Abstract
Abundance of ammonia-oxidizing Archaea (AOA) was found to be always greater than that of ammonia-oxidizing Bacteria along an estuarine salinity gradient, and AOA abundance was highest at intermediate salinity. However, AOA abundance did not correlate with potential nitrification rates. This lack of correlation may be due to methodological limitations or alternative energy sources.
Nitrification, the sequential oxidation of ammonia to nitrite and nitrate, is a critical step in the nitrogen cycle and is mediated by a suite of phylogenetically and physiologically distinct microorganisms. However, recent discoveries, such as anammox bacteria (33) and ammonia-oxidizing Archaea (20), have illustrated the fact that our current understanding of the depth and breadth of ammonia-oxidizing microorganisms is incomplete. Since their discovery several years ago, ammonia-oxidizing Archaea (AOA) have been reported to be present in a variety of environments, including various soils and sediments (13, 22, 34), oxic and suboxic marine layers (5, 10, 25, 36), estuaries (4, 9, 26, 31), subterranean environments (35), wastewater sludge (30), corals (6), and sponges (14). Much of the evidence for the presence of AOA in the environment comes from molecular studies of the distribution of presumptive archaeal ammonia monooxygenase genes thought to be homologs of the betaproteobacterial genes encoding the alpha subunit of ammonia monooxygenase (amoA). However, what remains less certain is the contribution to nitrification of AOA relative to ammonia-oxidizing Betaproteobacteria (β-AOB). In pelagic systems where AOA consistently outnumber β-AOB (10, 21, 25), there is strong evidence for archaeal nitrification based on molecular and biogeochemical data (5, 36). In estuarine systems, however, the data are more ambiguous (9, 27, 31), suggesting a more complex relationship in these systems.
In a previous study of the abundance of β-AOB and potential nitrification rates along a salinity gradient in the Plum Island Sound estuary (8), we found site-specific ammonia oxidation kinetics that correlated with salinity and β-AOB community structure. The highest rates, but lowest β-AOB abundance, were detected at the low-salinity site, suggesting that there may be other ammonia oxidizers contributing to nitrification or that there are significant differences in cell-specific oxidation kinetics of the resident β-AOB. Since the previous study did not include Archaea, we have now investigated whether the distribution of AOA could explain the various correlations between potential nitrification rates and β-AOB abundance along the salinity gradient.
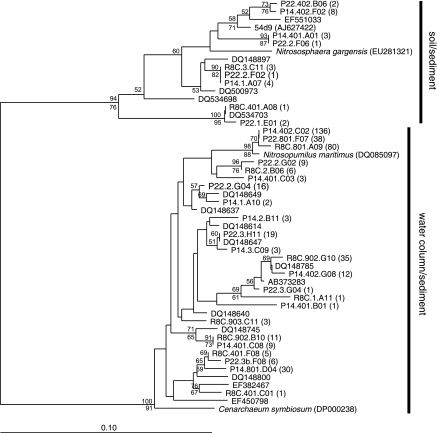
Diversity and abundance of AOA were measured from three sites representing low (0.5 to 8.7 practical salinity units [psu]), mid (6.3 to 24.7 psu), and high (20.5 to 31.7 psu) salinity in the Plum Island Sound estuary in northeastern Massachusetts. Site descriptions and sampling methods are described elsewhere (7, 8, 16). Archaeal amoA gene sequences were amplified, cloned, and analyzed as previously described (11, 20). A total of 14 clone libraries were generated from samples taken on at least three separate dates from each site (Table 1). Neighbor-joining and parsimony analyses were performed by using ARB software (23). Most of the sequences were affiliated with the water column/sediment cluster first described by Francis et al. (13) and were closely related to sequences recovered from other marine and estuarine environments (Fig. 1). The remaining sequences were affiliated with the soil/sediment cluster. Sequences most closely related to the cultivated marine archaeon, Nitrosopumilus maritimus, were the most frequently recovered sequence type from all three sites. Additionally, sequences from all three sites were distributed throughout the tree and showed little site specificity.
TABLE 1.
