Abstract
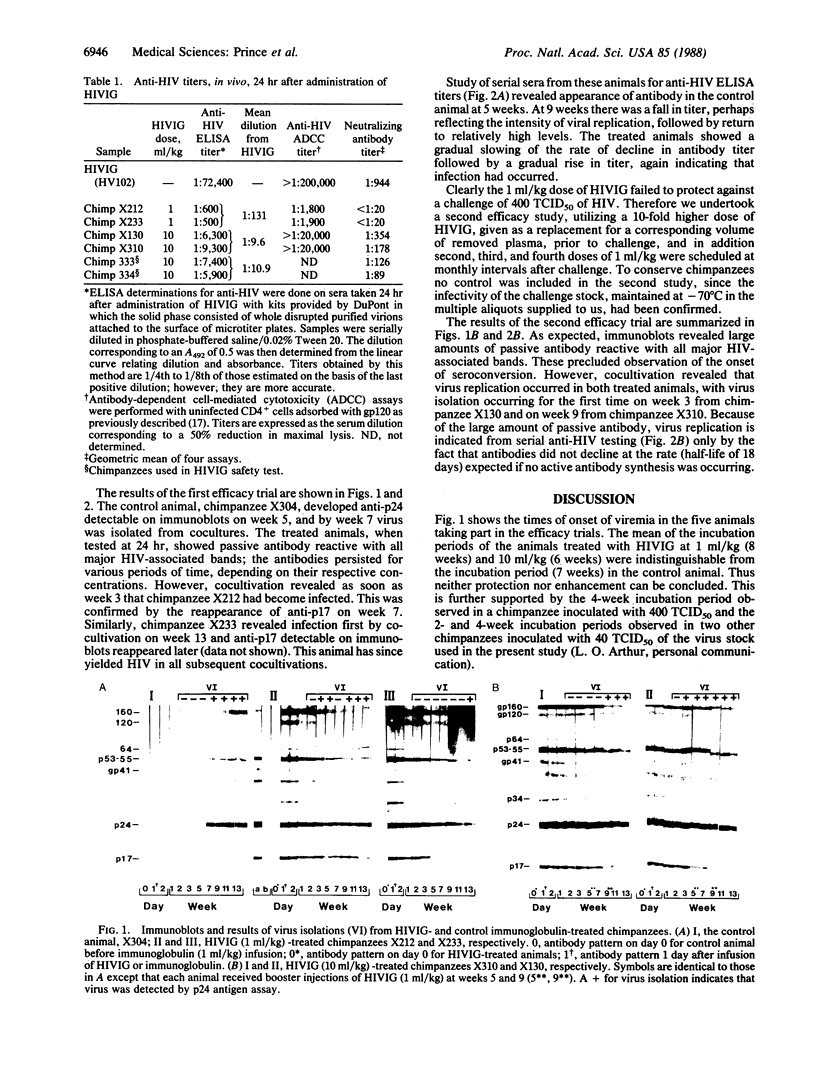
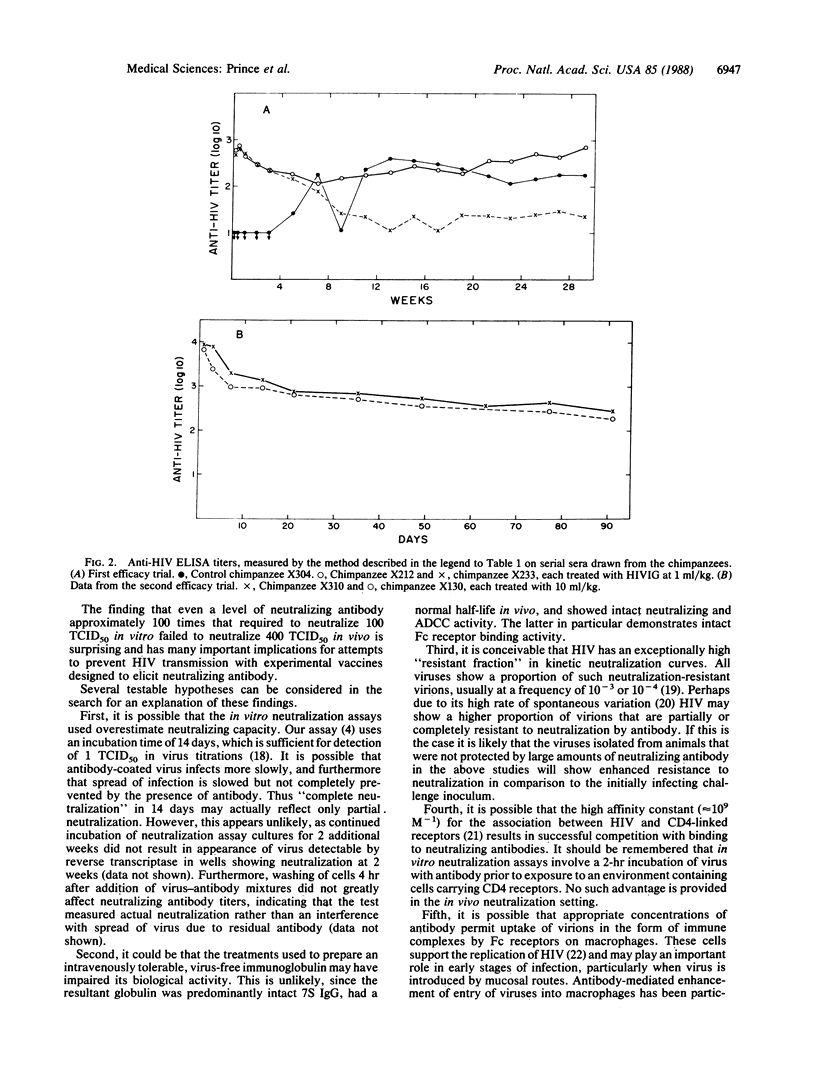
To assess the possible efficacy of passive immunization against human immunodeficiency virus (HIV) an immune globulin was prepared from plasma of HIV-seropositive donors selected to be among those having the top 12.5% of virus-neutralizing antibody titers. The immune globulin was treated with pepsin to render it intravenously tolerable. The preparation, which we termed HIVIG, neutralized 100 tissue culture 50% infective doses (TCID50) of HIV at an average dilution of 1:1000 in neutralization tests in vitro. During preparation HIVIG was subjected to virus inactivation and removal procedures that in theory resulted in a reduction in HIV infectivity by a factor of 10(25). At a dose of 9-10 ml/kg of body weight both the virus-inactivated source plasma and the final immunoglobulin preparation were noninfective and without adverse effect in two chimpanzees. Two chimpanzees inoculated intravenously with HIVIG at 1 ml/kg and two inoculated with 10 ml/kg were challenged intravenously 1 day later with 400 TCID50 of the same strain of HIV (HTLV-IIIb) used in neutralization assays in vitro. All animals became infected. Incubation periods to virus isolation (by cocultivation with human mononuclear cells) in HIVIG recipients did not differ significantly from the incubation period seen in a control animal that received a normal anti-HIV-free immunoglobulin. These findings may have implications for understanding the failure of experimental vaccines to protect against HIV challenge in chimpanzee experiments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman P. W., Groopman J. E., Gregory T., Clapham P. R., Weiss R. A., Ferriani R., Riddle L., Shimasaki C., Lucas C., Lasky L. A. Human immunodeficiency virus type 1 challenge of chimpanzees immunized with recombinant envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5200–5204. doi: 10.1073/pnas.85.14.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M., STRICKLAND A. G. A study of the basic aspects of neutralization of two animal viruses, western equine encephalitis virus and poliomyelitis virus. Virology. 1956 Apr;2(2):162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- Della-Porta A. J., Westaway E. G. A multi-hit model for the neutralization of animal viruses. J Gen Virol. 1978 Jan;38(1):1–19. doi: 10.1099/0022-1317-38-1-1. [DOI] [PubMed] [Google Scholar]
- Dorsett B., Cronin W., Chuma V., Ioachim H. L. Anti-lymphocyte antibodies in patients with the acquired immune deficiency syndrome. Am J Med. 1985 Apr;78(4):621–626. doi: 10.1016/0002-9343(85)90405-x. [DOI] [PubMed] [Google Scholar]
