Abstract
Associations between house dust-associated β-(1,3)-glucan exposure and airway inflammatory reactions have been reported, while such exposures in early childhood have been suggested to protect against asthma and wheezing. Most epidemiological studies have used reservoir dust samples and an inhibition enzyme immunoassay (EIA) for β-(1,3)-glucan exposure assessment. The objective of this study was to develop inexpensive but highly sensitive enzyme immunoassays to measure airborne β-(1,3)-glucans in low-exposure environments, like homes. Specificities of available anti-β-(1,3)-glucan antibodies were defined by direct and inhibition experiments. Three suitable antibody combinations were selected for sandwich EIAs. β-(1,3)-Glucans in passive airborne dust collected with an electrostatic dust fall collector (EDC) and floor dust from seven homes were measured with the three EIAs. Floor dust samples were additionally analyzed in the inhibition EIA. The sandwich EIAs were sensitive enough for airborne glucan measurement and showed different specificities for commercial glucans, while the β-(1,3)-glucan levels in house dust samples correlated strongly. The feasibility of measuring glucans in airborne dust with the recently introduced EDC method was further investigated by selecting the most suitable of the three EIAs to measure and compare β-(1,3)-glucan levels in the EDC and in floor and actively collected airborne dust samples of the previously performed EDC validation study. The EDC β-(1,3)-glucan levels correlated moderately with β-(1,3)-glucans in actively collected airborne dust and floor dust samples, while the glucan levels in the airborne dust and floor dust samples did not correlate. The combination of the newly developed β-(1,3)-glucan sandwich EIA with EDC sampling now allows assessment in large-scale population studies of exposure to airborne β-(1,3)-glucans in homes or other low-exposure environments.
β-(1,3)-Glucans are polysaccharides produced by plants, bacteria, and fungi. Their chain lengths, their degrees of branching, and the numbers and positions of their other glycosidic linkages, like β-(1,4)- and/or β-(1,6)-linkages, may vary largely. While β-(1,3)-(1,4)-glucan structures are typically found in plant material, β-(1,3)-(1,6)-chains are more prevalent in fungi and bacteria (31). Because they are typical microbe-associated molecular patterns (MAMPs), β-(1,3)-glucans activate cells of the innate immune system by binding to glucan-specific receptors like dectin-1 (1, 4, 6) and other cellular membrane receptors (5, 21). Associations between indoor β-(1,3)-glucan exposure and inflammatory reactions of the respiratory system have been reported (3, 10, 25, 33, 34, 40), but protective effects of glucan exposure in early childhood against the development of asthma and allergy have also been suggested (9, 13, 15, 29). β-(1,3)-Glucans are less potent inducers of inflammatory reactions than bacterial endotoxins (16, 30, 35), but since their total amounts in our environment may be much higher—glucans are measured in micrograms per milligram of house dust, whereas endotoxins are measured in nanograms per milligram of house dust (10, 14, 29, 37)—their proinflammatory impact may be similar to that of endotoxin exposure.
An inexpensive and relatively simple β-(1,3)-glucan-specific inhibition immunoassay was introduced in the mid-1990s by Douwes et al. (8). This assay has found wide application in large-scale population studies in which glucans have been routinely measured in dust from mattresses and living room and/or bedroom floors (9, 10, 12, 13, 29). However, while useful for quantification of β-(1,3)-glucans in extracts with >1 to 2% (wt/vol) floor or mattress dust, the sensitivity of the assay is usually too low for airborne measurements. Even in environments with high microbial contaminations, like the household waste recycling industry (36), β-(1,3)-glucan levels in airborne dust samples may often remain under the limit of detection. Until recently, the only published methods sensitive enough to measure β-(1,3)-glucans in airborne dust samples were the modified Limulus amebocyte lysate (LAL) assay (a modification of the endotoxin assay with which glucans can be specifically detected [11]) and two sandwich enzyme immunoassays (EIAs) (2, 23, 27). Due to its high cost, which is at least 5-fold higher than that of the inhibition EIA, the LAL assay has thus far hardly been used in epidemiological studies. The assay developed by Sander et al. (27) has been applied to only a limited number of samples from the work environment, and the EIA described by Blanc et al. (2) and Rao et al. (23) has been used only to analyze reservoir and airborne dust samples from heavily mold-contaminated houses in New Orleans after the hurricanes Katrina and Rita. A third sensitive EIA makes use of galactosyl ceramide, a receptor specific for β-(1,3)-glucans (41), as the capture reagent and of a monoclonal antibody specific for β-(1,3)-(1,6)-glucans as the detecting antibody (20). Application of this EIA in population studies has, however, not yet been reported.
Apart from the low sensitivity of the inhibition EIA and/or high cost of the modified LAL assay, the time, equipment, and budget needed for active sampling of airborne dust are reasons why epidemiological studies have relied mainly on β-(1,3)-glucan analyses of reservoir dust samples from floors or mattresses. β-(1,3)-Glucan levels in airborne dust samples may, however, be more representative of real inhalatory exposures.
The aim of this study was to develop new sensitive but inexpensive assays for β-(1,3)-glucans in airborne dust from homes or other locations with low exposure levels. We combined methods and reagents from three laboratories that previously developed and applied β-glucan EIAs (2, 8, 23, 27). The specificities of available antibodies to a panel of 13 different glucans were determined to assess whether it is possible to develop sandwich assays that would show clear differences in specificities toward glucans from different taxonomic sources—bacterial, fungal, or plant derived—and/or between glucans with different chemical structures.
Another objective of the present study was to explore the feasibility of using our recently developed passive airborne dust sampling method, the electrostatic dust fall collector (EDC) (22), for assessing exposure to glucans in airborne dust in the home environment, when combined with the new sensitive immunoassays.
MATERIALS AND METHODS
Glucans.
The commercial sources, origins, and structures of glucans used in this study are summarized in Table 1. All were available as dry powder and were >90% pure according to the suppliers' information. Stock solutions of 1 mg per ml were made, based on preliminary experiments in which solubilities were assessed. Baker's yeast (Saccharomyces cerevisiae) glucan and curdlan were dissolved in 0.05 M NaOH at room temperature. Barley glucan, laminarin, lichenan, and pustulan were dissolved by autoclaving them in ultrapure water; scleroglucan was dissolved by autoclaving it in 0.05 M NaOH; and oat glucan, xyloglucan, and pullulan were dissolved in ultrapure water at room temperature. Pachyman, paramylon, and schizophyllan were dissolved by autoclaving them in 0.05 M NaOH, followed by centrifugation at 1,000 × g for 15 min, and the supernatants were collected.
TABLE 1.
