Abstract
The presence of Vibrio parahaemolyticus in 123 oyster samples collected from an estuary on the southern coast of Sao Paulo state, Brazil, was investigated. Of the 123 samples, 99.2% were positive with densities ranging from <3 to 105 most probable number (MPN)/g. Densities correlated significantly with water temperature (r = 0.48; P < 0.001) but not with salinity (r = −0.09; P = 0.34). The effect of harvest site on counts was not significant (P > 0.05). These data provide information for the assessment of exposure of V. parahaemolyticus in oysters at harvest.
Infections caused by Vibrio parahaemolyticus have been reported in several countries (1, 3-5, 14, 15, 16, 17, 18, 19, 20, 22, 24, 26). Among other pathogenic features, V. parahaemolyticus strains produce a thermostable hemolysin, known as thermostable direct hemolysin (TDH), as well as TRH (a TDH-related hemolysin) (25, 29). However, not all strains are pathogenic, as less than 1% of food or environmental strains produce TDH or TRH (2, 7, 9, 10, 11).
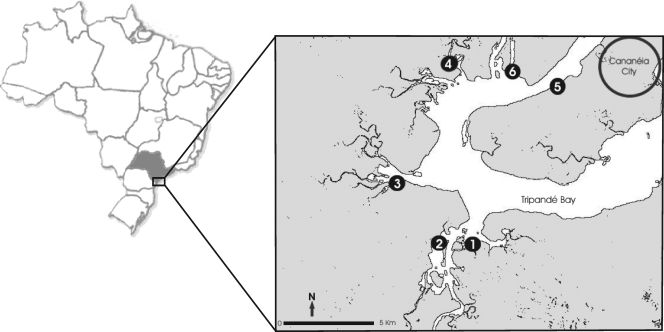
The most important vehicle for this microorganism is raw or partially cooked shellfish (8, 13, 25, 29). In this study, the densities of V. parahaemolyticus in oysters collected in six oyster bed sites in the estuary of Cananeia (25°S; 48°W) in the southern coastal area of Sao Paulo state, Brazil (Fig. 1) between May 2004 and June 2005 were determined using the most probable number (MPN) technique by the method of De Paola and Kaysner (12). Each sample consisted of 15 oysters, pooled in a plastic bag, and transported in a cold box to the laboratory located in the city of Sao Paulo, Brazil. The temperature during transportation did not exceed 13°C, and the travel time was around 5 h. In the laboratory, the oysters were kept under refrigeration (4 to 8°C) and analyzed within 24 h of collection. Oysters were cleaned and shucked by the method of Cook et al. (6). Identification of V. parahaemolyticus was based on traditional and API 20E strip biochemical tests (bioMérieux, France), using V. parahaemolyticus ATCC 17802 as the reference strain. The observed prevalence of this bacterium was high, as the microorganism was detected in 99.2% (122/123) of the samples and the densities varied between 0.78 and 5.04 log MPN/g.
FIG. 1.
Locations of oyster bed sites in the Cananeia estuary on the southern coast of Sao Paulo state, Brazil. (Courtesy of E. E. de Miranda and A. C. Coutinho [Embrapa Monitoramento por Satélite] [http://www.cdbrasil.cnpm.embrapa.br].)
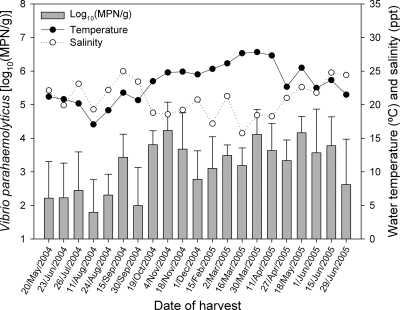
Strategies for the control of V. parahaemolyticus in oysters depend on understanding the seasonal and geographical distribution and the effects of environmental parameters on the growth of this pathogen. To verify the influence of salinity and temperature of seawater on the density of V. parahaemolyticus, samples of water (n = 123) were collected from the same depth of oyster beds using 250-ml plastic flasks. Salinity was determined using a salinometer (model RS10; Rosemount Analytical, Cedar Grove, NJ), and the temperature was determined at the time of collection using a digital thermometer (Hanna Instruments). The results are shown in Fig. 2.
