Abstract
We have analyzed the impact of surface-to-volume ratio on final bacterial concentrations after batch growth. We examined six bottle sizes (20 to 1,000 ml) using three independent enumeration methods to quantify growth. We found no evidence of a so-called volumetric bottle effect, thus contradicting numerous previous reports.
Microbial batch growth during confined incubation in bottles of various sizes is used daily in a broad variety of microbiological studies and methods, including bioassays such as the assimilable organic carbon (AOC) assay (6, 10, 18) and the analysis of pure culture or microbial community growth in freshwater (3, 11, 19, 20). In this context, “bottle effect” or “volume effect” is a term that has cropped up frequently in aquatic microbiology papers (e.g., references 12, 13, and 21) during the last 100 years to explain inexplicable phenomena and variations in results obtained from such batch growth studies. The uncertainty surrounding this apparent effect was clearly summarized in a recent paper by Pernthaler and Amann (16): “Such investigations are often plagued by the mysterious ‘bottle effect’, a hard-to-define concept that reflects the worry of whether phenomena observed in confined assemblages are nonspecific consequences of the confinement rather than a result of the planned manipulation.” The “bottle effect” alludes to an apparent reaction of bacteria to batchwise incubation in a confined environment, and this concept has intermittently been linked to influences on final cell concentrations (3) and grazing/bacterivory (13), a change in viability/activity parameters (9), a change in cultivability (5), and a change in population composition (1).
The fact that microbiological processes during confined incubation differ from those in the environment is indisputable. However, a particular section of “bottle effect” literature focuses specifically on a volumetric “bottle effect”, where the above-mentioned effects are linked specifically to the size (or surface-to-volume ratio) of the incubation vessel (3, 8, 11-13, 15, 21). One of the oldest and best-known studies summarized clearly: “It will be observed that the densest bacterial populations appear in the bottles of water which offer the largest area of glass surface per unit volume of water” (21). This idea has established itself as dogma during the last century, with only a few differing opinions (4). However, precious little empirical data that actually quantify and explain the volumetric “bottle effect” are ever presented. In one example, Bischofberger et al. (3) observed that incubation of groundwater led to significantly more growth (about 2 log units) in small bottles (100 ml) than in big ones (10 liters). More often, however, the “bottle effect” is merely mentioned, as if it is self-explanatory and indisputable (2, 11, 12). In the present study, we took a simple but detailed look at the effect of bottle size on the outcome of short-term (<5-day) batch growth assays and compared the data critically to information in the literature and current opinion on this topic.
Three batch growth experiments were conducted to assess the volumetric bottle effect on final cell concentrations after growth into stationary phase. Six different bottle sizes were used, covering the ranges most often reported in “bottle effect” literature. All glassware and Teflon-coated caps were cleaned comprehensively as described elsewhere (6) to remove any traces of organic carbon that might have been present on surfaces. The bottle sizes were as follows (water volumes and surface area-to-volume ratios [square centimeters to milliliters] are respectively included in parentheses): 1,000 ml (900 ml, 0.3:1), 500 ml (400 ml, 0.4:1), 250 ml (200 ml, 0.6:1), 100 ml (90 ml, 0.8:1), 40 ml (35 ml, 1.5:1), and 20 ml (15 ml, 2.4:1). In the first experiment, a sample of natural river water (dissolved organic carbon [DOC], 3.8 mg/liter; AOC, 0.3 mg/liter) from a small oligotrophic stream was obtained, filter sterilized with a 50-kDa dialysis filter (Fresenius Medical Care), and inoculated (at 103 cells/ml) with a microbial community used for AOC assays (19). In the second experiment, a sample of the effluent (DOC, 1.2 mg/liter; AOC, 0.03 mg/liter; total cell concentration [TCC], 3 × 105 cells/ml) from a granulated active carbon filter situated in a drinking water pilot plant (7) was collected and used directly for the experiment without additional treatment or inoculation. For the third experiment, sterile Luria-Bertani (LB) medium (diluted 1:10,000; DOC, 0.7 mg/liter; AOC, 0.46 mg/liter) was inoculated with Vibrio cholerae O1 (103 cells/ml) as described previously (19). The water from each experiment was distributed into triplicate flasks of each size and incubated (at 30°C) until stationary phase was reached. Stationary phase was indicated by no significant increase in the TCC (measured after 3, 4, and 5 days) on consecutive days. Samples from all experiments were analyzed (i) for TCCs after being stained with SYBR green I and subjected to flow cytometry (7, 19), (ii) for ATP by using a commercial luciferin-luciferase assay (Promega Corporation) (7), and (iii) for heterotrophic plate counts (HPC) on R2A agar by a pour plate method with incubation at 30°C for 10 days. Possible biofilm growth was checked by applying sonication to selected samples. However, no wall growth in bottles of any size was observed.