Producers, origins, and branching of the glucans used in this studya
| Glucan | Product identifier, source | Origin | Type | Backbone |
Branch | |
|---|---|---|---|---|---|---|
| Major | Minor | |||||
| Baker's yeast glucan | 49097, Sigma-Aldrich Chemie B.V., Zwijndrecht, the Netherlands | S. cerevisiae | Yeast | β-(1,3) | β-(1,6) | |
| Barley glucan | P-BGBL, Megazyme International Ireland Ltd., Wicklow, Ireland | Barley | Plant | β-(1,4) | β-(1,3) (∼25%) | |
| Curdlan | 032-09902, Wako Chemicals GmbH, Neuss, Germany | Alcaligenes faecalis | Bacterial | β-(1,3) | β-(1,6) (∼1.3%) | |
| Laminarin | 61430, Sigma-Aldrich Chemie B.V., Zwijndrecht, the Netherlands | Laminaria digitata | Algal | β-(1,3) | β-(1,6) | |
| Lichenan | P-LICHN, Megazyme International Ireland Ltd., Wicklow, Ireland | Cetraria islandica | Lichen | β-(1,4) | β-(1,3) (∼33%) | |
| Oat glucan | P-BGOM, Megazyme International Ireland Ltd., Wicklow, Ireland | Oat | Plant | β-(1,4) | β-(1,3) (∼25%) | |
| Pachyman | P-PACHY, Megazyme International Ireland Ltd., Wicklow, Ireland | Poria cocos | Fungal | β-(1,3) | β-(1,6) (∼2.5%) | |
| Paramylon | 89662, Sigma-Aldrich Chemie B.V., Zwijndrecht, the Netherlands | Euglena gracilis | Algal | β-(1,3) | ||
| Pullulan | P-PULLN, Megazyme International Ireland Ltd., Wicklow, Ireland | Pullularia pullulans | Fungal | α-(1,4) | α-(1,6) (∼25%) | |
| Pustulan | 540501, EMD Chemicals, Inc., Gibbstown, NJ | Umbilicaria papulosa | Lichen | β-(1,6) | ||
| Schizophyllan | Gift of U. Rau, Department of Biotechnology, TU Braunschweig, Germany | Schizophyllum commune | Fungal | β-(1,3) | β-(1,6) | |
| Scleroglucan | Gift of U. Rau, Department of Biotechnology, TU Braunschweig, Germany | Sclerotium rolfsii | Fungal | β-(1,3) | β-(1,6) | |
| Xyloglucan | P-XYGLN, Megazyme International Ireland Ltd., Wicklow, Ireland | Tamarindus indica | Plant | β-(1,4) | ||
Antiglucan antibodies.
Four different anti-β-(1,3)-glucan antibodies were available for this study: U1, an affinity-purified polyclonal rabbit antilaminarin IgG (batch no. 9810; Institute for Risk Assessment Sciences [IRAS]) (8); B2, biotinylated monoclonal mouse antilaminarin IgG (clone no. 2100 3F12; Research Institute of Occupational Medicine [Berufsgenossenschaftliches Forschungsinstitut für Arbeitsmedizin {BGFA}]) (27); P1, anti-β-(1,3)-(1,6)-glucan mouse monoclonal IgM (hybridoma cell line 10C6 413.01; Environmental Health Sciences Research Center [EHSRC]) (2, 23); and P2, the polyclonal Ig fraction from the serum of a rabbit immunized and boosted with scleroglucan (S83; Environmental Health Sciences Research Center [EHSRC]) (2, 23).
EIA reagents and materials.
As secondary reagents in the EIAs, we used peroxidase-labeled swine anti-rabbit IgG (SWARPO) (product no. P0399; Dako Denmark A/S, Glostrup, Denmark), RDI poly-horseradish peroxidase (HRP) diluent (product no. PHRPDIL; Fitzgerald Industries International, Concord, MA), poly-HRP streptavidin (product no. M2051; Sanquin Reagents, Amsterdam, the Netherlands), and peroxidase-labeled rabbit anti-mouse IgG (RAMPO) (product no. P02608; Dako Denmark A/S, Glostrup, Denmark). Tween 20 (product no. 8221840500; Merck KGaA, Darmstadt, Germany), bovine serum albumin (BSA) (product no. 05475; Sigma-Aldrich Chemie B.V., Zwijndrecht, the Netherlands), milk powder (Nutricia Nederland B.V., Zoetermeer, the Netherlands), H2O2 (product no. 10729; Merck KGaA, Darmstadt, Germany), and o-phenylenediamine (OPD) (product no. P 1526; Sigma-Aldrich Chemie B.V., Zwijndrecht, the Netherlands) were purchased from the indicated companies. Based on pilot tests, phosphate-buffered saline (PBS)-Tween 20 (PBT) plus 0.1% milk (PBTM) was used as the blocking and dilution buffer in the direct coating and inhibition EIAs with the U1, B2, and P1 antibodies, while the blocking and dilution buffer for P2 was PBS-Tween 20 plus 0.1% BSA (PBTB).
Direct-coating experiments.
Flat-bottom 96-well polystyrene microtiter plates with a high binding capacity (product no. 655061; Greiner Bio-One B.V., Alphen a/d Rijn, the Netherlands) were coated overnight at 4°C with 100-μl serial dilutions of the glucans in concentrations ranging from 0.06 to 16 μg/ml in PBS. Wells were washed with PBT and then incubated with the blocking buffer specified above under “EIA reagents and materials” for 1 h at 37°C. After another wash cycle, the wells were incubated for 1 h at 37°C with the antibodies at optimal concentrations. According to preliminary experiments, the resulting EIA signal was determined mainly by the types and concentrations of the coated glucans: U1 at 1/10,000, B2 at 1/7,500, P1 at 1/20,000, and P2 at 1/10,000. Binding of U1 or P2 was measured by using a 1/2,000-diluted peroxidase-labeled swine anti-rabbit antibody (SWARPO), binding of B2 with 1/20,000-diluted poly-peroxidase-labeled streptavidin, and binding of P1 with 1/1,500-diluted peroxidase-labeled rabbit anti-mouse antibody (RAMPO) as secondary antibodies, all of which were incubated for 1 h at 37°C, followed by two wash cycles and then incubation with OPD plus H2O2 as the peroxidase substrate. The reaction was stopped after 30 min by adding 50 μl 2 N HCl, and the optical density (OD) was read at 492 nm.
Inhibition experiments.
Inhibition experiments were conducted according to Douwes et al. (8), with a few modifications. Briefly, a coating of laminarin was used in the inhibition EIA with U1 and B2 antibodies, pachyman in the P1 inhibition EIA, and schizophyllan in the P2 inhibition EIA, all at 16 μg/ml. Glucans were tested as inhibitors in 2-fold serial dilutions with concentrations between 0.03 and 128 μg/ml, depending on their reactivity in the direct-coating experiments.
Glucan sandwich assays.