FIG. 2.
Total densities of Vibrio parahaemolyticus in oysters from the southern coast of Sao Paulo state, Brazil. Each bar or point represents the arithmetic mean of six sites, and each error bar represents the standard deviation.
Total V. parahaemolyticus densities did not correlate significantly with water salinity, as determined by Pearson coefficient (r = −0.09; P = 0.34). However, the mean salinity varied significantly according to the sampling site and season (P < 0.05) (Table 1). The highest mean salinity (24.2 ppt) was detected at site 5 and was 1.4 times higher than at site 2 (17.3 ppt), the lowest mean salinity detected in this study.
TABLE 1.
Seasonal distribution of the total density of Vibrio parahaemolyticus in oysters, water temperature, and salinity in the southern coastal area of Sao Paulo state, Brazil
| Variable | Season | No. of samples | Meana | SD | Range |
|---|---|---|---|---|---|
| Vibrio parahaemolyticus | Winter | 35 | 2.44 A | 1.06 | <0.48-4.38 |
| density (log10 | Spring | 29 | 3.26 B | 1.17 | 1.04-5.04 |
| MPN/g) | Summer | 24 | 3.47 B | 0.75 | 1.54-5.04 |
| Fall | 35 | 3.48 B | 1.01 | 1.04-5.04 | |
| Temp (°C) | Winter | 35 | 20.1 A | 1.9 | 14.4-24.0 |
| Spring | 29 | 23.6 B | 1.8 | 20.0-26.0 | |
| Summer | 24 | 26.7 C | 1.4 | 24.1-29.2 | |
| Fall | 35 | 23.9 B | 2.2 | 20.6-28.3 | |
| Salinity (ppt) | Winter | 35 | 22.3 A | 4.5 | 12.2-29.8 |
| Spring | 29 | 20.2 AB | 4.4 | 11.2-29.4 | |
| Summer | 24 | 18.2 B | 4.3 | 5.3-25.2 | |
| Fall | 35 | 21.8 A | 3.9 | 8.7-28.2 |
Values with different letters are significantly different (P < 0.05).
The weak correlation between water salinity and V. parahaemolyticus densities in oysters suggests that salinity per se is a secondary factor for growth of this bacterium, as are turbidity and chlorophyll content in water (27, 30). These results agree with those obtained by Deepanjali et al. (9) and Martinez-Urtiga et al. (21), who did not find correlation between these two parameters. However, they are in contrast with the results reported by DePaola et al. (11), who observed correlation (P < 0.05) between salinity and total density of V. parahaemolyticus.
The results of this study corroborate existing evidence (10, 11, 27, 30) indicating that the temperature of seawater has a significant correlation (r = 0.48; P < 0.001) on the densities of V. parahaemolyticus in oysters, but they are at odds with results reported by Deepanjali et al. (9), who observed no statistically significant correlation with seawater temperature. The temperature variations observed in the present study (15°C) were lower than those observed by DePaola et al. (22°C) (11) but higher than those reported by Deepanjali et al. (10°C) (9).
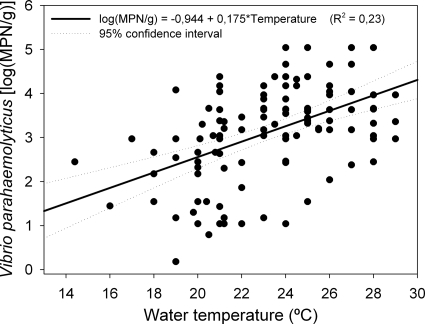
The relationship between V. parahaemolyticus density and water temperature and salinity were analyzed by multiple linear regression. Results showed that salinity was not significant either for linear effects or for squared effects (P > 0.05). For temperature, while the parameter of linear effect was significant (P < 0.05), the squared effect was not (P > 0.05). Considering the goodness of fit of the model, the following linear regression described the density in oysters the best (Fig. 3): log10 MPN V. parahaemolyticus/g = −0.944 + (0.175 × temperature). The lack of model fitness test was not significant and was considered adequate to express the relationship between V. parahaemolyticus density and seawater temperature, in spite of the low R2 (0.23).
