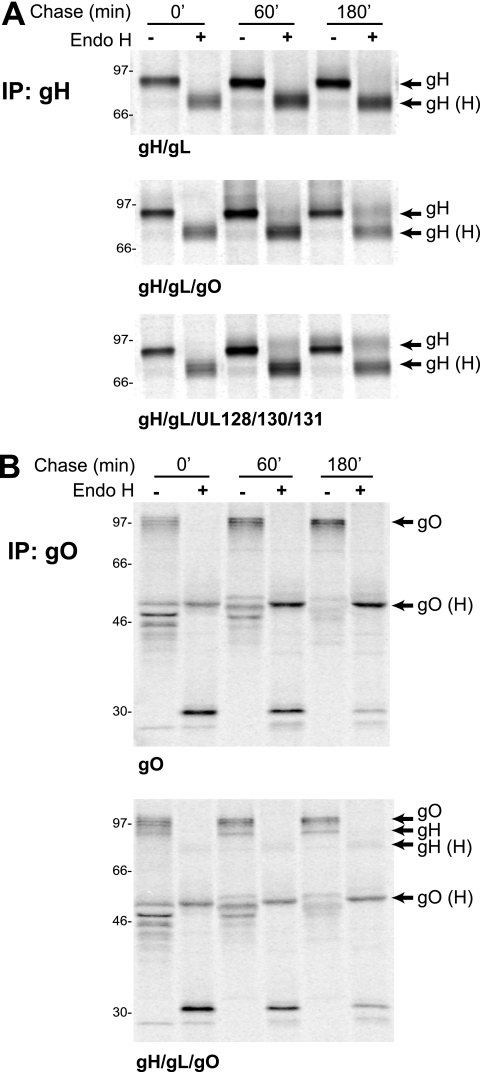
Previous experiments using Ad vectors to express gH/gL with the UL128-131 proteins showed that gH/gL was retained in the ER unless all of UL128-131 was coexpressed (42). However, in fibroblasts infected with TR mutants lacking the UL128-131 proteins, a substantial fraction of gH/gL became endo H resistant (42). These results suggested that gO could also facilitate ER export of gH/gL. To test this, gH/gL was expressed alone or in combination with gO or with UL128-131 using Ad vectors. U373 cells were used in these experiments because they infect well with Ad vectors, we have extensive experience using these cells in intracellular trafficking experiments involving short labeling periods, and previous results with U373 cells paralleled those obtained with HCMV-infected U373 cells and fibroblasts (17, 42). Cells were pulse-labeled with [35S]methionine/cysteine for 5 min, the label was chased for 0, 60, or 180 min, and then gH was immunoprecipitated and treated with endo H. When gH and gL were expressed alone, gH remained endo H sensitive through 180 min (Fig. 6A, top). As shown previously, when the UL128-131 proteins we coexpressed with gH/gL, a fraction of gH became endo H resistant (Fig. 6A, bottom). Similarly, when gO was expressed with gH and gL, a similar fraction of gH attained resistance to endo H (Fig. 6A, middle). There were no additive effects of expressing gO and UL128-131 with gH/gL (not shown). The quantities of gH that remain endo H sensitive even after 180 min may reflect more gH/gL expressed compared with expression of gO or UL128-131. However, we consistently observed low rates of gH/gL intracellular transport. In this regard, it is important to note that approximately half of gH produced in HCMV-infected cells remained endo H sensitive after 240 min (Fig. 3C).
FIG. 6.
Maturation of N-linked oligosaccharides associated with gH and gO expressed by Ad vectors. U373 cells were infected with the indicated combinations of Ad vectors expressing gH, gL, TR gO, UL128, UL130, and UL131. Cells were labeled for 5 min with [35S]methionine/cysteine, and then the label was chased for 0, 60, or 180 min. (A) gH was immunoprecipitated with anti-gH MAb 14-4b; (B) gO was immunoprecipitated using TR gO254 antibodies. Immunoprecipitated proteins were either left untreated or treated with endo H. Arrows indicate proteins corresponding to gH, gO, or endo H-sensitive forms of these proteins (H). Molecular weight markers are shown along the left side of each panel.