Number of archaeal amoA gene clones from each site and sampling date
| Site | No. of clones from date indicated |
Total no. of clones | ||||||
|---|---|---|---|---|---|---|---|---|
| April 2001 | August 2001 | April 2002 | September 2002 | April 2003 | September 2003 | Multiplea | ||
| P22 | 7 | 7 | 7 | 72 | 93 | |||
| P14 | 40 | 43 | 36 | 47 | 45 | 211 | ||
| R8C | 42 | 43 | 8 | 8 | 46 | 147 | ||
| Total | 89 | 93 | 43 | 8 | 47 | 8 | 163 | 451 |
Clones were generated from pooled DNA samples from multiple dates.
FIG. 1.
Phylogenetic relationships of representative deduced archaeal amoA protein sequences recovered from Plum Island Sound sediments representing low (P22 prefix), mid (P14 prefix), and high (R8C prefix) in situ salinity. The tree was inferred from 181 amino acid residues. Bootstrap values (≥50) based on 100 replicates for neighbor-joining (above the node) and parsimony (below the node) analyses are indicated. Numbers in parentheses indicate the number of similar sequences recovered from the same site. Water column/sediment and soil/sediment clusters are as designated by Francis et al. (13).
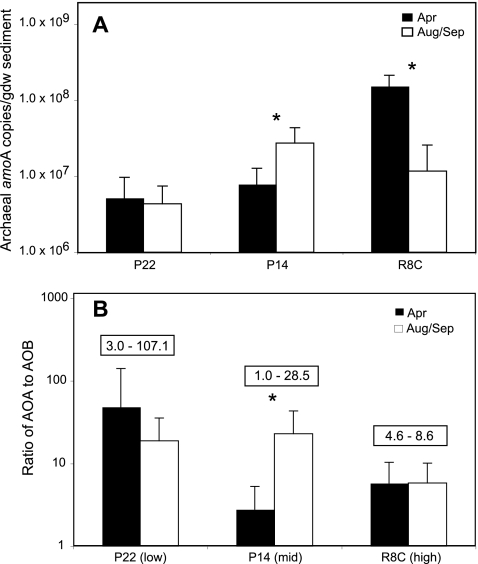
Abundances of AOA were measured from the same samples collected previously and were compared to the reported β-AOB abundances and potential nitrification rates (8). Archaeal amoA gene abundance, measured by real-time PCR as described by Moin et al. (26), ranged from 3.8 × 104 to 2.4 × 108 copies g (dry weight) of sediment−1 (Fig. 2A). The forward primer used in the real-time PCR assay is internal to the forward primer used for cloning and was a perfect match to 441 of the 451 archaeal amoA sequences recovered in this study. The other 10 sequences had a single mismatch that was either A to G or T to C, which are the most common Taq polymerase errors (12). Archaeal amoA gene abundance was greatest in April at the high-salinity site, and there were significant seasonal differences at the mid- and high-salinity sites. In general, archaeal amoA abundances in Plum Island are similar to values reported in other estuaries (26, 27, 31). Our highest values (108 gene copies g sediment−1) are quite high, but Moin et al. (26) reported archaeal amoA gene abundances as high as 109 gene copies g sediment−1 in a salt marsh. Additionally, Nelson et al. (28) reported abundances of 16S rRNA genes related to N. maritimus as high as 109 gene copies g sediment−1 in a salt marsh.
FIG. 2.
Average (±1 standard deviation) gene copy number of archaeal ammonia monooxygenase genes (amoA) in Plum Island Sound sediments (panel A) and the ratio of AOA to β-AOB abundance (panel B) in April and August-September averaged from duplicate (2001) or triplicate (2002 and 2003) cores from each site over 3 years. The range of ratios for each site is indicated in the box above the columns in panel B. Asterisks indicate a statistically significant difference between April and August-September values (determined by Student's t test using α = 0.05). Values of amoA abundance at R8C in April are from 2001 only. All other data are the averages of values from 3 years.
Ratios of AOA to β-AOB ranged from 1 to greater than 100 and were most variable at the low-salinity site (Fig. 2B). Significant seasonal differences in ratios were detected only at the mid-salinity site. Despite significantly different archaeal amoA abundances in April and August at the high-salinity site, ratios of AOA to β-AOB were relatively constant, suggesting similar responses of AOA and β-AOB communities to seasonal changes in the environment at this site. Ratios reported from other estuarine studies range from less than 1 to greater than 80 (9, 27, 31) and have been correlated to changes in salinity (27, 31). Similar to results of other estuary studies, the ratios were lower at higher salinities, but unlike results of other estuary studies (9, 27, 31), β-AOB were never more abundant than AOA.