FIG. 3.
Goodness of fit regression model of V. parahaemolyticus density in oysters and water temperature.
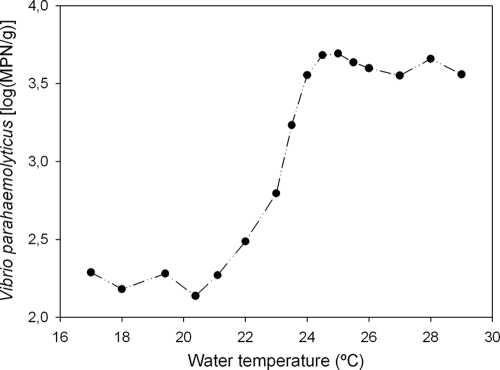
The effect of temperature was further summarized by rank correlation and the use of a smoothing technique (moving average) in which densities corresponding to temperatures within a range of 1°C were pooled to estimate an arithmetic mean of densities in successive intervals. The moving average was calculated using a length of three values. Although seawater temperature and V. parahaemolyticus densities were correlated in the present study, the mean densities reached a plateau at temperatures above 24°C and below 20°C (Fig. 4) where the density was not significantly influenced by temperature, consistent with observations reported also by DePaola et al. (11). Our findings could explain the lack of correlation among those parameters detected in tropical oysters by Deepanjali et al. (9) when the temperature varied from 25 to 35°C.
FIG. 4.
Relationship between the mean density of V. parahaemolyticus in oysters and seawater temperature in Cananeia estuary, Sao Paulo state, Brazil.
The influence of the season of the year and site of collection on the mean densities was assessed by analysis of variance and Tukey's test, when necessary. As shown in Table 1, the V. parahaemolyticus densities were similar in the samples collected during spring, summer, and autumn but differed significantly (P < 0.05) in those collected during winter. Densities among samples collected during summer varied less compared to other seasons. Densities above 105 MPN/g were detected in six (4.9%) oyster samples (three samples during spring, two samples during summer, and one sample during autumn) collected when the temperature was higher than 24°C and the salinity was higher than 15 ppt. The effect of harvest site on densities was not significant (P > 0.05).
Previous studies performed with oysters collected in the same region in Brazil have shown a low incidence of pathogenic V. parahaemolyticus (23, 28). Similar results were observed in the present study, as only one oyster sample (0.8%) and only one isolate among 2,243 isolates tested (0.044%) were Kanagawa and tdh positive. Besides the Kanagawa reaction (24), all strains have been tested for tlh, tdh, and trh genes using PCR (12). The pathogen-positive sample presented with a low density of V. parahaemolyticus (3 MPN/g) and was collected during winter, when the temperature was 21°C. Due to the low incidence of pathogenic strains in the samples, correlation between pathogenicity and water temperature or salinity could not be determined.
This study indicates that the presence of V. parahaemolyticus in oysters cultivated in the southern coast of Sao Paulo state, Brazil, is high, but pathogenic strains are seldom detected. These results on the ecology and characteristics of V. parahaemolyticus are valuable for future risk assessments related to this pathogen in oysters at harvest.
Acknowledgments
This study was supported by the Conselho Nacional de Pesquisa Cientifico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP). We are grateful to FAPESP for the scholarship to P.D.S.C.S.
We thank Brendan Niemira for comments.
Footnotes
Published ahead of print on 18 December 2009.
REFERENCES
- 1.Anonymous. 1999. Vibrio parahaemolyticus, Japan 1996-1998. Wkly. Epidemiol. Rep. 74:361-363. [PubMed] [Google Scholar]
- 2.Anonymous. 2005. Quantitative risk assessment on the public health impact of pathogenic Vibrio parahaemolyticus in raw oysters. U.S. Food and Drug Administration, Washington, DC.