TR gO expressed using Ad vectors, with or without gH/gL, remained endo H sensitive (Fig. 6B). In cells expressing gH, gL, and gO, a weak band of 85 to 90 kDa protein was coprecipitated with gO. This protein was gH, because the band was not present when gH was not expressed. This gH was entirely sensitive to endo H, contrasting with the direct immunoprecipitation (IP) of gH in which some gH became endo H resistant (Fig. 6A). Thus, gH that is precipitated with gO is largely or entirely endo H sensitive. Together with observations that gO is predominately endo H sensitive in TR-infected cells (Fig. 3A), these results suggest that TR gO does not traffic extensively to the Golgi apparatus where N-linked oligosaccharides are processed.
TR gO dissociates from gH/gL or is degraded before virion envelopment and egress.
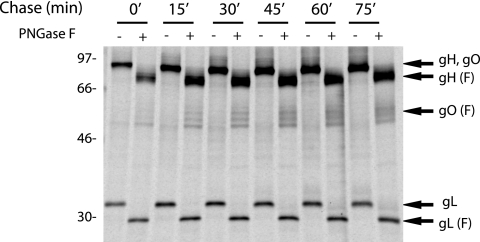
To characterize TR gO association with gH/gL, we performed experiments involving short pulse and chase periods. Cells were infected with Ad vectors expressing gH, gL, and gO, labeled for 15 min, and then label chased for 15, 30, 45, 60, or 75 min. Extracts were immunoprecipitated with an anti-gH MAb and then treated with PNGase F so that we could separate gH (∼75 kDa) from gO (a series of bands from 50 to 55 kDa). In the pulse samples (0-min chase), gL was coprecipitated with gH, indicating that association of gH and gL occurs very rapidly. Importantly, gL associated with gH/gL did not increase substantially beyond 15 min. Association of gO with gH/gL became evident after 15 min, and this increased until reaching 45 min and then remained relatively constant through 75 min. These results suggested that TR gO association with gH is slower than with gL, as has been described for AD169 gO (23).
In a second experiment involving Ad vectors, we attempted to characterize dissociation of gO from gH/gL by using longer chase periods. As shown in Fig. 7, after a 1-h chase, gO was found in complex with gH/gL (Fig. 8). At this time point, all three proteins were sensitive to endo H. Between 1 h and 3 h, a fraction of both gH and gL became endo H resistant. This fraction of endo H-resistant gH and gL did not increase substantially over the course of the 6- and 9-h chases. Importantly, it appeared that most of the gO associated with gH/gL remained endo H sensitive, as evidenced by the similar intensities of 55- to 60-kDa bands produced by both endo H and PNGase (Fig. 8). However, note that any small fraction of gO that became endo H resistant might be difficult to discern, given that this form of gO migrates in gels in a position similar to that of mature gH. Related to this point, the vast majority of total gO (not that bound to gH/gL) when coexpressed with gH/gL using Ad vectors remained endo H sensitive after 3 h (Fig. 6B). These results suggest that gH/gL processing is slow, and even after 9 h, much of the gH remained endo H sensitive. Given that substantial endo H-sensitive gH remained even after 9 h, it seems likely that gO remains bound to this endo H-sensitive fraction of gH/gL for relatively long periods.
FIG. 7.
Assembly of gH/gL/gO complexes expressed by Ad vectors. U373 cells were infected with Ad vectors expressing gH, gL, and TR gO [AdTRgO(co)] for 18 to 24 h and labeled for 5 min with [35S]methionine/cysteine, and then the label was chased for 0, 15, 45, 60, or 75 min. Cell extracts were immunoprecipitated using anti-gH MAb 14-4b, and proteins were either left untreated or treated with PNGase F. Arrows indicate bands corresponding to gH, gO, gL, or PNGase F-sensitive forms of these proteins (F). Molecular weight markers are shown on the left.
FIG. 8.
Stability of gH/gL/gO complexes expressed by Ad vectors. U373 cells were infected with Ad vectors expressing gH, gL, and TR gO [AdTRgO(co)] for 18 to 24 h and labeled with [35S]methionine/cysteine for 10 min, and the label was chased for 1, 3, 6, or 9 h. Cell extracts were immunoprecipitated with anti-gH MAb 14-4b, and then proteins were either left untreated or were treated with endo H or PNGase F. Arrows indicate bands corresponding to gH, gO, gL, or endo H- or PNGase F-sensitive forms of these proteins (H, F). Molecular weight markers are shown on the left.
