Abstract
Aerobic growth conditions significantly influenced anaerobic succinate production in two-stage fermentation by Escherichia coli AFP111 with knockouts in rpoS, pflAB, ldhA, and ptsG genes. At a low cell growth rate limited by glucose, enzymes involved in the reductive arm of the tricarboxylic acid cycle and the glyoxylate shunt showed elevated activities, providing AFP111 with intracellular redox balance and increased succinic acid yield and productivity.
Succinic acid is valued as one of the key basic chemicals used in the preparation of biodegradable polymers or as raw material for chemicals of the C4 family (8, 19). The fermentative production of succinic acid from renewable resources is environmentally acceptable and sustainable (3). A breakthrough in genetically engineering Escherichia coli (6, 7, 11, 18) for succinate production was the isolation of strain AFP111 (1, 4), a mutant of NZN111 with a spontaneous ptsG mutation (pflAB ldhA double mutant). The process involves a two-stage fermentation, with aerobic cell growth followed by anaerobic conditions for succinate production (16, 21, 22). The aerobically induced enzymes can maintain their activity during the anaerobic phase and significantly affect succinate fermentation (22, 23). Using the best transition time based on the activities of the key enzymes and other physiological states, a two-stage fermentation using the recombinant AFP111 strain harboring pTrc99A-pyc achieved a final succinic acid concentration and productivity of 99.2 g·liter−1 and 1.3 g·liter−1·h−1, respectively (21).
Aerobic cell growth is essential for the subsequent anaerobic fermentation. However, few studies have focused on the regulation of aerobic cell growth. As a regulation method, gluconeogenic carbon sources were used instead of glucose for the aerobic growth of Escherichia coli NZN111 and the activities of enzymes that are favorable for the anaerobic synthesis of succinate were enhanced (23, 24). Unfortunately, a gluconeogenic carbon source (e.g., sodium acetate) might increase the osmotic pressure of culture media, which would be detrimental to succinate production (23). As another regulation method, a glucose feeding strategy controlling the glucose concentration at about 0.5 g·liter−1 up to 1 g·liter−1 was reported to prevent excessive formation of acetic acid (16).
In this study, we investigated different glucose feeding strategies for the aerobic growth phase of the two-phase process for succinate production by E. coli AFP111. Specifically, we compared several growth rates by using glucose limitation in addition to maximum growth under conditions of excess glucose.
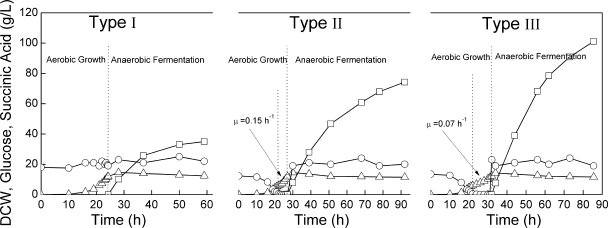
E. coli AFP111 [F+ λ− rpoS396(Am) rph-1 ΔpflAB::Cam ldhA::Kan ptsG] (4, 16), which was a kind gift from D. P. Clark (Southern Illinois University), was the only strain used in this study. Luria-Bertani (LB) medium (60 ml) was used for inoculum culture in 1,000-ml flasks, and 3 liters of chemically defined medium (13, 14) was used for two-stage culture in a 7-liter fermentor. Two-stage fermentations were divided into three types, based on the glucose feeding strategy used during the aerobic stage. For type I culture, the glucose concentration was maintained at about 20 g·liter−1 during aerobic cell growth. Type II and III cultures comprised a batch process and subsequent glucose-limited fed-batch process (Fig. 1). The batch process initially contained 13 g/liter of glucose. The fed-batch process began when the dry cell weight (DCW) reached about 6 g/liter, with type II and type III cultures using a 600 g/liter glucose feed to achieve cell growth rates of 0.15 h−1 and 0.07 h−1, respectively (10). When the DCW reached 12 g·liter−1, the aerobically grown cells were directly transferred to anaerobic conditions (Fig. 1). For the anaerobic process, oxygen-free CO2 was sparged at 0.5 liter·min−1, the pH was controlled between 6.4 and 6.8 with intermittent supplementation of solid magnesium carbonate hydroxide, and the glucose concentration was maintained at about 20 g·liter−1 by supplying glucose in an 800-g·liter−1 solution.
FIG. 1.
Concentrations of glucose (circles), DCW (triangles), and succinic acid (squares) in the three types of two-stage fermentation by AFP111. μ, growth rate.