- Edwards C. A., Piet M. P., Chin S., Horowitz B. Tri(n-butyl) phosphate/detergent treatment of licensed therapeutic and experimental blood derivatives. Vox Sang. 1987;52(1-2):53–59. doi: 10.1111/j.1423-0410.1987.tb02989.x. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- HAWKES R. A. ENHANCEMENT OF THE INFECTIVITY OF ARBOVIRUSES BY SPECIFIC ANTISERA PRODUCED IN DOMESTIC FOWLS. Aust J Exp Biol Med Sci. 1964 Aug;42:465–482. doi: 10.1038/icb.1964.44. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Gonda M. A., Shaw G. M., Popovic M., Hoxie J. A., Gallo R. C., Wong-Staal F. Genomic diversity of the acquired immune deficiency syndrome virus HTLV-III: different viruses exhibit greatest divergence in their envelope genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4813–4817. doi: 10.1073/pnas.82.14.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J., Allison A. C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977 Jul 1;146(1):218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977 Feb 24;265(5596):739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz B., Wiebe M. E., Lippin A., Stryker M. H. Inactivation of viruses in labile blood derivatives. I. Disruption of lipid-enveloped viruses by tri(n-butyl)phosphate detergent combinations. Transfusion. 1985 Nov-Dec;25(6):516–522. doi: 10.1046/j.1537-2995.1985.25686071422.x. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Fultz P. N., McClure H. M., Eichberg J. W., Thomas E. K., Zarling J., Singhal M. C., Kosowski S. G., Swenson R. B., Anderson D. C. Effect of immunization with a vaccinia-HIV env recombinant on HIV infection of chimpanzees. Nature. 1987 Aug 20;328(6132):721–723. doi: 10.1038/328721a0. [DOI] [PubMed] [Google Scholar]
- Iglehart J. D., Weinhold K. J., Ward E. C., Matthews T. J., Langlois A. J., Schäfer W., Bolognesi D. P. Prospects for the immunological management of lethal tumors. Cancer Invest. 1983;1(5):409–421. doi: 10.3109/07357908309048509. [DOI] [PubMed] [Google Scholar]
- Jason J. M., McDougal J. S., Dixon G., Lawrence D. N., Kennedy M. S., Hilgartner M., Aledort L., Evatt B. L. HTLV-III/LAV antibody and immune status of household contacts and sexual partners of persons with hemophilia. JAMA. 1986 Jan 10;255(2):212–215. [PubMed] [Google Scholar]
- Kloster B. E., Tomar R. H., Spira T. J. Lymphocytotoxic antibodies in the acquired immune deficiency syndrome (AIDS). Clin Immunol Immunopathol. 1984 Feb;30(2):330–335. doi: 10.1016/0090-1229(84)90066-7. [DOI] [PubMed] [Google Scholar]