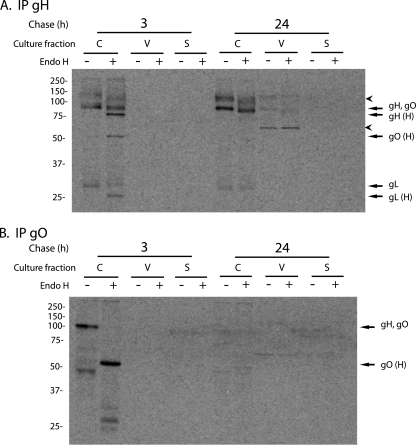
It was of interest to characterize the interactions of gO and gH/gL in HCMV-infected cells, cells that differ from Ad-infected cells in that they express other HCMV proteins and produce HCMV assembly compartments and virus particles. TR-infected fibroblasts were labeled for 1 h with [35S]methionine/cysteine, and then the label was chased for 3 or 24 h. At each of these times, infected cells and cell culture supernatants were harvested, and HCMV particles were pelleted from the cell supernatants through 20% sorbitol, producing the following three fractions: cells, extracellular virus particles, and supernatants with virus removed. Each fraction was disrupted with 1% Triton X-100, and gH or gO were immunoprecipitated. After the 3-h chase, there was no obvious gH in extracellular virions, and about half of gH in the cells was endo H resistant (Fig. 9A). After the 3-h chase, gO was found in cells and was largely or entirely endo H sensitive (Fig. 9B), as was the gO immunoprecipitated with gH/gL (Fig. 9A). Again, note that endo H-resistant gO would comigrate with gH.
FIG. 9.
Pulse-chase analysis of HCMV glycoproteins expressed in cells and incorporated into extracellular virus particles. Human fibroblasts (NHDF) were infected with 3 IU/cell of HCMV TR for 4 days. The cells were labeled for 1 h with [35S]methionine/cysteine (500 uCi/ml), and then the label was chased for 3 or 24 h. The following three fractions were prepared: cells (C), virus particles derived from cell culture supernatants by centrifugation through 20% sorbitol cushions (V), and the cell culture supernatants after viruses were pelleted (S). Each fraction was extracted with 1% Triton X-100, and then gH or gO was immunoprecipitated using anti-gH MAb 14-4b (A) or anti-TR gO254 antibodies (B). Immunoprecipitated proteins were left untreated or treated with endo H. Arrows indicate bands corresponding to gH, gO, gL, or endo H-sensitive forms of these proteins (H). Arrowheads mark unidentified proteins that coprecipitated with gH. Molecular weight markers are shown along the left side of each panel.
A protein larger than gH or gO of ∼140 to 150 kDa was coprecipitated with gH (top arrowhead in Fig. 9A). This protein was sensitive to endo H and not detected in cells in which gH/gL was expressed (without other HCMV proteins) using Ad vectors. We also detected this glycoprotein in virus particles after the 24-h chase (Fig. 9A, left panels). It was formally possible that this protein was a previously unrecognized form of gO. However, the 140- to 150-kDa protein was substantially larger than any form of gO detected in any of our experiments or in previous studies. Moreover, this protein was not recognized by TR gO254 sera (Fig. 9B). We previously coprecipitated HCMV gB with gH/gL using this same anti-gH antibody (50). This result and the absence of gO reactivity lead us to believe that this protein is gB that coprecipitates with gH/gL from HCMV-infected cells and extracellular particles.
All of gH found in HCMV-infected cells after the 24-h chase migrated at ∼85 to 90 kDa and was entirely or largely endo H resistant (a small mobility shift was observed, as described above). Moreover, a fraction of this gH had been released into the supernatant in the form of pelleted virus particles, and this gH was also endo H resistant. After the 24-h chase, we did not detect gO in any of the fractions, either by direct immunoprecipitation of gO (Fig. 9B) or in association with gH/gL (Fig. 9A). Note that there was a prominent band at ∼65 kDa in viral particles (bottom arrowhead in Fig. 9A) that migrated slower than gO and was likely pp65, a protein that is frequently nonspecifically immunoprecipitated from HCMV-infected cells and virions. Together, the results involving both Ad vectors and HCMV-infected cells showed that gH/gL was slowly processed to an endo H-resistant form and that gO was not stably bound to gH/gL. Instead, gO is lost from cells, not secreted into cell culture supernatants either in virus particles or as a soluble protein, and most likely degraded.