Combinations of antibodies with similar reaction patterns were selected to develop sandwich assays. Preliminary trials were conducted to optimize the choice of buffers and antibody dilutions/concentrations that produced the highest sensitivities with acceptable background OD levels. This resulted in the combinations of U1 as the coating antibody and B2 as the detection antibody (U1B2 assay) and of P1 as the coating antibody and either U1 or B2 as the detecting antibody (P1U1 or P1B2 assay, respectively). Optimal concentrations of the coating and detection antibodies were defined by pilot experiments. Microtiter plates were coated overnight at 4°C with 100 μl of either U1 (protein concentration, 0.5 μg/ml) or P1 (0.3 μg/ml) in PBS (pH 7). All wells were blocked for 1 h at 37°C with 200 μl of RDI diluent. In all subsequent steps, PBT was used as the diluent and each reagent was used in 100-μl portions per well. As the calibration standard, we used baker's yeast glucan in eight 2-fold dilutions with starting concentrations of 20, 200, and 500 ng/ml in the U1B2, P1U1, and P1B2 EIAs, respectively. Samples were incubated in four dilutions (see below, “Dust extraction and analysis”) in PBT. The binding of detecting antibody U1 (1/2,000) or B2 (1/1,000) was quantified with either SWARPO or poly-HRP streptavidin as in the direct-coating EIAs. The cutoff level for the U1B2 EIA was defined as the OD measured for the 0.3-ng/ml concentration of the standard baker's yeast glucan. The corresponding cutoff levels for the P1U2 and P1B2 EIAs were the ODs measured at 3 ng/ml and 8 ng/ml of the standard, respectively.
Sampling procedures.
Passive airborne dust sampling and floor dust sampling were performed in the homes of seven students. In each home, two floor dust samples were taken with a vacuum cleaner equipped with a nylon sample sock (Allied Filter Fabrics, Sydney, Australia) with a 25-μm pore size (28). An area of 1 m2 of carpeted or 2 m2 of smooth floor was vacuumed for 2 min. Passive airborne dust sampling was conducted with the EDC as previously described (22). Briefly, settling dust was collected on four electrostatic cloths mounted in a 40- by 30-cm plastic folder left for 14 days in a horizontal position with the cloths exposed to the air. The folder was kept closed before and after sampling and during transport and storage. In total, 14 floor dust and 28 EDC samples were collected.
Samples used for comparing levels of dust in the EDC, actively collected airborne, and floor dust samples were collected as described before (22).
Dust extraction and analysis.
Prior to glucan extraction, all samples were extracted for endotoxins and allergens as described previously (22, 28). To extract glucans, samples were heated for 1 h at 120°C (8). All extracts were stored at −20°C prior to analysis.
Glucan extracts of airborne samples were tested at dilutions of 1/5, 1/10, 1/20, and 1/40 in the three glucan sandwich assays, and floor dust extracts were tested at dilutions of 1/500, 1/1,000, 1/2,000, and 1/4,000 in the U1B2 and P1U1 EIAs and at dilutions of 1/20, 1/40, 1/80, and 1/160 in the P1B2 EIA. In the inhibition assay, the floor dust samples were tested at dilutions of 1/20, 1/60, 1/180, and 1/540.
All samples were additionally analyzed at the same dilutions as mentioned above with the assay described by Sander et al. (27) at the BGFA laboratory (BGFA EIA), with a reported limit of detection (LOD) of 0.36 ng/ml, according to the carboxymethyl-curdlan (CM-curdlan) standard. The assay applied previously by Blanc et al. (2) and Rao et al. (23) at the EHSRC in Iowa (EHSRC EIA) had a LOD of 20 to 40 ng/ml according to the scleroglucan standard. In that assay, EDC samples were analyzed undiluted and at dilutions of 1/5, 1/25, and 1/125 and floor dust samples were analyzed at dilutions ranging from 1/10 to 1/1,250.
RESULTS
Specificities of antibodies.
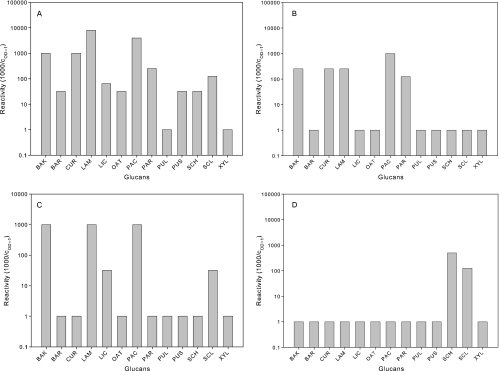
The immunoreactivities of affinity-purified polyclonal rabbit anti-laminarin IgG (U1), biotinylated monoclonal mouse anti-laminarin IgG (B2), anti-β-(1,3)-(1,6)-glucan mouse monoclonal IgM (P1), and antiscleroglucan rabbit polyclonal Ig (P2) to a panel of 13 glucans were tested (Fig. 1). Reactivity is expressed as 1,000 divided by the coating concentration (μg/ml) that is needed to achieve a color reaction with an OD of 1.0 (cOD = 1). Clearly positive but weakly reactive glucans for which the OD values remained <1.0 at all tested coating concentrations were assigned a cOD = 1 of 32 μg/ml and thus a reactivity of 31.25, while glucans not showing any reactivity, even at 16 μg/ml, were assigned a cOD = 1 of 1,000 μg/ml and thus a reactivity of 1.0.
FIG. 1.
Reactivities of antibodies to different coated glucans. Reactivity is expressed as 1,000 divided by the coating concentration (μg/ml) that is needed to achieve an OD of 1. Starting reactions not yet reaching an OD of <1 at 16 μg/ml were assigned a cOD = 1 of 32 μg/ml, while glucans not showing any reactivity at 16 μg/ml were assigned a cOD = 1 of 1,000. BAK, baker's yeast glucan; BAR, barley glucan; CUR, curdlan; LAM, laminarin; LIC, lichenan; OAT, oat glucan; PAC, pachyman; PAR, paramylon; PUL, pullulan; PUS, pustulan; SCH, schizophyllan; SCL, scleroglucan; XYL, xyloglucan. (A) Polyclonal rabbit anti-laminarin IgG (U1); (B) biotinylated monoclonal mouse antilaminarin IgG (B2); (C) anti-β-(1,3)-(1,6)-glucan mouse monoclonal IgM (P1); (D) anti-scleroglucan rabbit polyclonal Ig (P2).
There are clear differences in the reactivities of the four antibodies to the coated glucans. The U1 antibody showed the broadest reactivity. For 11 of the 13 glucans, the cOD = 1 could be determined by interpolation (Fig. 1A), with strongest reactions (cOD = 1, ≤1 μg/ml; reactivity, ≥1,000) to baker's yeast glucan, curdlan, laminarin, and pachyman. A moderate reactivity (cOD = 1, 4 to 16 μg/ml; reactivity, 60 to 250) to U1 was seen with lichenan, paramylon, and scleroglucan, while barley, oat glucan, pustulan, and schizophyllan showed only weak reactions to U1.
The B2 and P1 antibody showed a more limited reaction profile, with dose-response curves reaching an OD of 1.0 at any coating concentration for only five glucans (Fig. 1B and C). B2 reacted most strongly with pachyman and moderately (reactivity, 100 to 250) with baker's yeast glucan, curdlan, laminarin, and paramylon, while P1 reacted strongly with baker's yeast glucan, laminarin, and pachyman and moderately to lichenan and to scleroglucan. P2 showed a highly selective and quite different reaction profile. Only two glucans reached an OD of 1 (Fig. 1D), with only moderate (cOD = 1 = 2 to 8 μg/ml) reactivity (100 to 500) to schizophyllan and scleroglucan. As expected, none of the four antibodies showed any reactivity with xyloglucan, a pure β-(1,4)-glucan, or with pullulan, an α-(1,4)-(1,6)-glucan.