The optical density at 600 nm was used to monitor cell growth, and this value was correlated to DCW. The concentration of glucose was assayed with an enzyme electrode analyzer, and organic acids were quantified by high-performance liquid chromatography (HPLC). The intracellular concentrations of NADH and NAD+ were assayed with a cycling method (12). The activities of isocitrate lyase (ICL) (20), pyruvate kinase (PYK) (17), phosphoenolpyruvate (PEP) carboxykinase (PCK) (20, 23), PEP carboxylase (PPC) (23), and malate dehydrogenase (MDH) (23) were measured spectrophotometrically at the end of the aerobic phase and 12 h after the onset of the anaerobic phase.
All three types of fermentations were terminated when the succinate concentration increased less than 1 g·liter−1 in 5 h. Type III fermentation was terminated at a final succinic acid concentration of 101.2 g·liter−1 and an anaerobic-phase productivity of 1.89 g·liter−1·h−1 (Fig. 1). Trace amounts of by-products (such as acetate, ethanol, and pyruvate) accumulated and did not follow any trend in the anaerobic phase (data not shown).
At the end of the aerobic culture phase, the specific enzyme activities of PCK, PYK, and ICL in type III culture were 2.9, 2.5, and 11.4 times higher, respectively, than the activities in type I culture (Table 1) . This phenomenon is consistent with published reports that suggest that the expression of enzymes involved in anaplerotic metabolism and the glyoxylate shunt (5, 15) is elevated in E. coli grown under glucose-limited conditions. These enzymes maintained their activities in the subsequent anaerobic phase (Table 1) and would be central to succinate production (22, 23). The elevated levels of PCK and PPC would provide the reductive branch of the tricarboxylic acid (TCA) cycle with oxaloacetate (OAA) at a higher rate (9), thereby supplying both malate and citrate (Table 1).
TABLE 1.
Activities of enzymes at the end of the aerobic culture phase and 12 h after the onset of the anaerobic phase
| Fermentation typea | Stageb | Mean sp act of enzyme ± SD (U/mg protein)c |
||||
|---|---|---|---|---|---|---|
| PCK | PPC | MDH | PYK | ICL | ||
| I | Aerobic | 0.82 ± 0.05 | 0.22 ± 0.05 | 21.97 ± 0.15 | 1,175 ± 11.38 | 0.12 ± 0.00 |
| Anaerobic | 0.55 ± 0.02 | 0.19 ± 0.00 | 18.27 ± 1.05 | 978 ± 12.33 | 0.09 ± 0.00 | |
| II | Aerobic | 1.46 ± 0.10 | 0.23 ± 0.04 | 25.69 ± 0.37 | 2,053 ± 3.65 | 0.73 ± 0.03 |
| Anaerobic | 1.09 ± 0.01 | 0.20 ± 0.01 | 35.55 ± 0.78 | 1,430 ± 13.78 | 0.41 ± 0.02 | |
| III | Aerobic | 2.38 ± 0.11 | 0.16 ± 0.00 | 23.5 ± 0.13 | 2,955 ± 8.77 | 1.37 ± 0.00 |
| Anaerobic | 1.75 ± 0.03 | 0.21 ± 0.01 | 43.8 ± 0.62 | 2,501 ± 10.15 | 1.02 ± 0.01 | |
Fermentation types were mentioned in culture conditions section.
“Aerobic” represents the data obtained at the end of aerobic culture; “Anaerobic” represents those obtained 12 h after transition to anaerobic fermentation.
The standard deviations (SD) were calculated from triplicate samples of the same run.
The reductive branch of the TCA cycle consumes 4 mol of electrons to form 2 mol of succinate based on 1 mol of glucose (1, 4). Therefore, the conversion of glucose to succinate through the reductive arm of the TCA cycle alone will lead to an intracellular imbalance of reducing equivalents (2, 18). Fortunately, the glyoxylate shunt (2, 18, 22) is available to provide 10 mol of electrons by converting 1 mol of glucose to 1 mol of succinate and 2 mol of CO2 (22). In the case of the ptsG mutant strain AFP111, when the molar flux at the PEP branch point flowing to OAA versus flowing to pyruvate reaches a ratio of 5:2, the intracellular redox balance is satisfied and the maximum theoretical mass yield of 1.12 g·g−1 succinic acid is achieved (22). Based on the elevated activities of PCK, PYK, and ICL (Table 1), both pathways leading to succinate were enhanced after glucose-limited growth. The succinic acid yields of 1.03 to 1.07 g·g−1 in the two glucose-limited processes approached the maximum theoretical yield for AFP111 (22), and these yields were about two times greater than the yield in the type I fermentation (Table 2).