DISCUSSION
It is now well established that beta- and gammaherpesviruses assemble multiple gH/gL complexes that function to mediate entry into different cell types (4, 21, 25, 34, 43). The HCMV gH/gL/UL128-131 complex mediates entry into epithelial and endothelial cells, apparently by binding cellular receptors, and all five proteins of this complex are required for ER export and function (40-42). Much less was known about the gH/gL/gO complex, which is apparently distinct from gH/gL/UL128-131 in the virion envelope, at least for the AD169 strain (22, 53). Given the differences between laboratory and clinical strains of HCMV with respect to UL128-131, it was important to characterize a gO molecule expressed by a low-passage clinical strain. Moreover, whether gH/gL/gO functions in virus entry has not been tested. It was clear that efficient HCMV infection of fibroblasts and cell-to-cell spread requires gO, yet a HCMV gO-null mutant could spread normally between endothelial cells (27). Based on this relatively incomplete data, one prevailing model in the field suggested that gH/gL/UL128-131 mediates entry into epithelial/endothelial cells, whereas gH/gL/gO promotes infection of fibroblasts. This model was tested in this paper in biochemical and cell trafficking studies of TR gO and in the accompanying paper by Wille et al. (54) characterizing a TR gO-null mutant.
The major observation we report here is that the low-passage clinical strain TR expresses a gO molecule that was not detected in extracellular virions. Using TR gO254 antibodies, we estimated that <1% of total gO (in cells and virus) was present in extracellular virions. In contrast, other HCMV glycoproteins were present in virions at 38 to 67% of the total protein. One might argue that gO epitopes recognized by TR gO254 antibodies were lost after gO was incorporated into virions, so that we failed to detect mature gO in virions. This seems unlikely, given that a second antipeptide sera, TR gO110, also failed to detect gO in virions. Since these antibodies were both anti-peptide antibodies and used in Western blots in which gO was denatured, it would necessary for the maturation of gO to include posttranslational, covalent modifications at both sites including residues 110 to 127 and 254 to 275 for both antibodies to fail to recognize mature gO. It seems highly unlikely that these modifications would occur at both sites and also mask antibody recognition in both cases.
We also obtained evidence that TR gO was largely or entirely endo H sensitive and, thus, ER retained in both pulse-chase experiments and steady-state Western blot analyses. Since incorporation of gO in the virion envelope is thought to occur in a post-ER Golgi apparatus or TGN compartment, these results strongly support the hypothesis that TR gO is not incorporated into the virion envelope. TR gO remained bound to a fraction of gH/gL for long periods (6 to 9 h) in both Ad vector- and HCMV-infected cells. Apparently, gH/gL that was associated with gO over these long time periods was, itself, endo H sensitive. However, slowly, a fraction of gH/gL attained endo H resistance, and this mature, endo H-resistant gH/gL was found in extracellular virions after a 24-h chase. Therefore, gO remains bound to gH/gL in the ER, but when gH/gL is slowly transported to the Golgi apparatus and post-Golgi compartments for assembly, the gO is removed from gH/gL. This was very different from AD169 gO, which was detected in the envelope of extracellular particles in amounts similar to those found in gH and gL, as previously reported (22). As expected, AD169 gO in extracellular virions was endo H resistant, and about half of the glycoprotein in infected cells became endo H resistant. Thus, clinical strain TR and lab strain AD169 differ in how gO is transported in infected cells and on whether gO is stably incorporated into the virion envelope. These results challenge the hypothesis that TR gH/gL/gO mediates entry into fibroblasts for clinical HCMV, though it is possible that AD169 gH/gL/gO is involved in entry of AD169.
We do not currently understand whether these observations with TR gO will extend to gO molecules expressed by other clinical HCMV strains. Several different gO anti-peptide antibodies (produced using the TR gO sequences) did not recognize HCMV gO molecules from low-passage Merlin and FIX or lab strains AD169, Towne, and Toledo. Moreover, AD169-specific gO antibodies did not recognize TR, Merlin, FIX, or Towne gO. However, the AD169 gO antibodies did weakly recognize Toledo gO, which appeared to be present in extracellular virions. Toledo possesses a gene inversion that disrupts expression of UL128 (15) and, thus, does not assemble gH/gL/UL128-131. Based on this limited correlation, it is tempting to speculate that the formation of a stable gH/gL/gO complex and its incorporation into extracellular virions might be related to the loss of expression of gH/gL/UL128-131. As noted above, loss of UL128-131 can occur rapidly during passage of HCMV on fibroblasts (2). Moreover, gO is highly diverse among different HCMV strains, with differences up to 25% in amino acid sequences, making gO among the most variable of HCMV proteins (10, 36, 37). Some of the diversity in gO might theoretically stem from the rapid changes in gH/gL complexes that occur when virus is propagated on fibroblasts, i.e., loss of UL128-131 might promote changes in gO. However, the salient point is that TR can infect epithelial and endothelial cells and monocyte-macrophages, whereas AD169 cannot. Therefore, in the absence of other information, we consider TR to be the wild type with regard to how gO behaves and expect that this property will be found with other clinical or low-passage HCMV. To clarify this point, we are generating anti-Merlin gO antibodies.