To exclude the possibility that the selective reaction patterns with the coated glucans were biased by a differential presentation of epitopes in coated, denatured glucans, we additionally performed inhibition experiments to assess the binding of the antibodies to the glucans in solution. The results of these inhibition EIAs confirmed the specificities and potencies found in the direct-coating experiments, with only one clear exception. The reaction of P1, the anti-β-(1,3)-(1,6)-glucan mouse monoclonal IgM, with pachyman as the coated glucan could not be inhibited by laminarin, whereas in the direct-coating experiments, laminarin and pachyman showed similar strong reactivities. In all other cases, a moderate to strong correlation was found between the reactivity in inhibition EIAs (expressed as c50, the concentrations giving 50% inhibition) and the reactivity in the direct-coating EIA, with Pearson correlation coefficients (r) of >0.9 for U1, B2, and P2 and 0.43 for P1 (data not shown).
Sandwich assays.
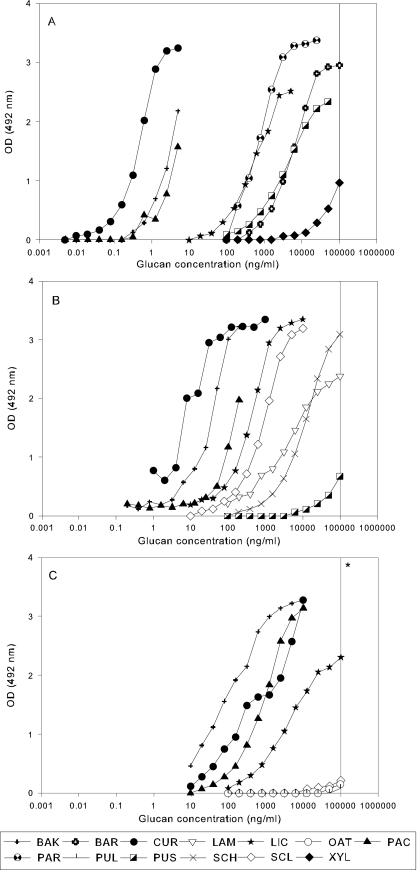
Sandwich EIAs were assessed with the three-antibody combination U1B2, P1U1, and P1B2, where the first letter represents the capturing antibody and the second letter the detecting antibody. The reactivities of the 13 glucans in the three EIAs are presented in Fig. 2.
FIG. 2.
Reactions of the 13 glucans in sandwich EIAs. See the legend to Fig. 1 for abbreviations of the glucans. (A) U1B2; (B) P1U1; (C) P1B2. Glucans showing reactions at an OD of <0.2 at 100 μg/ml (*) are not presented.
In the U1B2 EIA (Fig. 2A), curdlan, baker's yeast glucan, and pachyman showed the strongest reactions, with dose-response curves between 0.01 and 10 ng/ml, while barley glucan, lichenan, paramylon, and pustulan were less reactive, with dose-response curves at concentrations above 10 ng/ml. Xyloglucan reacted weakly between 10 and 100 μg/ml; oat beta glucan, schizophyllan, and scleroglucan started to react only at 100 μg/ml; while laminarin and pullulan showed no reaction at all at concentrations up to 100 μg/ml (not shown).
Curdlan and baker's yeast glucan also showed the strongest reactivity in the P1U1 EIA (Fig. 1B) but with dose-response curves at concentrations between 1 and 100 ng/ml. Pachyman, lichenan, and scleroglucan were moderately reactive, while laminarin and schizophyllan reacted only at higher concentrations (above 1 μg). Pustulan started to react only at those concentrations, and barley glucan, paramylon, pullulan, oat glucan, and xyloglucan were completely nonreactive at concentrations up to 100 μg/ml (not shown).
In the P1B2 EIA (Fig. 2C) baker's yeast glucan, pachyman, and curdlan were again the most strongly reacting glucans, with dose-response curves at concentrations of >10 ng/ml. The only other glucan showing a clear dose-response curve in the tested concentration range was lichenan at levels of >100 ng/ml; only weak reactions at high concentrations (100 μg/ml) were noted for laminarin, oat glucan, pullulan, and scleroglucan, and barley glucan, paramylon, pustulan, schizophyllan, and xyloglucan were completely nonreactive (not shown). Compared to results of the U1B2 EIA, all dose-response curves for glucans with positive reactions in the P1U1 and the P1B2 EIAs were shifted to higher concentrations. In addition, the differences between the dose-response curves of the single glucans appeared to be less pronounced in the P1U1 and P1B2 assays than in the U1B2 assay (compare Fig. 2B and C with A).
The reactivities of the glucans in the sandwich EIAs are summarized in Table 2 as the concentrations of glucans at which a cOD = 1 is reached. The data presented in the table confirm the high sensitivity of the U1B2 EIA, especially for the detection of β-(1,3)-glucan structures in baker's yeast glucan, curdlan, and pachyman. The plant glucans, namely, xyloglucan, a β-(1,4)-glucan, and the β-(1,3)-(1,4)-glucans from oat and barley, as well as pullulan, a fungal α-(1,4)-(1,6)-glucan, and the algal glucan laminarin, a β-(1,3)-(1,6)-glucan, did not react or only weakly reacted in the sandwich EIAs. Schizophyllan and scleroglucan, two fungal β-(1,3)-(1,6)-glucans, in the tested concentration range were reactive only in the P1U1 EIA. Because of the strong reactivity of baker's yeast glucan in all three antiglucan sandwich EIAs, we selected it as the standard preparation in the subsequent analyses of airborne and floor dust samples.
TABLE 2.
Glucan concentration at which an OD of 1 is reached in three glucan sandwich EIAs
| Glucan | Concn (ng/ml)a in indicated assay |
||
|---|---|---|---|
| U1B2 | P1U1 | P1B2 | |
| Baker's yeast glucan | 2.16 | 19.48 | 41.8 |
| Barley glucan | 2,806 | Neg. | Neg. |
| Curdlan | 0.3 | 0.63 | 215 |
| Laminarin | Neg. | 2,862 | Neg. |
| Lichenan | 325 | 238 | 2,932 |
| Oat glucan | >105 | Neg. | >105 |
| Pachyman | 3.16 | 94.45 | 541 |
| Paramylon | 394 | Neg. | Neg. |
| Pullulan | Neg. | Neg. | Neg. |
| Pustulan | 2,116 | >105 | Neg. |
| Schizophyllan | >105 | 6,188 | Neg. |
| Scleroglucan | >105 | 548 | >105 |
| Xyloglucan | >105 | Neg. | Neg. |
Neg., negative. >105 indicates that a weak reaction was shown at concentrations above 100,000 ng/ml.
Glucan measurements in house dust.