- 3.Centers for Disease Control and Prevention. 1999. Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters and clams harvested from Long Island Sound—Connecticut, New Jersey, and New York, 1998. MMWR Morb. Mortal. Wkly. Rep. 48:48-51. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1998. Outbreak of Vibrio parahaemolyticus infections associated with eating raw oysters—Pacific Northwest, 1997. MMWR Morb. Mortal. Wkly. Rep. 47:457-462. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2006. Vibrio parahaemolyticus infections associated with consumption of raw shellfish—three states, 2006. MMWR Morb. Mortal. Wkly. Rep. 55:854-856. [PubMed] [Google Scholar]
- 6.Cook, D. W., W. Burkhardt, A. DePaola, S. A. McCarthy, and K. R. Calci. 2001. Molluscan shellfish: oysters, mussels, and clams, p. 507-514. In F. P. Downes and K. Ito (ed.), Compendium of methods for the microbiological examination of foods, 4th ed. American Public Health Association, Washington, DC.
- 7.Cook, D. W., J. D. Bowers, and A. DePaola. 2002. Density of total and pathogenic (tdh+) Vibrio parahaemolyticus in Atlantic and Gulf Coast molluscan shellfish at harvest. J. Food Prot. 65:1873-1880. [DOI] [PubMed] [Google Scholar]
- 8.Daniels, N. A., L. MacKinnon, R. Bishop, S. Altekruse, B. Ray, R. M. Hammond, S. Thompson, S. Wilson, N. H. Bean, P. M. Griffin, and L. Slutsker. 2000. Vibrio parahaemolyticus infections in the United States, 1973-1998. J. Infect. Dis. 181:1661-1666. [DOI] [PubMed] [Google Scholar]
- 9.Deepanjali, A., H. S. Kumar, I. Karunasagar, and I. Karunasagar. 2005. Seasonal variation in abundance of total and pathogenic Vibrio parahaemolyticus bacteria in oysters along the southwest coast of India. Appl. Environ. Microbiol. 71:3575-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DePaola, A., L. H. Hopkins, J. T. Peeler, B. Wentz, and R. M. McPhearson. 1990. Incidence of Vibrio parahaemolyticus in U.S. coastal waters and oysters. Appl. Environ. Microbiol. 56:2299-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DePaola, A., J. L. Nordstrom, J. C. Bowers, J. G. Wells, and D. W. Cook. 2003. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl. Environ. Microbiol. 69:1521-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DePaola, A., and C. A. Kaysner. 2004. Vibrio, chapter 9. In Food and Drug Administration (ed.), Bacteriological analytical manual, 8th ed., revision A, 1998. U.S. Food and Drug Administration, Washington, DC. http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/default.htm.
- 13.Drake, S. L., A. DePaola, and L. A. Jaykus. 2007. An overview of Vibrio vulnificus and Vibrio parahaemolyticus. Compr. Rev. Food Sci. Food Safety 6:120-144. [Google Scholar]
- 14.Fuenzalida, L., C. Hernandez, J. Toro, M. L. Rioseco, J. Romero, and R. T. Espejo. 2006. Vibrio parahaemolyticus in shellfish and clinical samples during two large epidemics of diarrhoea in southern Chile. Environ. Microbiol. 8:675-683. [DOI] [PubMed] [Google Scholar]
- 15.Gil, A. I., H. Miranda, C. F. Lanata, A. Prada, E. R. Hall, C. M. Barreno, S. Busrin, N. A. Bhuiyan, D. A. Sack, and G. B. Nair. 2007. O3:K6 serotype of Vibrio parahaemolyticus identical to the global pandemic clone associated with diarrhea in Peru. Int. J. Infect. Dis. 11:324-328. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Escalona, N., V. Cachicas, C. Acevedo, M. L. Rioseco, J. A. Vergara, F. Cabello, J. Romero, and R. T. Espejo. 2005. Vibrio parahaemolyticus diarrhea, Chile, 1998 and 2004. Emerg. Infect. Dis. 11:129-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara-Kudo, Y., K. Sugiyama, M. Nishibuchi, A. Chowdhury, J. Yatsuyanagi, Y. Ohtomo, A. Saito, H. Nagano, T. Nishina, H. Nakagawa, H. Konuma, M. Miyahara, and S. Kumagai. 