Several lines of evidence suggest that TR gO functions as a chaperone to promote ER export of gH/gL and incorporation of gH/gL into the virion envelope. First, gO was largely or entirely endo H sensitive, whether expressed in HCMV-infected cells or by using Ad vectors. Second, coexpression of gO with gH/gL promoted endo H resistance of gH/gL. Third, in the accompanying paper by Wille et al., we showed that the gO-null TR mutant incorporated very little gH/gL (5% compared with that of wild-type HCMV) into extracellular virions (54). UL128-131 binding to gH/gL also promotes ER export (42). Thus, it appears that gH/gL present in the ER is bound by either gO or the UL128-131 proteins, and changes in the structure of gH/gL associated with these interactions allow export from the ER to the Golgi apparatus. From the Golgi apparatus, it is likely that gH/gL complexes are transported to assembly compartments. Although the gO-null mutant incorporated very little gH/gL into extracellular virions, the small quantity of gH/gL present in virions exhibited increased amounts of UL128-131. This further suggests that gO and UL128-131 compete for binding to gH/gL. Consistent with this hypothesis, coexpression of gO with gH/gL decreased binding of UL128-131 in experiments involving Ad vectors (B. J. Ryckman, unpublished observations). However, the ER export and incorporation of gH/gL into virions appears to be largely influenced by gO, as evidenced by the phenotype of the HCMV gO-null mutant. Perhaps, gO binds gH/gL much better than UL128-131, or gO might be expressed at higher molar ratios than UL128-131.
Since gO is not present in extracellular particles of TR, it follows that the interaction between TR gO and gH/gL was transient, i.e., gO dissociated from gH/gL or was degraded. When gH, gL, and gO were coexpressed using Ad vectors or in HCMV-infected cells, maturation of gH/gL (acquisition of endo H resistance) was slow, and about half of gH was endo H resistant even after a 9-h chase period. The gO that coprecipitated with gH/gL was probably associated with that fraction of gH/gL that remained endo H sensitive. Pulse-chase experiments involving HCMV-infected cells and long (24-h) chase periods showed endo H-resistant gH present in extracellular virions, but there was no gO in these virions. Therefore, gO is not stably associated with that fraction of gH/gL that is incorporated into virions that are secreted. There was no evidence of gO in cell culture supernatants centrifuged to remove virus particles, suggesting that gO was degraded in either the ER or some post-ER compartment. Note that it was relatively difficult to establish whether there was a minor fraction of endo H-resistant gO associated with gH/gL in pulse-chase experiments, because endo H-resistant gO comigrates with gH in gels. However, a comparison the intensity of gO bands in endo H-treated samples with that in PNGase-treated samples suggested that the endo H-resistant fraction of gO was not large.
AD169 gO is disulfide linked to gH/gL (23, 48), but whether TR gO is disulfide linked to gH/gL is not yet clear. Assuming this, it is possible that TR gO is proteolyzed while still in association with gH/gL complexes. The slow assembly and disassembly of gH/gL complexes and the potential proteolysis of gO are reminiscent of the intracellular transport of major histocompatibility complex (MHC) class II complexes. Class II α and β chains assemble in the ER with a third protein, the invariant chain (Ii) (reviewed in reference 8). Ii acts as a molecular chaperone, promoting ER export of class II complexes and blocking the active site (peptide-binding groove) of class II dimers. Ii is proteolytically removed from α/β complexes following transport to Golgi apparatus and acidic endosomes, where antigenic peptides are loaded onto class II proteins. As with gH/gL complexes, intracellular transport of class II α/β/Ii complexes and Ii proteolysis are slow, taking 6 to 12 h (9, 18).