In seven homes, two floor dust samples were collected by vacuuming and four settling airborne dust samples were collected with the new EDC method (22). Thus, in total, 14 living room floor dust and 28 EDC airborne dust samples were tested in the newly developed sandwich EIAs. The floor dust samples were additionally analyzed in the inhibition EIA (8). Duplicate aliquots of the sample extracts were also tested in the two other laboratories, BGFA and the EHSRC, with the there-developed sandwich EIAs (2, 23, 27).
All glucan extracts from airborne dust as well as floor dust samples showed measurable glucan levels in the U1B2 EIA, while in the P1U1 and P1B2 EIAs, two and five EDC samples, respectively, showed no reaction above the cutoff OD value. In the BGFA EIA, one EDC sample was below the detection limit, and in the EHSRC EIA, β(1,3)-glucans could be measured only in undiluted extracts of the EDC samples.
Geometric means (GM) and geometric standard deviations (GSD) were calculated for the glucan levels of EDC and floor dust samples measured in the different sandwich EIAs and additionally for the floor dust samples measured in the inhibition EIA (Table 3). For both EDC and floor dust extracts, similar glucan levels were found in the P1U1 and P1B2 EIAs, while the levels measured with the U1B2 EIA were ∼5 times lower for floor dust and ∼2.8 times lower for EDC samples. The inhibition assay resulted in glucan levels in floor dust samples that were ∼9 times higher than those measured in the U1B2 assay.
TABLE 3.
Geometric means and geometric standard deviations for glucan levels in floor dust and electrostatic dust fall samplesa
| EIA | EDC sampling |
Floor dust sampling |
||||
|---|---|---|---|---|---|---|
| n/N | GM glucan level (GSD) (μg/m2) | Range (μg/m2) | n/N | GM glucan level (GSD) (μg/m2) | Range (μg/m2) | |
| U1B2 | 24/24 | 26 (2.4) | 7-100 | 14/14 | 52 (2.5) | 12-160 |
| P1U1 | 22/24 | 71 (2.0) | 20-210 | 14/14 | 269 (3.8) | 33-1,800 |
| P1B2 | 19/24 | 75 (2.5) | 18-350 | 14/14 | 260 (3.1) | 34-930 |
| Inhibition | 14/14 | 480 (3.9) | 88-4,500 | |||
| BGFA | 23/24 | 5.9 (2.5) | 0.9-29 | 14/14 | 11 (2.3) | 3-39 |
| EHSRC | 24/24 | 53 (1.8) | 24-155 | 14/14 | 46 (5.9) | 4-600 |
The three newly developed sandwich assays were calculated with reference to baker's yeast as the standard. The floor dust in the inhibition EIA was calculated with reference to laminarin as the standard, the BGFA EIA was calculated with reference to CM-curdlan, and the EHSRC was calculated with reference to scleroglucan. n/N, number of samples with a result greater than the LOD/total number of samples.
Levels of β-(1,3)-glucans in EDC and floor dust extracts measured in the BGFA EIA were on average 4.5 times lower than levels measured in the U1B2 EIA. The measured levels in floor dust in the EHSRC EIA were similar to those in the U1B2 assay, and in EDC samples, they were 2 times higher than in the U1B2 assay.
Pearson correlation coefficients were calculated for log-transformed β(1,3)-glucan levels (ng/ml) measured in the samples with the various sandwich assays and, where appropriate, with the inhibition assay (Table 4). Correlations between results of the three newly developed sandwich EIAs were high for both floor dust and EDC samples (r ≥ 0.8) (Table 4). EDC dust glucan levels measured in the BGFA EIA showed moderate to strong correlations with the results of other sandwich assays, while floor dust levels showed only weak to moderate correlations. EDC and floor dust β-(1,3)-glucan levels measured in the EHSRC EIA were moderately to strongly correlated with the results of the other sandwich assays. Compared to the previously described inhibition assay (8), the three new glucan sandwich EIAs and the EHSRC EIA showed a moderate (r > 0.6) and the BGFA EIA only a weak (r = 0.24) correlation for β(1,3)-glucan levels measured in floor dust samples.
TABLE 4.
Pearson correlations between β-(1,3)-glucan levels measured in the newly developed sandwich EIAs, the BGFA and EHSRC EIAs, and the inhibition EIA in EDC and/or floor dust samples
| EIA | Sample | Pearson correlation in indicated assay |
||||
|---|---|---|---|---|---|---|
| U1B2 | P1U1 | P1B2 | BGFA | EHSRC | ||
| U1B2 | EDC | 0.82 | 0.96 | 0.83 | 0.75 | |
| Floor | 0.80 | 0.97 | 0.39 | 0.68 | ||
| P1U1 | EDC | 0.88 | 0.62 | 0.49 | ||
| Floor | 0.83 | 0.46 | 0.91 | |||
| P1B2 | EDC | 0.74 | 0.65 | |||
| Floor | 0.41 | 0.66 | ||||
| BGFA | EDC | 0.71 | ||||
| Floor | 0.38 | |||||
| Inhibition | Floor | 0.64 | 0.71 | 0.62 | 0.24 | 0.71 |
Repeated measurements in the same extracts but on different days were conducted for the U1B2, P1U1, and P1U1 EIAs and resulted in average interday coefficients of variation (CVs) of <25% for both floor and EDC samples in the U1B2 and P1B2 EIAs, while for the P1U1 EIA, the average CV was up to 40%.
Validation of the EDC for airborne dust glucan measurements.
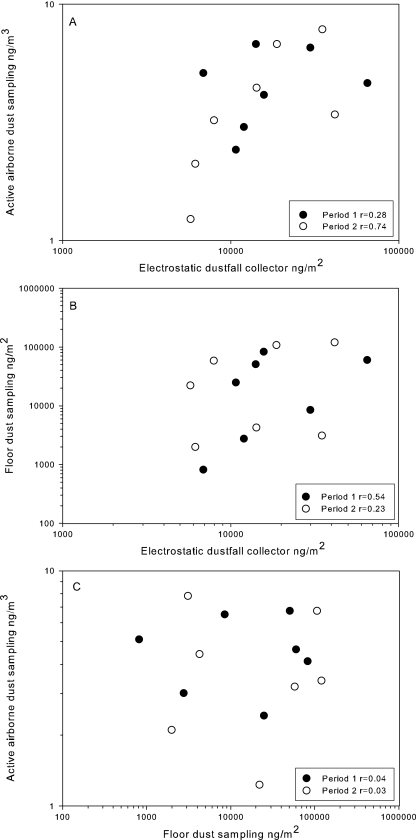
In a previous study (22), we compared the endotoxin levels of dust collected in two sampling periods, with a break of 14 days between them. We applied and compared EDC sampling and active airborne dust sampling as well as floor dust sampling to determine whether the EDC is suitable for airborne endotoxin exposure assessment. In order to establish whether the EDC is also suitable for airborne glucan exposure assessment, we tested extracts of those samples in the U1B2 EIA, the most sensitive and reproducible EIA of the three new glucan sandwich assays.