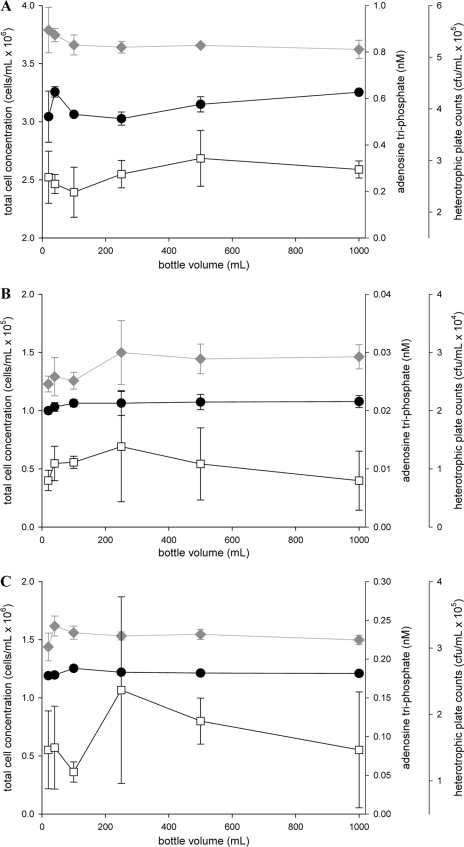
Growth was observed in all three experiments. The results show the net growth after subtraction of the initial cell/ATP/HPC concentrations from the final concentrations (Fig. 1). The proposed concept of the volumetric bottle effect implies that more growth should occur in smaller bottles. All data sets were subjected to regression analysis, and we observed no significant correlation (P < 0.01) between bottle size and final growth in any of the experiments by any of the three independent methods used for quantification. Figure 1A shows the batch growth results for a natural microbial community in prefiltered river water. This experimental setup is reflective of a typical AOC assay (6) or batch cultivation of natural microbial communities (20). Figure 1B shows the results for direct incubation of a treated drinking water sample. This sample and experimental setup were chosen specifically to assess any potential volumetric “bottle effect” on an indigenous microbial community in a biologically stable water sample, where only limited growth is expected. Indeed, the final cell concentration in the sample was only about 25% higher than the original cell concentration. The cultivability (HPC/TCC × 100) at day 0 was 0.4%, and at the end of the experimental period it had increased to 2.5%. This points to increased cultivability as a result of growth during confinement (5), yet it does not relate at all to the size of the incubation vessel. Figure 1C shows the data for V. cholerae grown in sterile LB medium (diluted 1:10,000) to stationary phase. Again, this particular setup is of specific relevance since a recently published paper on the growth of V. cholerae referred directly to the volumetric “bottle effect” to explain rather large differences between growth results from two separate studies (11, 19). The data from Fig. 1C suggest at least that a “bottle effect” should be ruled out as an interfering factor in this case.
FIG. 1.