Previously, we characterized gH/gL/UL128-131 using interference, demonstrating that expression of gH/gL/UL128-131 significantly (95%) inhibited HCMV entry into epithelial cells (40). This suggested saturable receptors for gH/gL/UL128-131 in epithelial cells. Expression of gH/gL alone did not cause interference in epithelial cells, although there was some (40 to 50%) interference with gH/gL in fibroblasts (40). Recently, we coexpressed gO with gH/gL in fibroblasts and obtained more efficient interference than with expression of gH/gL alone, i.e., <5% of the cells were infected (M. C. Chase, B. J. Ryckman, and D. C. Johnson, unpublished observations). Previously, we showed that gH/gL and gB are both necessary and sufficient for cell-cell fusion (50). Apparently, the relatively low levels of gH/gL that can reach cell surfaces without gO are sufficient for cell-cell fusion. However, recent results showed that gO coexpression with gH/gL and gB substantially increases cell-cell fusion (M. C. Chase, unpublished results). Therefore, gO apparently increases interference and cell-cell fusion by increasing gH/gL ER export and surface expression. Nevertheless, it is important to consider the functional complex as gH/gL and not as gH/gL/gO.
In summary, the data here and in the accompanying report by Wille et al. (54) provide a different view of how HCMV gO functions compared with those from previous studies of AD169 gO (21-23, 29, 48, 53). We speculate that lab strains of HCMV such as AD169 adapted to the fibroblast entry pathway by mutating UL128-131 and also by altering gO so that the glycoprotein is present in virions. TR gO binds gH/gL, promotes ER export and incorporation into the virion envelope, but dissociates or is degraded before virions are secreted. Thus, gO is a bona fide chaperone, unlike gL that remains bound to gH. These studies are the first to support the notion that functionally important gH/gL (lacking both gO and UL128-131) is found in virions. Together with the data in the accompanying paper (54), it appears that gH/gL is required for entry into all three cell types as follows: epithelial and endothelial cells and fibroblasts. Therefore, our current model suggests that HCMV requires both gH/gL and gH/gL/UL128-131 for entry into epithelial and endothelial cells. This differs significantly from what has been reported for EBV, HHV-6, and HHV-7, which utilize one or other of two different gH/gL complexes to enter different cells (4, 25, 34, 43).
Acknowledgments
This work was supported by a grant from the National Institutes of Health, R01AI081517 to D.C.J., and a Ruth L. Kirschstein National Research Service Award from the NEI (EY015965) to B.J.R.
We are grateful to William J. Britt (University of Alabama—Birmingham) and Teresa Compton (Novartis) for supplying important antibodies. We thank Richard Stanton and Gavin Wilkinson (Cardiff University) and Tom Shenk (Princeton University) for providing BAC clones of various HCMV strains. Tiffani Howard prepared the graphics. Finally, we appreciate advice and support from Adam Vanarsall and Paul Wille and other members of the Johnson and Nelson laboratories.
Footnotes
Published ahead of print on 23 December 2009.
REFERENCES
- 1.Adler, B., L. Scrivano, Z. Ruzcics, B. Rupp, C. Sinzger, and U. Koszinowski. 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 87:2451-2460. [DOI] [PubMed] [Google Scholar]
- 2.Akter, P., C. Cunningham, B. P. McSharry, A. Dolan, C. Addison, D. J. Dargan, A. F. Hassan-Walker, V. C. Emery, P. D. Griffiths, G. W. Wilkinson, and A. J. Davison. 2003. Two novel spliced genes in human cytomegalovirus. J. Gen. Virol. 84:1117-1122. [DOI] [PubMed] [Google Scholar]
- 3.Bogner, E., M. Reschke, B. Reis, E. Reis, W. Britt, and K. Radsak. 1992. Recognition of compartmentalized intracellular analogs of glycoprotein H of human cytomegalovirus. Arch. Virol. 126:67-80. [DOI] [PubMed] [Google Scholar]
- 4.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. [DOI] [PubMed] [Google Scholar]
- 5.Britt, W. J., and C. A. Alford. 1996. Cytomegaloviruses, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven Press, Philadelphia, PA.