Pearson correlation coefficients for glucan levels after different sampling periods were high for all dust sampling methods (r > 0.7). The EDC levels correlated moderately (r = 0.57) with the airborne dust levels in actively collected samples (Fig. 3A). A weak correlation was found between EDC and floor dust glucan levels (r = 0.37) (Fig. 3B), while we could not find any correlation between results of the active airborne dust sampling and the floor dust sampling (Fig. 3C).
FIG. 3.
Comparison of glucan levels measured by different dust sampling methods. (A) Active airborne dust sampling versus EDC; (B) floor dust sampling versus EDC; (C) active airborne dust sampling versus floor dust sampling.
We further validated the EDC for airborne glucan assessment with the U1B2 EIA by calculating the CV for glucan levels measured in parallel cloths from the same EDC (intrasampler CV). Comparison of glucan yields on single cloths within one sampler resulted in a low average intrasampler CV of 15%.
DISCUSSION
We developed three new sensitive glucan sandwich assays with combinations of one polyclonal (U1) and two monoclonal (P1, B2) anti β-(1,3)-glucan antibodies. Best results—high sensitivity and acceptable background reactions—were achieved with the antibody combinations U1B2, P1U1, and P1B2. All of these were applicable for airborne dust β-(1,3)-glucan measurements in a home environment. The specificities of the new EIAs were tested with a panel of 13 different glucans (Table 1). The strongest reactions in all EIAs were noted for baker's yeast glucan, curdlan, and pachyman. Lichenan was the only other β-(1,3)-glucan that was detected in all three EIAs. Therefore, these β-(1,3)-glucans are used here to compare the sensitivities of the assays. The U1B2 EIA is the most sensitive, detecting 10- and 20-times-lower concentrations of baker's yeast glucan, 2- and 700-times-lower concentrations of curdlan, and 30- and 180-times-lower concentrations of pachyman than concentrations detected by the P1U1 and P1B2 EIAs. The reactivity of lichenan is moderate, and levels of reactivity were similar in the U1B2 and P1U1 assays and 10 times lower in the P1B2 EIA. The P1B2 EIA is clearly the least sensitive of the new EIAs.
Sensitivities for glucans in dust collected with the EDC were in concordance with the order of sensitivities found with purified glucans. All EDC samples were clearly positive in the U1B2 EIA, while in the P1U1 and P1B2 EIAs, two and five EDC samples, respectively, had levels below the detection limit.
The specificities of the three-antibody combination U1B2, P1U1, and P1B2 used in the sandwich EIAs differed, but all reacted only with β-(1,3)-glucans and not with xyloglucan, a β-(1,4)-glucan, or pullulan, an α-(1,4)-(1,6)-glucan. There was, however, no clear binding preference for a certain type of additional linking in the β-(1,3)-glucans. In fact, the glucans with the strongest reactivity differ in their primary structures; both curdlan and pachyman are linear β-(1,3)-glucans, with a small amount of internal β-(1,6)-linkages (26), while baker's yeast glucan is a highly branched β-(1,3)-(1,6) glucan (18). We therefore conclude that all three new sandwich EIAs are specific for β-(1,3)-glucans irrespective of additional types of glycosidic linkages.
With regard to the taxonomic origins of the glucans, the observed specificities are to some extent more conclusive. The P1B2 combination shows the most restricted specificity, reacting with only two fungal glucans, baker's yeast glucan and pachyman, and with curdlan, a bacterial glucan. Lichenan, reacting moderately in the P1B2 EIA, is a glucan derived from a lichen symbiosis of an alga and a fungal partner. In the P1U1 EIA, reactions were seen with the same four glucans as in the P1B2 EIA and additionally with scleroglucan, a fungus-derived glucan, and (although at high concentrations only) with the fungal glucan schizophyllan and algal glucan laminarin. The U1B2 EIA detected, apart from the three strongly reactive glucans and lichenan, paramylon, an alga-derived glucan. Weak reactions were observed in the U1B2 assay for pustulan, another lichen glucan which, according to the producer, consists mainly of β-(1,6)-linked glucose moieties and the plant-derived barley glucan. We thus conclude that the P1B2 and P1U1 EIAs are apparently specific for microbial glucans, while the reactivity of dust samples in the U1B2 EIA may in some cases be due to the presence of large amounts of plant glucans. This may occur in environments like bakeries, farms, or flour industrial plants (14, 32), where large quantities of plant glucans can be expected.
To assess the applicability of the new EIAs in population studies, we measured EDC and floor dust glucan levels in samples from home environments. We decided to use baker's yeast glucan as the standard, since it reacted very strongly in all three new sandwich EIAs. Due to the frequent use of baker's yeast (S. cerevisiae) in the food-producing industry (beer, bread, etc.), it can be assumed to commonly occur in home environments. The mean glucan levels measured in the U1B2 EIA were lower than in the two other sandwich EIAs, and all three sandwich EIAs produced lower glucan levels than the inhibition assay.
We also compared the new EIAs with the two sandwich EIAs developed and applied previously in the BGFA and EHSRC laboratories (2, 8, 23, 27). Mean glucan levels measured in EDCs with the EHSRC EIA were similar to those measured by the P1U1 and P1B2 EIAs, while the mean levels in floor dust were comparable with those measured in the U1B2 EIA. The BGFA EIA measured lower levels in all samples than any of the other EIAs. This and the differences mentioned in the previous paragraph may depend primarily on the standard preparations used, baker's yeast glucan in the three sandwich EIAs (Fig. 2), CM-curdlan in the BGFA EIA, scleroglucan in the EHSRC EIA, and laminarin in the inhibition EIA. In addition, we observed relatively large differences in the reactivities of the standard preparation, baker's yeast glucan, in the three sandwich EIAs (Fig. 2), whereas the dose-response curves for EDC and floor dust extracts in the various assays showed much less pronounced quantitative differences. This indicates that the “average antigenic structure” of β-(1,3)-glucans or β-(1,3)-glucan-like immunoreactive components in house dust extracts—most likely a mixture from various sources—differs significantly from that of baker's yeast glucan and probably also those from the other standard preparations. As a consequence, the glucan concentration units given in ng or μg per m2 or g dust should as yet not be used as absolute units but as relative values depending on both the assay in which they are obtained and the calibration standard.
The levels measured in house dust samples with the various EIAs correlated moderately to strongly for both EDC and floor dust. The most pronounced exception is the weak correlation between results of the BGFA EIA and the inhibition EIA. In the BGFA and EHSRC EIAs, we further frequently noticed dose-response curves for sample extracts that were not parallel to the standard calibration curve and therefore resulted in high intra-assay CV values. This possibly also led to lower correlations when calculated average concentrations were compared with levels found in other assays. The compared assays could, however, not be used to further differentiate between glucan types in home environments. Relations between (airborne) glucan exposures and health effects can thus as yet be studied only in a general dose-response-dependent manner.
The applicability of exposure measurements in population studies also requires a sufficient level of reproducibility. We found that repeated measurements of the same dust extracts on different days in the U1B2 and P1B2 EIAs gave for both sample types very similar results (interday CV, <25%), while for the P1U1 EIA, the average CV was clearly higher (interday CV, <40%).