2003. Prevalence of pandemic thermostable direct hemolysin-producing Vibrio parahaemolyticus O3:K6 in seafood and the coastal environment in Japan. Appl. Environ. Microbiol. 69:3883-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harth, E., L. Matsuda, C. Hernández, M. L. Rioseco, J. Romero, N. González-Escalon, J. Martinez-Urtaza, and R. T. Espejo. 2009. Epidemiology of Vibrio parahaemolyticus outbreaks, southern Chile. Emerg. Infect. Dis. 15:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leal, N. C., S. C. da Silva, V. O. Cavalcanti, Â. C. T. Figueiroa, V. V. F. Nunes, I. S. Miralles, and E. Hofer. 2008. Vibrio parahaemolyticus serovar O3:K6 gastroenteritis in northeast Brazil. J. Appl. Microbiol. 105:691-697. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Urtaza, J., B. Huapava, R. G. Gavilan, V. Blanco-Abad, J. Ansede-Bermejo, C. Cadarso-Suarez, A. Figueiras, and J. Trinanes. 2008. Emergence of Asiatic vibrio diseases in South America in phase with El Niño. Epidemiology 19:829-837. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Urtaza, J., A. Lozano-Leon, J. Varela-Pet, J. Trinanes, Y. Pazos, and O. Garcia-Martin. 2008. Environmental determinants of the occurrence and distribution of Vibrio parahaemolyticus in the Rias of Galicia, Spain. Appl. Environ. Microbiol. 74:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Urtaza, J., L. Simental, D. Velasco, A. DePaola, M. Ishibashi, Y. Nakaguchi, M. Nishibuchi, D. Carrera-Flores, C. Rey-Alvarez, and A. Pousa. 2005. Pandemic Vibrio parahaemolyticus O3:K6, Europe. Emerg. Infect. Dis. 11:1319-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matté, G. R., M. H. Matté, I. G. Rivera, and M. T. Martins. 1994. Distribution of potentially pathogenic vibrios in oysters from a tropical region. J. Food Prot. 57:870-873. [DOI] [PubMed] [Google Scholar]
- 24.Olea, A. M., C. Gonzalez, M. Chiu, C. Vallebuona, M. Labraña, and F. Martiniello. 2005. Brote de gastroenteritis por Vibrio parahaemolyticus en Chile. Rev. Chilena Salud Pública 9:51-53. [Google Scholar]
- 25.Oliver, J. D., and J. B. Kaper. 2001. Vibrio species, p. 263-300. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers, 2nd ed. ASM Press, Washington, DC.
- 26.Pan, T. M., T. K. Wang, C. L. Lee, S. W. Chien, and C. B. Horng. 1997. Food-borne disease outbreaks due to bacteria in Taiwan, 1986 to 1995. J. Clin. Microbiol. 35:1260-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips, A. M. B., A. DePaola, J. Bowers, S. Ladner, and D. J. Grimes. 2007. An evaluation of the use of remotely sensed parameters for prediction of incidence and risk associated with Vibrio parahaemolyticus in Gulf Coast oysters (Crassostrea virginica). J. Food Prot. 70:879-884. [DOI] [PubMed] [Google Scholar]
- 28.Ristori, C. A., S. T. Iaria, D. S. Gelli, and I. N. G. Rivera. 2007. Pathogenic bacteria associated with oysters (Crassostrea brasiliana) and estuarine water along the south coast of Brazil. Int. J. Environ. Health Res. 17:259-269. [DOI] [PubMed] [Google Scholar]
- 29.Su, Y.-C., and C. Liu. 2007. Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol. 24:549-558. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman, A. M., A. DePaola, J. C. Bowers, J. A. Krantz, J. L. Nordstrom, C. N. Johnson, and D. J. Grimes. 2007. Variability of total and pathogenic Vibrio parahaemolyticus densities in northern Gulf of Mexico water and oysters. Appl. Environ. Microbiol. 73:7589-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]