TABLE 2.
Succinic acid production during anaerobic fermentation phasea
| Fermentation type | Mean ± SD |
|||||
|---|---|---|---|---|---|---|
| Succinic acid (g·liter−1) | Yield (g·g−1) | Productivity (g·liter−1·h−1) | Specific productivity at 12 h (mg·g−1·h−1) | NADH at 12 h mmol·(g DCW)−1 | NADH/NAD+ ratio at 12 h | |
| I | 35.0 ± 0.74 | 0.43 ± 0.05 | 0.98 ± 0.04 | 105 ± 15 | 0.88 ± 0.07 | 0.55 ± 0.08 |
| II | 74.3 ± 3.24 | 1.03 ± 0.01 | 1.32 ± 0.05 | 160 ± 8 | 1.95 ± 0.11 | 1.05 ± 0.10 |
| III | 101.2 ± 1.04 | 1.07 ± 0.02 | 1.89 ± 0.07 | 227 ± 11 | 1.97 ± 0.15 | 1.27 ± 0.13 |
The data were calculated only for the anaerobic stage. The standard deviations (SD) were calculated from two independent two-stage fermentations.
In addition to differences in succinic acid yields, the glucose-limited and type I fermentations each resulted in significantly different specific succinic acid productivities (Table 2). A specific succinic acid productivity of 227 mg·g−1·h−1 was obtained at 12 h in type III fermentation. Because two pathways are needed for succinate production due to redox constraints, and enzyme activities in both pathways were elevated by glucose limitation, the results suggest that operating with glucose limitation provides the cells with greater metabolic flexibility to achieve a redox balance. Furthermore, the results suggest that one or more of these enzymes are limiting succinate formation under batch conditions (type I fermentation). Considering the NADH/NAD+ assays (Table 2), the results would support the hypothesis that succinate production was limited by insufficient NADH (2, 18).
In summary, our study presented an efficient method of aerobic cell cultivation for two-stage succinate fermentation by engineered E. coli. Since the physiological state of aerobically grown cells was essential for their subsequent anaerobic succinate fermentation, some other environmental and physiology factors in the aerobic growth phase may also play an important role in improving succinate production.
Acknowledgments
The English of the manuscript was checked by Long-an Shang. Also, we were greatly helped by the thoughtful comments of one of the reviewers.
This work was supported by the National Natural Science Foundation of China (grant no. 20606017), the 973 Program of China (grant no. 2009CB724701), and the Qing Lan Project of Jiangsu Province.
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Chatterjee, R., C. S. Millard, K. Champion, D. P. Clark, and M. I. Donnelly. 2001. Mutation of the ptsG gene results in increased production of succinate in fermentation of glucose by Escherichia coli. Appl. Environ. Microbiol. 67:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark, D. P. 1989. The fermentation pathways of Escherichia coli. FEMS Microbiol. Rev. 63:223-234. [DOI] [PubMed] [Google Scholar]
- 3.Cukalovic, A., and C. V. Stevens. 2008. Feasibility of production methods for succinic acid derivatives: a marriage of renewable resources and chemical technology. Biofuels Bioprod. Bioref. 2:505-529. [Google Scholar]
- 4.Donnelly, M. I., C. S. Millard, D. P. Clark, M. J. Chen, and J. W. Rathke. 1998. A novel fermentation pathway in an Escherichia coli mutant producing succinic acid, acetic acid and ethanol. Appl. Biochem. Biotechnol. 72:187-198. [DOI] [PubMed] [Google Scholar]
- 5.Fischer, E., and U. Sauer. 2003. A novel metabolic cycle catalyzes glucose oxidation and anaplerosis in hungry Escherichia coli. Biol. Chem. 278:46446-46451. [DOI] [PubMed] [Google Scholar]
- 6.Jantama, K., M. J. Haupt, S. A. Svoronos, X. Zhang, J. C. Moore, K. T. Shanmugam, and L. O. Ingram. 2008. Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of E. coli C that produce succinate and malate. Biotechnol. Bioeng. 99:1140-1153. [DOI] [PubMed] [Google Scholar]
- 7.Jantama, K., X. Zhang, J. C. Moore, K. T. Shanmugam, S. A. Svoronos, and L. O. Ingram. 2008. Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol. Bioeng. 101:881-893. [DOI] [PubMed] [Google Scholar]