- 6.Cha, T. A., E. Tom, G. W. Kemble, G. M. Duke, E. S. Mocarski, and R. R. Spaete. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191:387-395. [DOI] [PubMed] [Google Scholar]
- 8.Cresswell, P. 1994. Assembly, transport, and function of MHC class II molecules. Annu. Rev. Immunol. 12:259-293. [DOI] [PubMed] [Google Scholar]
- 9.Cresswell, P. 1996. Invariant chain structure and MHC class II function. Cell 84:505-507. [DOI] [PubMed] [Google Scholar]
- 10.Dolan, A., C. Cunningham, R. D. Hector, A. F. Hassan-Walker, L. Lee, C. Addison, D. J. Dargan, D. J. McGeoch, D. Gatherer, V. C. Emery, P. D. Griffiths, C. Sinzger, B. P. McSharry, G. W. Wilkinson, and A. J. Davison. 2004. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 85:1301-1312. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. U. S. A. 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerna, G., E. Percivalle, D. Lilleri, L. Lozza, C. Fornara, G. Hahn, F. Baldanti, and M. G. Revello. 2005. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J. Gen. Virol. 86:275-284. [DOI] [PubMed] [Google Scholar]
- 14.Hahn, G., H. Khan, F. Baldanti, U. H. Koszinowski, M. G. Revello, and G. Gerna. 2002. The human cytomegalovirus ribonucleotide reductase homolog UL45 is dispensable for growth in endothelial cells, as determined by a BAC-cloned clinical isolate of human cytomegalovirus with preserved wild-type characteristics. J. Virol. 76:9551-9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn, G., M. G. Revello, M. Patrone, E. Percivalle, G. Campanini, A. Sarasini, M. Wagner, A. Gallina, G. Milanesi, U. Koszinowski, F. Baldanti, and G. Gerna. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 78:10023-10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlow, E., and D. Lane. 1999. Using antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 17.Hegde, N. R., C. Dunn, D. M. Lewinsohn, M. A. Jarvis, J. A. Nelson, and D. C. Johnson. 2005. Endogenous human cytomegalovirus gB is presented efficiently by MHC class II molecules to CD4+ CTL. J. Exp. Med. 202:1109-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegde, N. R., R. A. Tomazin, T. W. Wisner, C. Dunn, J. M. Boname, D. M. Lewinsohn, and D. C. Johnson. 2002. Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: a novel mechanism for evading major histocompatibility complex class II antigen presentation. J. Virol. 76:10929-10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobom, U., W. Brune, M. Messerle, G. Hahn, and U. H. Koszinowski. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 74:7720-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homman-Loudiyi, M., K. Hultenby, W. Britt, and C. Soderberg-Naucler. 2003. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-golgi network 46, and mannosidase II. J. Virol. 77:3191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber, M. T., and T. Compton. 1997. Characterization of a novel third member of the human cytomegalovirus glycoprotein H-glycoprotein L complex. J. Virol. 71:5391-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber, M. T., and T. Compton. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72:8191-8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber, M. T., and T. Compton. 1999. Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus. J. Virol. 73:3886-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber, M. T., T. W. Wisner, N. R. Hegde, K. A. Goldsmith, D. A. Rauch, R. J. Roller, C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and D. C. Johnson. 2001. Herpes simplex virus with highly reduced gD levels can efficiently enter and spread between human keratinocytes. J. Virol. 75:10309-10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutt-Fletcher, L. M. 2007. Epstein-Barr virus entry. J. Virol. 81:7825-7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis, M. A., T. R. Jones, D. D. Drummond, P. P. Smith, W. J. Britt, J. A. Nelson, and C. J. Baldick. 2004. Phosphorylation of human cytomegalovirus glycoprotein B (gB) at the acidic cluster casein kinase 2 site (Ser900) is required for localization of gB to the trans-Golgi network and efficient virus replication. J. Virol. 78:285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang, X. J., B. Adler, K. L. Sampaio, M. Digel, G. Jahn, N. Ettischer, Y. D. Stierhof, L. Scrivano, U. Koszinowski, M. Mach, and C. Sinzger. 2008. UL74 of human cytomegalovirus contributes to virus release by promoting secondary envelopment of virions. J. Virol. 82:2802-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaye, J. F., U. A. Gompels, and A. C. Minson. 1992. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J. Gen. Virol. 73:2693-2698. [DOI] [PubMed] [Google Scholar]
- 29.Kinzler, E. R., R. N. Theiler, and T. Compton. 2002. Expression and reconstitution of the gH/gL/gO complex of human cytomegalovirus. J. Clin. Virol. 25(Suppl. 2):S87-S95. [DOI] [PubMed] [Google Scholar]