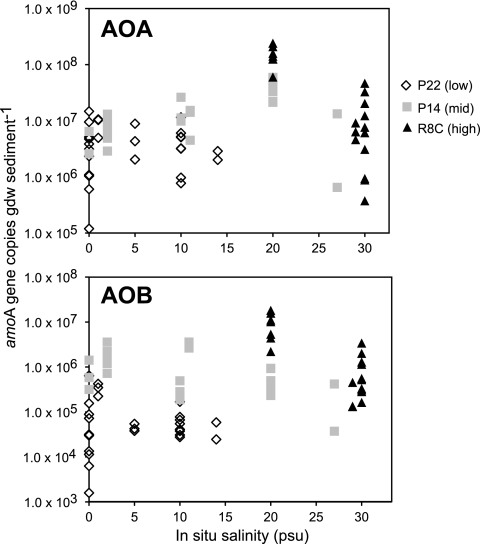
Although we did not detect a linear relationship between AOA abundance and salinity, there was a pattern of highest AOA abundance at 20 psu (Fig. 3, upper panel). The pattern was similar for β-AOB, but only in samples from the high-salinity site (Fig. 3, lower panel). Whether salinity or some other factor that covaries with salinity is a controlling variable remains unclear. Previous researchers have found the highest nitrifying activity at intermediate salinities with high turbidity (18, 32), and it has been hypothesized that the high turbidity provides the optimal substrate concentrations and habitat for estuarine nitrifiers (2). However, additional studies with cultivated isolates or enrichment cultures are necessary to more fully elucidate the ecophysiology of estuarine AOA and β-AOB.
FIG. 3.
Abundance of amoA genes from ammonia-oxidizing Archaea (AOA) and ammonia-oxidizing Betaproteobacteria (AOB) in relation to in situ salinity (psu). Symbols represent individual P22 (low-salinity), P14 (mid-salinity), and R8C (high-salinity) samples collected on different dates.
We also investigated relationships among AOA, β-AOB, and potential nitrification rates. AOA and β-AOB abundances were significantly and positively correlated when samples from all sites were included (Table 2). However, the correlation appears to be driven primarily by the strong relationship between AOA and β-AOB abundances at the high-salinity site. We observed a similar pattern of correlation between AOA and potential nitrification rates. Although the correlation coefficients for AOA abundance versus potential nitrification rates and AOA versus β-AOB abundances were significant, they were lower than the correlation coefficient for β-AOB abundance versus potential nitrification rates. Additionally, when AOA and β-AOB abundances were combined (as ammonia-oxidizing prokaryotes [AOP] in Table 2) and correlated with potential nitrification rates, the correlation was much lower than when only β-AOB abundance was included in the correlation. The high-salinity site was the only site showing a strong positive correlation between AOA abundance and potential rates, but the relationship between β-AOB abundance only and potential rates was still much stronger.
TABLE 2.
Pearson's correlation coefficients describing the relationships between AOA and β-AOB abundance and potential nitrification ratesa
| Comparison | Pearson's correlation coefficient (r) for indicated samples |
|||
|---|---|---|---|---|
| All | P22 (low salinity) | P14 (mid salinity) | R8C (high salinity) | |
| AOA versus β-AOB | 0.78* | 0.26 | −0.13 | 0.79* |
| AOA versus ratesb | 0.50* | 0.34 | −0.22 | 0.81* |
| β-AOBb versus rates | 0.70* | 0.81* | 0.87* | 0.96* |
| AOP versus rates | 0.53* | 0.42* | −0.17 | 0.83* |
AOA and β-AOB abundances were measured by real-time PCR quantification of the archaeal or betaproteobacterial amoA gene, respectively. AOP represents the combined abundance of AOA and β-AOB. Asterisks indicate statistically significant regressions using an α value of 0.05.
Data are from Bernhard et al. (7). rates, potential nitrification rates.