- 8.Jiang, M., K. Chen, Z. Liu, P. Wei, H. Ying, and H. Chang. 14 April 2009, posting date. Succinic acid production by Actinobacillus succinogenes using spent brewer's yeast hydrolysate as a nitrogen source. Appl. Biochem. Biotechnol. doi: 10.1007/s12010-009-8649-1. [DOI] [PubMed]
- 9.Kim, P., M. Laivenieks, C. Vieille, and J. G. Zeikus. 2004. Effect of overexpression of Actinobacillus succinogenes phosphoenolpyruvate carboxykinase on succinic acid production in Escherichia coli. Appl. Environ. Microbiol. 70:1238-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lara, A. R., L. Caspeta, G. Gosset, F. Bolívar, and O. T. Ramírez. 2008. Utility of an Escherichia coli strain engineered in the substrate uptake system for improved culture performance at high glucose and cell concentrations: an alternative to fed-batch cultures. Biotechnol. Bioeng. 99:893-901. [DOI] [PubMed] [Google Scholar]
- 11.Lee, D. Y., T. Y. Kim, B. H. Kim, J. Lee, and S. Y. Lee. 2005. Metabolic engineering of Escherichia coli for enhanced production of succinic acid, based on genome comparison and in silico gene knockout simulation. Appl. Environ. Microbiol. 71:7880-7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonardo, M. R., Y. Dailly, and D. P. Clark. 1996. Role of NAD in regulating the adhE gene of Escherichia coli. J. Bacteriol. 178:6013-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu, S., M. A. Eiteman, and E. Altman. 2009. pH and base counterion affect succinate production in dual-phase Escherichia coli fermentations. J. Ind. Microbiol. Biotechnol. 36:1101-1109. [DOI] [PubMed] [Google Scholar]
- 14.Lu, S., M. A. Eiteman, and E. Altman. 2009. Effect of CO2 on succinate production in dual-phase Escherichia coli fermentations. J. Biotechnol. 143:213-223. [DOI] [PubMed] [Google Scholar]
- 15.Nanchen, A., A. Schicker, and U. Sauer. 2006. Nonlinear dependency of intracellular fluxes on growth rate in miniaturized continuous cultures of Escherichia coli. Appl. Environ. Microbiol. 72:1164-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nghiem, N. P., M. Donnelly, C. S. Millard, and L. Stols. February 1999. U.S. patent 5,869,301.
- 17.Peng, L., and K. Shimizu. 2003. Global metabolic regulation analysis for Escherichia coli K12 based on protein expression by 2-dimensional electrophoresis and enzyme activity measurement. Appl. Microbiol. Biotechnol. 61:163-178. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez, A. M., G. N. Bennett, and K. Y. San. 2005. Novel pathway engineering design of the anaerobic central metabolic pathway in Escherichia coli to increase succinate yield. Metab. Eng. 7:229-239. [DOI] [PubMed] [Google Scholar]
- 19.Song, H., and S. Y. Lee. 2006. Production of succinic acid by bacterial fermentation. Enzyme Microb. Technol. 39:352-361. [Google Scholar]
- 20.Van der Werf, M. J., M. V. Guettler, M. K. Jain, and J. G. Zeikus. 1997. Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch. Microbiol. 168:332-342. [DOI] [PubMed] [Google Scholar]
- 21.Vemuri, G. N., M. A. Eiteman, and E. Altman. 2002. Succinate production in dual-phase Escherichia coli fermentations depends on the time of transition from aerobic to anaerobic conditions. J. Ind. Microbiol. Biotechnol. 28:325-332. [DOI] [PubMed] [Google Scholar]
- 22.Vemuri, G. N., M. A. Eiteman, and E. Altman. 2002. Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli. Appl. Environ. Microbiol. 68:1715-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu, H., L. Z. Min, L. Zhou, and Q. Ye. 2007. Improved succinic acid production in the anaerobic culture of an Escherichia coli pflB ldhA double mutant as a result of enhanced anaplerotic activities in the preceding aerobic culture. Appl. Environ. Microbiol. 73:7837-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu, H., L. Z. Min, L. Zhou, X. J. Lin, and Q. Ye. 2009. Enhanced anaerobic succinic acid production by Escherichia coli NZN111 aerobically grown on gluconeogenic carbon sources. Enzyme Microb. Technol. 44:165-169. [Google Scholar]