Abstract
Neurotoxic paralytic shellfish poisoning (PSP) toxins, anatoxin-a (ATX), and hepatotoxic cylindrospermopsin (CYN) have been detected in several lakes in northeast Germany during the last 2 decades. They are produced worldwide by members of the nostocalean genera Anabaena, Cylindrospermopsis, and Aphanizomenon. Although no additional sources of PSP toxins and ATX have been identified in German water bodies to date, the observed CYN concentrations cannot be produced solely by Aphanizomenon flos-aquae, the only known CYN producer in Germany. Therefore, we attempted to identify PSP toxin, ATX, and CYN producers by isolating and characterizing 92 Anabaena, Aphanizomenon, and Anabaenopsis strains from five lakes in northeast Germany. In a polyphasic approach, all strains were morphologically and phylogenetically classified and then tested for PSP toxins, ATX, and CYN by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and enzyme-linked immunosorbent assay (ELISA) and screened for the presence of PSP toxin- and CYN-encoding gene fragments. As demonstrated by ELISA and LC-MS, 14 Aphanizomenon gracile strains from Lakes Melang and Scharmützel produced four PSP toxin variants (gonyautoxin 5 [GTX5], decarbamoylsaxitoxin [dcSTX], saxitoxin [STX], and neosaxitoxin [NEO]). GTX5 was the most prevalent PSP toxin variant among the seven strains from Lake Scharmützel, and NEO was the most prevalent among the seven strains from Lake Melang. The sxtA gene, which is part of the saxitoxin gene cluster, was found in the 14 PSP toxin-producing A. gracile strains and in 11 non-PSP toxin-producing Aphanizomenon issatschenkoi, A. flos-aquae, Anabaena planktonica, and Anabaenopsis elenkinii strains. ATX and CYN were not detected in any of the isolated strains. This study is the first confirming the role of A. gracile as a PSP toxin producer in German water bodies.
Neurotoxic saxitoxins, also known as paralytic shellfish poisoning (PSP) toxins, as well as neurotoxic anatoxin-a (ATX) and hepatotoxic cylindrospermopsin (CYN), have been detected in several northeast German lakes in the last 2 decades (3, 35). In a survey conducted in 1995 and 1996, ATX was present in 26% of 78 German lakes and PSP toxins were present in 34% of 29 lakes (3). In 2004, a qualitative survey showed that CYN was present in 50% of 127 German lakes investigated (8). Aphanizomenon flos-aquae Ralfs ex Born. et Flah. has been identified as producer of CYN in these lakes (33), but sources of PSP toxins and ATX have yet to be identified in German water bodies.
PSP toxins are potent neurotoxic alkaloids produced by marine dinoflagellates and filamentous freshwater cyanobacteria (1, 2, 42). The 21 currently known PSP toxin variants belong to four groups: carbamoyl toxins, decarbamoyl toxins, N-sulfocarbamoyl toxins, and deoxydecarbamoyl toxins (15). Carbamoyl toxins are the most potent PSP toxins, including saxitoxin (STX) and neosaxitoxin (NEO), while deoxydecarbamoyl toxins comprise the least potent PSP toxins (38). PSP toxins block neural sodium ion channels, leading to death through respiratory failure (1).
Cyanobacteria belonging to the orders Oscillatoriales and Nostocales, including members of the genera Cylindrospermopsis, Anabaena, and Aphanizomenon, have been identified as PSP toxin producers in freshwater habitats (4). Aphanizomenon gracile Lemmermann and Aphanizomenon flos-aquae strains from China, Portugal, and the United States have been described as PSP toxin producers (9, 23, 31). Both species are abundant members of the Nostocales and are widely distributed in phytoplankton communities in oligotrophic, mesotrophic, and eutrophic water bodies throughout northeast Germany (35).
Regarding saxitoxins, Cylindrospermopsis raciborskii (Woloszyńska) Seenayya et Subba Raju strain T3 was recently found to contain a new candidate saxitoxin gene cluster containing around 35 kb of DNA and comprising more than 26 genes (16). This saxitoxin gene cluster was also found in Anabaena circinalis Rabenhorst ex Bornet & Flahault strains from Australia, in Aphanizomenon sp. strain NH5, and in Lyngbya wollei (Farlow ex Gomont) comb. nov. (16).