- Langlois A. J., Matthews T. J., Weinhold K. J., Chaffee S., Hershfield M., Bolognesi D. P. Detection of HIV-1 neutralizing antibodies by a simple, rapid, colorimetric assay. AIDS Res Hum Retroviruses. 1988 Feb;4(1):63–69. doi: 10.1089/aid.1988.4.63. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Legrain P., Goud B., Buttin G. Increase of retroviral infection in vitro by the binding of antiretroviral antibodies. J Virol. 1986 Dec;60(3):1141–1144. doi: 10.1128/jvi.60.3.1141-1144.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A. M., Horowitz B., Brotman B. Sterilisation of hepatitis and HTLV-III viruses by exposure to tri(n-butyl)phosphate and sodium cholate. Lancet. 1986 Mar 29;1(8483):706–710. doi: 10.1016/s0140-6736(86)91101-3. [DOI] [PubMed] [Google Scholar]
- Prince A. M., Horowitz B., Dichtelmueller H., Stephan W., Gallo R. C. Quantitative assays for evaluation of HTLV-III inactivation procedures: tri(N-butyl)phosphate:sodium cholate and beta-propiolactone. Cancer Res. 1985 Sep;45(9 Suppl):4592s–4594s. [PubMed] [Google Scholar]
- Prince A. M., Pascual D., Kosolapov L. B., Kurokawa D., Baker L., Rubinstein P. Prevalence, clinical significance, and strain specificity of neutralizing antibody to the human immunodeficiency virus. J Infect Dis. 1987 Aug;156(2):268–272. doi: 10.1093/infdis/156.2.268. [DOI] [PubMed] [Google Scholar]
- Rey F., Barré-Sinoussi F., Schmidtmayerova H., Chermann J. C. Detection and titration of neutralizing antibodies to HIV using an inhibition of the cytopathic effect of the virus on MT4 cells. J Virol Methods. 1987 Jun;16(3):239–249. doi: 10.1016/0166-0934(87)90008-5. [DOI] [PubMed] [Google Scholar]
- Wells M. A., Wittek A. E., Epstein J. S., Marcus-Sekura C., Daniel S., Tankersley D. L., Preston M. S., Quinnan G. V., Jr Inactivation and partition of human T-cell lymphotrophic virus, type III, during ethanol fractionation of plasma. Transfusion. 1986 Mar-Apr;26(2):210–213. doi: 10.1046/j.1537-2995.1986.26286152919.x. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Masur H., Spira T. J. Lymphocyte-reactive antibodies in acquired immune deficiency syndrome. J Clin Immunol. 1984 Mar;4(2):118–123. doi: 10.1007/BF00915045. [DOI] [PubMed] [Google Scholar]
- de Noronha F., Schäfer W., Essex M., Bolognesi D. P. Influence of antisera to oncornavirus glycoprotein (gp71) on infections of cats with feline leukemia virus. Virology. 1978 Apr;85(2):617–621. doi: 10.1016/0042-6822(78)90467-1. [DOI] [PubMed] [Google Scholar]