Effects of bottle size on bacterial batch growth of a natural microbial community in filter-sterilized surface water (A), growth of bacteria during direct incubation of water from a drinking water treatment plant (B), and batch growth of a V. cholerae pure culture in diluted LB medium (C). Growth (expressed as the net growth) was quantified by flow cytometric total cell counting (circles), total ATP analysis (diamonds), and conventional plating (squares). All data points represent averages of triplicate measurements.
The results presented in this study clearly dispute the concept of a volumetric “bottle effect” on the outcome of short-term batch growth assays, be it for pure cultures or natural microbial communities. These findings contradict evidence reported by many other researchers (3, 8, 11-13, 15, 21). Although the volumetric “bottle effect” is often cited as a somewhat mysterious occurrence, it is imperative that clear experimental data are required for the critical appraisal thereof. The main experimental theory behind the phenomenon is that organic carbon adsorbs to clean glass surfaces, thus locally concentrating the carbon and creating more favorable growth conditions (2, 14). This adsorption and the fact that bacteria can utilize such adsorbed carbon have been demonstrated experimentally (14). What has, in our opinion, not been shown conclusively is that these effects can be so dramatic that they would alter the growth of samples to the extent that different sizes of bottles would render different final cell numbers after growth. Since we have not observed any volumetric “bottle effect” in our work, we can only speculate on the possible reasons why this has been observed previously. One explanation may be that glassware contaminated with organic carbon can contribute to the perception of a volumetric “bottle effect,” as large surface-to-volume ratios (found in small bottles) would account for increased contamination compared to that in bottles with smaller ratios. Hence, more additional available carbon would be introduced into smaller bottles, giving rise to higher final cell numbers after growth. In this context, it is essential that a comprehensive glassware-cleaning protocol be followed, including heating to a high temperature (>500°C) and storage away from volatile organics (6). In addition, it is important that such experiments at low carbon concentrations are complemented with the inclusion of correct and sensitive controls to assess potential organic carbon contamination. For example, the use of deionized water as a negative control should be avoided, since the absence of inorganic nutrients is bound to lead to no growth and thus false-negative results (10). A good negative control would be water that is only carbon limited, e.g., bottled drinking water (17). Moreover, the use of multiple tools for analyzing growth, including cultivation-independent methods, is encouraged.
In conclusion, we did not observe evidence of a volumetric bottle effect on short-term (<5-day) batch incubations. The findings of this study suggest that reference to the so-called volumetric bottle effect should be considered carefully unless supported by clear experimental data. This study does not dispute the fact that many authors have observed results implying apparent bottle effects during growth studies, but it questions the interpretation and understanding of this concept and the random use of the term “bottle effect” to explain uncertainty in results, specifically in relation to bottle size. Hopefully, these data will assist with experimental setups and comparison of data among different groups and stimulate discussion of and future research on this interesting, but slightly controversial, topic.
Acknowledgments
The research presented herein was financially supported by the 6th Framework European project TECHNEAU (grant no. 018320).
Footnotes
Published ahead of print on 18 December 2009.