Contrary to our initial hypothesis, the inclusion of AOA as part of the ammonia-oxidizing community does not fully explain previously reported differences in ammonia oxidation kinetics related to changes in salinity. In a previous work, there was a significant decrease in potential nitrification rates and β-AOB abundance from spring to late summer repeatedly over 3 years (8). However, in the current study, AOA abundance decreased in late summer only at the high-salinity site, while abundance remained constant at the low-salinity site and was significantly greater in late summer at the mid-salinity site. Thus, there is no consistent relationship between AOA abundance and either potential nitrification rates or β-AOB abundance patterns. In a previous study of estuarine AOA and β-AOB, Caffrey et al. (9) also found no correlation between potential nitrification rates and AOA abundance at four of six sites. Although further studies are necessary to confirm AOA nitrification activity in the estuary, our data do not support a significant AOA contribution, particularly at the low- or mid-salinity sites, despite relatively high AOA abundance.
The lack of correlation between AOA and potential nitrification rates has several possible explanations. It may be that AOA are inactive, perhaps transient, members of the estuarine community, and the high rates are due to very active AOB. It is also possible that AOA use alternative energy sources and thus are not actively nitrifying in the estuary. Several studies have reported evidence of heterotrophic metabolism among at least some marine Crenarchaeota (1, 17, 29), suggesting the possibility of either mixed populations of autotrophic and heterotrophic Crenarchaeota or a community of mixotrophic Crenarchaeota. Chemoautotrophic growth on ammonia oxidation has been demonstrated for Nitrosopumilus maritimus (20), but there is only circumstantial evidence of archaeal nitrification activity in marine environments, with the most convincing data being from open ocean studies (5, 36). Additionally, although increased archaeal amoA gene expression after ammonium additions has been reported for soil microcosms (34), the specific role of the putative archaeal ammonia monooxygenase remains to be confirmed.
Since potential nitrification rates are typically measured under nonlimiting oxygen and substrate conditions, an alternative explanation for the observed lack of correlation is that high substrate concentrations or other conditions of the potential rate experiments may inhibit AOA, particularly since we now know that N. maritimus has a very high affinity for ammonium (24). The ammonium added to our incubations (300 μM) was within the ranges found in situ (7) and is considerably lower than the 2 to 3 mM concentrations of ammonium that have been shown to at least partially inhibit AOA from hot springs (15) and pure cultures of N. maritimus (24). However, it is still possible that the ammonium additions were too high for activity of the resident AOA in our study. Additionally, AOA activity may also be inhibited by agitation (24), which was used in our potential rate measurements to maintain nonlimiting oxygen conditions. Alternatively, archaeal amoA gene abundance may not be an appropriate indicator of their nitrifying activity, based on a recent study of AOA and β-AOB in nitrogen-rich agricultural soils, in which β-AOB were primarily responsible for the oxidation of ammonia despite a high abundance of archaeal amoA genes (19).
We must also consider the possibility that we still have not accounted for the presence of all organisms capable of oxidizing ammonia, including methane oxidizers and heterotrophic nitrifiers. Methane has been reported to be a significant component of the C cycle at the low-salinity site (16) and may support populations of methane oxidizers that are capable of ammonia oxidation under certain environmental conditions (3). To our knowledge, the role of heterotrophic nitrifiers in estuarine sediments has not been investigated. Additionally, previous attempts to amplify genes representing the gammaproteobacterial ammonia oxidizers in Plum Island Sound were unsuccessful (7).
In summary, AOA outnumbered β-AOB along the salinity gradient and were most abundant at intermediate salinity. Additionally, our results do not support a major contribution of AOA to nitrification in the Plum Island Sound estuarine sediments. The lack of correlation between AOA abundance and potential nitrification rates may be a methodological artifact or may reflect alternative energy sources used by AOA. In situ rate measurements in future studies will be important to resolve the role of archaeal contributions to nitrification in estuarine systems.
Nucleotide sequence accession numbers.
Sequences were deposited in GenBank under accession numbers GQ499386 to GQ499836.
Acknowledgments
This work was supported in part by National Science Foundation awards MCB-0457183 and DEB-0814586 (A.E.B.), MCB-0604448 (D.A.S. and J.R.D.L.T.), and OCE-0623174 (D.A.S.) and by NSF-LTER program award OCE-0423565 (A.E.G.). Additional support was provided by the George and Carol Milne Endowment at Connecticut College.