Since the U1B2 EIA appeared to be the most sensitive and provided the most reproducible results, we chose this EIA for further analyses of airborne dust samples. We previously applied and compared the results of EDC sampling and active airborne dust sampling, as well as floor dust sampling, to determine whether the EDC is suitable for airborne endotoxin exposure assessment (22). β-(1,3)-Glucan levels measured in extracts of EDC dust samples were moderately correlated to those in actively collected airborne dust samples and weakly correlated to those in floor dust samples, while active airborne dust sampling and floor dust sampling did not show any correlation at all. Therefore, we conclude that the EDC dust sampling method in combination with the U1B2 EIA can be used as a tool to assess airborne glucan exposures in home environments. This method is easy for the participants of a study to use, can be sent by mail, and is a cheap and reliable method for collecting airborne dust. The EDC is a potential alternative to or a complement for vacuum dust sampling in large-scale epidemiological studies.
In summary, three new sensitive glucan sandwich assays for assessment of exposure to airborne glucans in low-exposure environments have been developed. The glucan levels measured in samples from home environments correlated with results from existing methods (2, 8, 23, 27), and unlike with existing assays, dose-response curves for dust sample extracts were always steep and more parallel to the standard calibration curve in the newly developed EIAs, thus giving more precise measurements. The passive airborne dust sampling method EDC is applicable not only for endotoxin (22) but also for glucan exposure assessment in combination with the U1B2 EIA. We thus now have the tools to assess airborne glucan exposure in large population studies in a time- and cost-effective way.
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Adams, E. L., P. J. Rice, B. Graves, H. E. Ensley, H. Yu, G. D. Brown, S. Gordon, M. A. Monteiro, E. Papp-Szabo, D. W. Lowman, T. D. Power, M. F. Wempe, and D. L. Williams. 2008. Differential high affinity interaction of Dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side chain branching. J. Pharmacol. Exp. Ther. 325:115-123. [DOI] [PubMed] [Google Scholar]
- 2.Blanc, P. D., M. D. Eisner, P. P. Katz, I. H. Yen, C. Archea, G. Earnest, S. Janson, U. B. Masharani, P. J. Quinlan, S. K. Hammond, P. S. Thorne, J. R. Balmes, L. Trupin, and E. H. Yelin. 2005. Impact of the home indoor environment on adult asthma and rhinitis. J. Occup. Environ. Med. 47:362-372. [DOI] [PubMed] [Google Scholar]
- 3.Bonlokke, J. H., G. Stridh, T. Sigsgaard, S. K. Kjaergaard, H. Lofsted, K. Andersson, E. C. Bonefeld-Jørgensen, M. N. Jayatissa, L. Bodin, J. E. Juto, and L. Molhave. 2006. Upper-airway inflammation in relation to dust spiked with aldehydes or glucan. Scand. J. Work Environ. Health 32:374-382. [DOI] [PubMed] [Google Scholar]
- 4.Brown, G. D. 2006. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6:33-43. [DOI] [PubMed] [Google Scholar]
- 5.Brown, G. D., and S. Gordon. 2005. Immune recognition of fungal beta-glucans. Cell. Microbiol. 7:471-479. [DOI] [PubMed] [Google Scholar]
- 6.Brown, J., C. A. O'Callaghan, A. S. Marshall, R. J. Gilbert, C. Siebold, S. Gordon, G. D. Brown, and E. Y. Jones. 2007. Structure of the fungal beta-glucan-binding immune receptor dectin-1: implications for function. Protein Sci. 16:1042-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuah, C. T., A. Sarko, Y. Deslandes, and R. H. Marchessault. 1983. Packing analysis of carbohydrates and polysaccharides. Part 14. Triple-helical crystalline structure of curdlan and paramylon hydrates. Macromolecules 16:1375-1382. [Google Scholar]
- 8.Douwes, J., G. Doekes, R. Montijn, D. Heederik, and B. Brunekreef. 1996. Measurement of beta(1→3)-glucans in occupational and home environments with an inhibition enzyme immunoassay. Appl. Environ. Microbiol. 62:3176-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douwes, J., R. van Strien, G. Doekes, J. Smit, M. Kerkhof, J. Gerritsen, D. Postma, J. de Jongste, N. Travier, and B. Brunekreef. 2006. Does early indoor microbial exposure reduce the risk of asthma? The Prevention and Incidence of Asthma and Mite Allergy birth cohort study. J. Allergy Clin. Immunol. 117:1067-1073. [DOI] [PubMed] [Google Scholar]
- 10.Douwes, J., A. Zuidhof, G. Doekes, S. C. van der Zee, I. Wouters, M. H. Boezen, and B. Brunekreef. 2000. (1→3)-β-d-Glucan and endotoxin in house dust and peak flow variability in children. Am. J. Respir. Crit. Care Med. 162:1348-1354. [DOI] [PubMed] [Google Scholar]
- 11.Foto, M., J. Plett, J. Berghout, and J. D. Miller. 2004. Modification of the Limulus amebocyte lysate assay for the analysis of glucan in indoor environments. Anal. Bioanal. Chem. 379:156-162. [DOI] [PubMed] [Google Scholar]
- 12.Gehring, U., J. Douwes, G. Doekes, A. Koch, W. Bischof, B. Fahlbusch, K. Richter, H. E. Wichmann, and J. Heinrich. 2001. Beta(1→3)-glucan in house dust of German homes: housing characteristics, occupant behavior, and relations with endotoxins, allergens, and molds. Environ. Health Perspect. 109:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehring, U., J. Heinrich, G. Hoek, M. Giovannangelo, E. Nordling, T. Bellander, J. Gerritsen, J. C. de Jongste, H. A. Smit, H. E. Wichmann, M. Wickman, and B. Brunekreef. 2007. Bacteria and mould components in house dust and children's allergic sensitisation. Eur. Respir. J. 29:1144-1153. [DOI] [PubMed] [Google Scholar]
- 14.Halstensen, A. S., K. C. Nordby, I. M. Wouters, and W. Eduard. 2007. Determinants of microbial exposure in grain farming. Ann. Occup. Hyg. 51:581-592. [DOI] [PubMed] [Google Scholar]
- 15.Iossifova, Y. Y., T. Reponen, D. I. Bernstein, L. Levin, H. Kalra, P. Campo, M. Villareal, J. Lockey, G. K. Hershey, and G. LeMasters. 2007. House dust (1-3)-beta-d-glucan and wheezing in infants. Allergy 62:504-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krüger, T., T. Sigsgaard, and E. C. Bonefeld-Jørgensen. 2004. Ex vivo induction of cytokines by mould components in whole blood of atopic and non-atopic volunteers. Cytokine 25:73-84. [DOI] [PubMed] [Google Scholar]