- 30.Landolfo, S., M. Gariglio, G. Gribaudo, and D. Lembo. 2003. The human cytomegalovirus. Pharmacol. Ther. 98:269-297. [DOI] [PubMed] [Google Scholar]
- 31.Li, L., J. A. Nelson, and W. J. Britt. 1997. Glycoprotein H-related complexes of human cytomegalovirus: identification of a third protein in the gCIII complex. J. Virol. 71:3090-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews, D. A., D. Cummings, C. Evelegh, F. L. Graham, and L. Prevec. 1999. Development and use of a 293 cell line expressing lac repressor for the rescue of recombinant adenoviruses expressing high levels of rabies virus glycoprotein. J. Gen. Virol. 80:345-353. [DOI] [PubMed] [Google Scholar]
- 33.Molesworth, S. J., C. M. Lake, C. M. Borza, S. M. Turk, and L. M. Hutt-Fletcher. 2000. Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells. J. Virol. 74:6324-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori, Y., P. Akkapaiboon, S. Yonemoto, M. Koike, M. Takemoto, T. Sadaoka, Y. Sasamoto, S. Konishi, Y. Uchiyama, and K. Yamanishi. 2004. Discovery of a second form of tripartite complex containing gH-gL of human herpesvirus 6 and observations on CD46. J. Virol. 78:4609-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 100:14976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy, E., I. Rigoutsos, T. Shibuya, and T. E. Shenk. 2003. Reevaluation of human cytomegalovirus coding potential. Proc. Natl. Acad. Sci. U. S. A. 100:13585-13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paterson, D. A., A. P. Dyer, R. S. Milne, E. Sevilla-Reyes, and U. A. Gompels. 2002. A role for human cytomegalovirus glycoprotein O (gO) in cell fusion and a new hypervariable locus. Virology 293:281-294. [DOI] [PubMed] [Google Scholar]
- 38.Plachter, B., C. Sinzger, and G. Jahn. 1996. Cell types involved in replication and distribution of human cytomegalovirus. Adv. Virus Res. 46:195-261. [DOI] [PubMed] [Google Scholar]
- 39.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryckman, B. J., M. C. Chase, and D. C. Johnson. 2008. HCMV gH/gL/UL128-131 interferes with virus entry into epithelial cells: evidence for cell type-specific receptors. Proc. Natl. Acad. Sci. U. S. A. 105:14118-14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryckman, B. J., M. A. Jarvis, D. D. Drummond, J. A. Nelson, and D. C. Johnson. 2006. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J. Virol. 80:710-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryckman, B. J., B. L. Rainish, M. C. Chase, J. A. Borton, J. A. Nelson, M. A. Jarvis, and D. C. Johnson. 2008. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J. Virol. 82:60-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadaoka, T., K. Yamanishi, and Y. Mori. 2006. Human herpesvirus 7 U47 gene products are glycoproteins expressed in virions and associate with glycoprotein H. J. Gen. Virol. 87:501-508. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinzger, C., A. Grefte, B. Plachter, A. S. Gouw, T. H. The, and G. Jahn. 1995. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 76:741-750. [DOI] [PubMed] [Google Scholar]
- 46.Smith, I. L., I. Taskintuna, F. M. Rahhal, H. C. Powell, E. Ai, A. J. Mueller, S. A. Spector, and W. R. Freeman. 1998. Clinical failure of CMV retinitis with intravitreal cidofovir is associated with antiviral resistance. Arch. Ophthalmol. 116:178-185. [DOI] [PubMed] [Google Scholar]
- 47.Stanton, R., D. Westmoreland, J. D. Fox, A. J. Davison, and G. W. Wilkinson. 2005. Stability of human cytomegalovirus genotypes in persistently infected renal transplant recipients. J. Med. Virol. 75:42-46. [DOI] [PubMed] [Google Scholar]
- 48.Theiler, R. N., and T. Compton. 2002. Distinct glycoprotein O complexes arise in a post-Golgi compartment of cytomegalovirus-infected cells. J. Virol. 76:2890-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomazin, R., J. Boname, N. R. Hegde, D. M. Lewinsohn, Y. Altschuler, T. R. Jones, P. Cresswell, J. A. Nelson, S. R. Riddell, and D. C. Johnson. 1999. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 5:1039-1043. [DOI] [PubMed] [Google Scholar]
- 50.Vanarsdall, A. L., B. J. Ryckman, M. C. Chase, and D. C. Johnson. 2008. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J. Virol. 82:11837-11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp II, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, D., and T. Shenk. 2005. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J. Virol. 79:10330-10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, D., and T. Shenk. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. U. S. A. 102:18153-18158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wille, P. T., A. J. Knoche, J. A. Nelson, M. A. Jarvis, and D. C. Johnson. 2010. A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells. J. Virol. 84:2585-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wisner, T., C. Brunetti, K. Dingwell, and D. C. Johnson. 2000. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J. Virol. 74:2278-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]