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Agogue, H., M. Brink, J. Dinasquet, and G. J. Herndl. 2008. Major gradients in putatively nitrifying and non-nitrifying Archaea in the deep North Atlantic. Nature 456:788-792. [DOI] [PubMed] [Google Scholar]
- 2.Balls, P. W., N. Brockie, J. Dobson, and W. Johnston. 1996. Dissolved oxygen and nitrification in the upper Forth Estuary during summer (1982-92): patterns and trends. Estuar. Coast. Shelf Sci. 42:117-134. [Google Scholar]
- 3.Bedard, C., and R. Knowles. 1989. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol. Rev. 53:68-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beman, J. M., and C. A. Francis. 2006. Diversity of ammonia-oxidizing archaea and bacteria in the sediments of a hypernutrified subtropical estuary: Bahía del Tóbari, Mexico. Appl. Environ. Microbiol. 72:7767-7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beman, J. M., B. N. Popp, and C. A. Francis. 2008. Molecular and biogeochemical evidence for ammonia oxidation by marine Crenarchaeota in the Gulf of California. ISME J. 2:429-441. [DOI] [PubMed] [Google Scholar]
- 6.Beman, J. M., K. J. Roberts, L. Wegley, F. Rohwer, and C. A. Francis. 2007. Distribution and diversity of archaeal ammonia monooxygenase genes associated with corals. Appl. Environ. Microbiol. 73:5642-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhard, A. E., T. Donn, A. E. Giblin, and D. A. Stahl. 2005. Loss of diversity of ammonia-oxidizing bacteria correlates with increasing salinity in an estuary system. Environ. Microbiol. 7:1289-1297. [DOI] [PubMed] [Google Scholar]
- 8.Bernhard, A. E., J. Tucker, A. E. Giblin, and D. A. Stahl. 2007. Functionally distinct communities of ammonia-oxidizing bacteria along an estuarine salinity gradient. Environ. Microbiol. 9:1439-1447. [DOI] [PubMed] [Google Scholar]
- 9.Caffrey, J. M., N. Bano, K. Kalanetra, and J. T. Hollibaugh. 2007. Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISME J. 1:660-662. [DOI] [PubMed] [Google Scholar]
- 10.Coolen, M. J. L., B. Abbas, J. Van Bleijswijk, E. C. Hopmans, M. M. M. Kuypers, S. G. Wakeham, and J. S. Sinninghe Damste. 2007. Putative ammonia-oxidizing Crenarchaeota in suboxic waters of the Black Sea: a basin-wide ecological study using 16S ribosomal and functional genes and membrane lipids. Environ. Microbiol. 9:1001-1016. [DOI] [PubMed] [Google Scholar]
- 11.de la Torre, J. R., C. B. Walker, A. E. Ingalls, M. Konneke, and D. A. Stahl. 2008. Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ. Microbiol. 10:810-818. [DOI] [PubMed] [Google Scholar]
- 12.Dunning, A. M., P. Talmud, and S. E. Humphries. 1988. Errors in the polymerase chain reaction. Nucleic Acids Res. 16:10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis, C. A., K. J. Roberts, J. M. Beman, A. E. Santoro, and B. B. Oakley. 2005. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Nat. Acad. Sci. U. S. A. 102:14683-14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallam, S. J., T. J. Mincer, C. Schleper, C. M. Preston, K. Roberts, P. M. Richardson, and E. F. DeLong. 2006. Pathways of carbon assimilation and ammonia oxidation suggested by environmental genomic analyses of marine Crenarchaeota. PLoS Biol. 4:e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatzenpichler, R., E. V. Lebedeva, E. Spieck, K. Stoecker, A. Richter, H. Daims, and M. Wagner. 2008. A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc. Nat. Acad. Sci. U. S. A. 105:2134-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkinson, C. S. J., A. E. Giblin, J. Tucker, and R. H. Garritt. 1999. Benthic metabolism and nutrient cycling along an estuarine salinity gradient. Estuaries 22:863-881. [Google Scholar]
- 17.Ingalls, A. E., S. R. Shah, R. L. Hansman, L. I. Aluwihare, G. M. Santos, E. R. M. Druffel, and A. Pearson. 2006. Quantifying archaeal community autotrophy in the mesopelagic ocean using natural radiocarbon. Proc. Nat. Acad. Sci. U. S. A. 103:6442-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iriarte, A., I. de Madariaga, F. Diez-Garagarza, M. Revilla, and E. Orive. 1996. Primary plankton production, respiration and nitrification in a shallow temperate estuary during summer. J. Exp. Mar. Biol. Ecol. 208:127-151. [Google Scholar]