- 17.Kulicke, W. M., A. I. Lettau, and H. Thielking. 1997. Correlation between immunological activity, molar mass, and molecular structure of different (1→3)-beta-d-glucans. Carbohydr. Res. 297:135-143. [DOI] [PubMed] [Google Scholar]
- 18.Manners, D. J., A. J. Masson, J. C. Patterson, H. Bjorndal, and B. Lindberg. 1973. The structure of a beta-(1-6)-d-glucan from yeast cell walls. Biochem. J. 135:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntosh, M., B. A. Stone, and V. A. Stanisich. 2005. Curdlan and other bacterial (1→3)-beta-d-glucans. Appl. Microbiol. Biotechnol. 68:163-173. [DOI] [PubMed] [Google Scholar]
- 20.Milton, D. K., K. U. Alwis, L. Fisette, and M. Muilenberg. 2001. Enzyme-linked immunosorbent assay specific for (1→6) branched, (1→3)-beta-d-glucan detection in environmental samples. Appl. Environ. Microbiol. 67:5420-5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller, A., J. Raptis, P. J. Rice, J. H. Kalbfleisch, R. D. Stout, H. E. Ensley, W. Browder, and D. L. Williams. 2000. The influence of glucan polymer structure and solution conformation on binding to (1→3)-beta-d-glucan receptors in a human monocyte-like cell line. Glycobiology 10:339-346. [DOI] [PubMed] [Google Scholar]
- 22.Noss, I., I. M. Wouters, M. Visser, D. J. Heederik, P. S. Thorne, B. Brunekreef, and G. Doekes. 2008. Evaluation of a low cost electrostatic dust fall collector for indoor air endotoxin exposure assessment. Appl. Environ. Microbiol. 74:5621-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao, C. Y., M. A. Riggs, G. L. Chew, M. L. Muilenberg, P. S. Thorne, D. Van Sickle, K. H. Dunn, and C. Brown. 2007. Characterization of airborne molds, endotoxins, and glucans in homes in New Orleans after Hurricanes Katrina and Rita. Appl. Environ. Microbiol. 73:1630-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rau, U. 2004. Glucans secreted by fungi. Turkish Electron. J. Biotechnol. 2:30-36. [Google Scholar]
- 25.Rylander, R. 1999. Indoor air-related effects and airborne (1→3)-beta-d-glucan. Environ. Health Perspect. 107(Suppl. 3):501-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito, H., A. Misaki, and T. Harada. 1968. A comparison of the structure of curdlan and pachyman. Agr. Biol. Chem. 32:1261-1269. [Google Scholar]
- 27.Sander, I., C. Fleischer, G. Borowitzki, T. Brüning, and M. Raulf-Heimsoth. 2008. Development of a two-site enzyme immunoassay based on monoclonal antibodies to measure airborne exposure to (1→3)-beta-d-glucan. J. Immunol. Methods 337:55-62. [DOI] [PubMed] [Google Scholar]
- 28.Schram-Bijkerk, D., G. Doekes, M. Boeve, J. Douwes, J. Riedler, E. Ublagger, E. von Mutius, M. Benz, G. Pershagen, M. Wickman, T. Alfven, C. Braun-Fahrländer, M. Waser, and B. Brunekreef. 2006. Exposure to microbial components and allergens in population studies: a comparison of two house dust collection methods applied by participants and fieldworkers. Indoor Air 16:414-425. [DOI] [PubMed] [Google Scholar]
- 29.Schram-Bijkerk, D., G. Doekes, J. Douwes, M. Boeve, J. Riedler, E. Ublagger, E. von Mutius, M. R. Benz, G. Pershagen, M. van Hage, A. Scheynius, C. Braun-Fahrländer, M. Waser, and B. Brunekreef. 2005. Bacterial and fungal agents in house dust and wheeze in children: the PARSIFAL study. Clin. Exp. Allergy 35:1272-1278. [DOI] [PubMed] [Google Scholar]
- 30.Sigsgaard, T., E. C. Bonefeld-Jørgensen, S. K. Kjaergaard, S. Mamas, and O. F. Pedersen. 2000. Cytokine release from the nasal mucosa and whole blood after experimental exposures to organic dusts. Eur. Respir. J. 16:140-145. [DOI] [PubMed] [Google Scholar]
- 31.Stone, B. A., and A. E. Clarke. 1992. Chemistry and biology of (1→3)-β-glucans. La Trobe University Press, Bundoora, Victoria, Australia.
- 32.Stuurman, B., T. Meijster, D. Heederik, and G. Doekes. 2008. Inhalable beta(1→3)glucans as a non-allergenic exposure factor in Dutch bakeries. Occup. Environ. Med. 65:68-70. [DOI] [PubMed] [Google Scholar]
- 33.Thorn, J., L. Beijer, and R. Rylander. 1998. Airways inflammation and glucan exposure among household waste collectors. Am. J. Ind. Med. 33:463-470. [DOI] [PubMed] [Google Scholar]
- 34.Thorn, J., and R. Rylander. 1998. Airways inflammation and glucan in a rowhouse area. Am. J. Respir. Crit. Care Med. 157:1798-1803. [DOI] [PubMed] [Google Scholar]
- 35.Wouters, I. M., J. Douwes, P. S. Thorne, D. Heederik, and G. Doekes. 2002. Inter- and intraindividual variation of endotoxin- and beta(1→3)-glucan-induced cytokine responses in a whole blood assay. Toxicol. Ind. Health 18:15-27. [DOI] [PubMed] [Google Scholar]
- 36.Wouters, I. M., S. K. Hilhorst, P. Kleppe, G. Doekes, J. Douwes, C. Peretz, and D. Heederik. 2002. Upper airway inflammation and respiratory symptoms in domestic waste collectors. Occup. Environ. Med. 59:106-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wouters, I. M., S. Spaan, J. Douwes, G. Doekes, and D. Heederik. 2006. Overview of personal occupational exposure levels to inhalable dust, endotoxin, β(1->3)-glucan and fungal extracellular polysaccharides in the waste management chain. Ann. Occup. Hyg. 50:39-53. [DOI] [PubMed] [Google Scholar]
- 38.Wu, J., Y. Zhang, L. Wang, B. Xie, H. Wang, and S. Deng. 2006. Visualization of single and aggregated hulless oat (Avena nuda L.) (1→3),(1→4)-beta-d-glucan molecules by atomic force microscopy and confocal scanning laser microscopy. J. Agric. Food Chem. 54:925-934. [DOI] [PubMed] [Google Scholar]
- 39.Young, S. H., W. J. Dong, and R. R. Jacobs. 2000. Observation of a partially opened triple-helix conformation in 1→3-beta-glucan by fluorescence resonance energy transfer spectroscopy. J. Biol. Chem. 275:11874-11879. [DOI] [PubMed] [Google Scholar]
- 40.Young, S. H., G. R. Ostroff, P. C. Zeidler-Erdely, J. R. Roberts, J. M. Antonini, and V. Castranova. 2007. A comparison of the pulmonary inflammatory potential of different components of yeast cell wall. J. Toxicol. Environ. Health A 70:1116-1124. [DOI] [PubMed] [Google Scholar]
- 41.Zimmerman, J. W., J. Lindermuth, P. A. Fish, G. P. Palace, T. T. Stevenson, and D. E. DeMong. 1998. A novel carbohydrate-glycosphingolipid interaction between a beta-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 273:22014-22020. [DOI] [PubMed] [Google Scholar]