REFERENCES
- 1.Agis, M., A. Granda, and J. R. Dolan. 2007. A cautionary note: examples of possible microbial community dynamics in dilution grazing experiments. J. Exp. Mar. Biol. Ecol. 341:176-183. [Google Scholar]
- 2.Amy, P. S., and H. D. Hiatt. 1989. Survival and detection of bacteria in an aquatic environment. Appl. Environ. Microbiol. 55:788-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischofberger, T., S. K. Cha, R. Schmitt, B. König, and W. Schmidt-Lorenz. 1990. The bacterial flora of non-carbonated, natural mineral water from the springs to reservoir and glass and plastic bottles. Int. J. Food Microbiol. 11:51-71. [DOI] [PubMed] [Google Scholar]
- 4.Butterfield, C. T. 1933. Observations on changes in numbers of bacteria in polluted water. J. Sew. Works 5:600-622. [Google Scholar]
- 5.Ferguson, R. L., E. N. Buckley, and A. V. Palumbo. 1984. Response of marine bacterioplankton to differential filtration and confinement. Appl. Environ. Microbiol. 47:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammes, F. A., and T. Egli. 2005. New method for assimilable organic carbon determination using flow-cytometric enumeration and a natural microbial consortium as inoculum. Environ. Sci. Technol. 39:3289-3294. [DOI] [PubMed] [Google Scholar]
- 7.Hammes, F., M. Berney, Y. Wang, M. Vital, O. Köster, and T. Egli. 2008. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 42:269-277. [DOI] [PubMed] [Google Scholar]
- 8.Heukelekian, H., and A. Heller. 1940. Relation between food concentration and surface for bacterial growth. J. Bacteriol. 40:547-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jürgens, K., J. M. Gasol, and D. Vaqué. 2000. Bacteria-flagellate coupling in microcosm experiments in the Central Atlantic Ocean. J. Exp. Mar. Biol. Ecol. 245:127-147. [Google Scholar]
- 10.Kaplan, L. A., T. L. Bott, and D. J. Reasoner. 1993. Evaluation and simplification of the assimilable organic carbon nutrient bioassay for bacterial growth in drinking water. Appl. Environ. Microbiol. 59:1532-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirschner, A. K., J. Schlesinger, A. H. Farnleitner, R. Hornek, B. Süss, B. Golda, A. Herzig, and B. Reitner. 2008. Rapid growth of planktonic Vibrio cholerae non-O1/non-O139 strains in a large alkaline lake in Austria: dependence on temperature and dissolved organic carbon quality. Appl. Environ. Microbiol. 74:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krammer, M., B. Velimirov, U. Fischer, A. H. Farnleitner, A. Herzig, and A. K. Kirschner. 2008. Growth response of soda lake bacterial communities to simulated rainfall. Microb. Ecol. 55:194-211. [DOI] [PubMed] [Google Scholar]
- 13.Marrase, C. L., L. Lim, and D. A. Caron. 1992. Seasonal and daily changes in bacterivory in a coastal plankton community. Mar. Ecol. Prog. Ser. 82:281-289. [Google Scholar]
- 14.Marshall, K. C. 1996. Adhesion as a strategy for access to nutrients, p. 59-87. In M. Fletcher (ed.), Bacterial adhesion: molecular and ecological diversity. Wiley-Liss, New York, NY.
- 15.Morita, R. Y. 1997. Bacteria in oligotrophic environments. Chapman & Hall, New York, NY.
- 16.Pernthaler, J., and R. Amann. 2005. Fate of heterotrophic microbes in pelagic habitats: focus on populations. Microbiol. Mol. Biol. Rev. 69:440-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Servais, P., A. Anzil, and C. Ventresque. 1989. Simple method for the determination of biodegradable dissolved organic carbon in water. Appl. Environ. Microbiol. 55:2732-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van der Kooij, D. 1992. Assimilable organic carbon as indicator for bacterial regrowth. J. Am. Water Works Assoc. 84:57-65. [Google Scholar]
- 19.Vital, M., H. P. Füchslin, F. Hammes, and T. Egli. 2007. Growth of Vibrio cholerae O1 Ogawa Eltor in freshwater. Microbiology 153:1993-2001. [DOI] [PubMed] [Google Scholar]
- 20.Wang, Y., F. Hammes, N. Boon, and T. Egli. 2007. Quantification of the filterability of freshwater bacteria through sterile 0.45, 0.22 and 0.1 μm pore size filters and shape-dependent enrichment of filterable bacterial communities. Environ. Sci. Technol. 41:7080-7086. [DOI] [PubMed] [Google Scholar]
- 21.ZoBell, C. E., and Q. Anderson. 1936. Observations on the multiplication of bacteria in different volumes of stored sea water and the influence of oxygen tension and solid surfaces. Biol. Bull. 71:324-342. [Google Scholar]