- 19.Jia, Z., and R. Conrad. 2009. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 11:1658-1671. [DOI] [PubMed] [Google Scholar]
- 20.Koenneke, M., A. E. Bernhard, J. R. de la Torre, C. B. Walker, J. B. Waterbury, and D. A. Stahl. 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543-546. [DOI] [PubMed] [Google Scholar]
- 21.Lam, P., M. M. Jensen, G. Lavik, D. F. McGinnis, B. Muller, C. J. Schubert, R. Amann, B. Thamdrup, and M. M. M. Kuypers. 2007. Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proc. Nat. Acad. Sci. U. S. A. 104:7104-7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leininger, S., T. Urich, M. Schloter, L. Schwark, J. Qi, G. W. Nicol, J. I. Prosser, S. C. Schuster, and C. Schleper. 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806-809. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Förster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. König, T. Liss, R. Lüßmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K.-H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martens-Habbena, W., P. M. Berube, H. Urakawa, J. R. de la Torre, and D. A. Stahl. 2009. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461:976-981. [DOI] [PubMed] [Google Scholar]
- 25.Mincer, T. J., M. J. Church, L. T. Taylor, C. Preston, D. M. Karl, and E. F. DeLong. 2007. Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ. Microbiol. 9:1162-1175. [DOI] [PubMed] [Google Scholar]
- 26.Moin, N. S., K. A. Nelson, A. Bush, and A. E. Bernhard. 2009. Distribution and diversity of archaeal and bacterial ammonia-oxidizers in salt marsh sediment. Appl. Environ. Microbiol. 75:7461-7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosier, A. C., and C. A. Francis. 2008. Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ. Microbiol. 10:3002-3016. [DOI] [PubMed] [Google Scholar]
- 28.Nelson, K. A., N. S. Moin, and A. E. Bernhard. 2009. Archaeal diversity and the prevalence of Crenarchaeota in salt marsh sediments. Appl. Environ. Microbiol. 75:4211-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouverney, C. C., and J. A. Fuhrman. 2000. Marine planktonic Archaea take up amino acids. Appl. Environ. Microbiol. 66:4829-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park, H.-D., G. F. Wells, H. Bae, C. S. Criddle, and C. A. Francis. 2006. Occurrence of ammonia-oxidizing archaea in wastewater treatment plant bioreactors. Appl. Environ. Microbiol. 72:5643-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoro, A. E., C. A. Francis, N. R. De Sieyes, and A. B. Boehm. 2008. Shifts in the relative abundance of ammonia-oxidizing bacteria and archaea across physicochemical gradients in a subterranean estuary. Environ. Microbiol. 10:1068-1079. [DOI] [PubMed] [Google Scholar]
- 32.Somville, M. 1984. Use of nitrifying activity measurements for describing the effect of salinity on nitrification in the Scheldt Estuary. Appl. Environ. Microbiol. 47:424-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strous, M., J. A. Fuerst, E. H. M. Kramer, S. Logemann, G. Muyzer, K. T. van de Pas-Schoonen, R. Webb, J. G. Kuenen, and M. S. M. Jetten. 1999. Missing lithotroph identified as new planctomycete. Nature 400:446-449. [DOI] [PubMed] [Google Scholar]
- 34.Treusch, A. H., S. Leininger, A. Kietzin, S. C. Schuster, H. P. Klenk, and C. Schleper. 2005. Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ. Microbiol. 7:1985-1995. [DOI] [PubMed] [Google Scholar]
- 35.Weidler, G. W., M. Dornmayr-Pfaffenhuemer, F. W. Gerbl, W. Heinen, and H. Stan-Lotter. 2007. Communities of Archaea and Bacteria in a subsurface radioactive thermal spring in the Austrian Central Alps, and evidence of ammonia-oxidizing Crenarchaeota. Appl. Environ. Microbiol. 73:259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wuchter, C., B. Abbas, M. J. L. Coolen, L. Herfort, J. Van Bleijswijk, P. Timmers, M. Strous, E. Teira, G. J. Herndl, J. J. Middleburg, S. Schouten, and J. S. Sinninghe Damste. 2006. Archaeal nitrification in the ocean. Proc. Nat. Acad. Sci. U. S. A. 103:12317-12322. [DOI] [PMC free article] [PubMed] [Google Scholar]