Anatoxin-a, a neurotoxic bicyclic alkaloid, has been detected in freshwater bodies worldwide (4). Anatoxin-a production has been found in Anabaena, Aphanizomenon, Cylindrospermum, Oscillatoria sp., and Phormidium strains (4). Anatoxin-a is a potent agonist for the nicotinic acetylcholine receptor. Its toxic effects include muscle fasciculation, gasping, convulsions, and death by respiratory arrest in vertebrates (2).
Cylindrospermopsin is a potent alkaloid hepatotoxin produced by planktonic cyanobacteria of the order Nostocales. It was first detected in Australian Cylindrospermopsis raciborskii strains (12) and is additionally produced by Anabaena bergii Ostenfeld (36), Umezakia natans M. Watanabe (11), Aphanizomenon ovalisporum (Forti) (37), and A. flos-aquae (33). CYN results in liver, kidney, intestinal, and lung damage (13) and inhibits protein synthesis (40).
Overall knowledge of the cyanobacterial sources of PSP toxins, ATX, and CYN is scarce. To identify the producers of such toxins, we isolated and investigated 92 Aphanizomenon, Anabaena, and Anabaenopsis strains from five northeast German water bodies dominated by cyanobacteria of the order Nostocales. All strains were morphologically and phylogenetically classified and screened for the presence of toxin-encoding genes and for the ability to produce cyanobacterial toxins using a polyphasic approach including enzyme-linked immunosorbent assay (ELISA) and liquid chromatography with tandem mass spectrometry (LC-MS/MS).
MATERIALS AND METHODS
Isolation of strains and morphological characterization.
Ninety-two Aphanizomenon, Anabaena, and Anabaenopsis strains were isolated from five lakes in northeast Germany (Table 1). The isolated filaments were washed 5 times and placed in microtiter plates containing 300 μl Z8 medium (20). After successful growth, they were placed in 50-ml flasks containing 20 ml Z8 medium. All strains investigated in this study were maintained at 22°C and a photon flux density of 80 μmol of photons m−2s−1. Strains were classified based on morphological traits according to Komárek (17), Komárková-Legnerová and Eloranta (19), and Komárek and Komárkova (18). Morphological studies were conducted using an Olympus BX51 light microscope and color view imaging system (Olympus, Germany).
TABLE 1.
Strains of the order Nostocales isolated from different German lakes
| Species | No. of strains | Lake |
|---|---|---|
| Aphanizomenon gracile | 34 | Langer, Scharmützel, Melang |
| Aphanizomenon flos-aquae | 18 | Stechlin, Scharmützel |
| Aphanizomenon issatschenkoi | 6 | Langer |
| Anabaena crassa | 1 | Langer |
| Anabaena flos-aquae | 2 | Langer, Scharmützel |
| Anabaena lemmermanii | 3 | Nehmitz |
| Anabaena planktonica | 22 | Langer, Scharmützel, Stechlin |
| Anabaenopsis elenkinii | 2 | Langer |
| Anabaena bergii | 3 | Melang |
| Anabaena sp. | 1 | Stechlin |
Genomic DNA extraction/PCR amplification and sequencing.
Fresh culture material of all cyanobacterial strains was frozen and thawed three times and then boiled for 5 min and subsequently centrifuged for 5 min (13,000 rpm or ∼16,000 × g). The supernatants were discarded. Each pellet was resuspended in 100 μl distilled water and vortexed for 1 min. Genomic DNA was extracted using the MoleStrips DNA blood kit and the DNA-Cyano protocol on GeneMole (Mole Genetics, Lysaker, Norway) according to the manufacturer's instructions.
All PCRs were performed on a Peltier thermal cycler PTC 200 (MJ Research, Inc., San Francisco, CA) using the Taq PCR core kit (Qiagen GmbH, Hilden, Germany). The reaction mixture contained 0.1 μl Taq DNA polymerase (5 U/μl), 0.5 μl deoxynucleoside triphosphate mix (10 mM), 2 μl Qiagen PCR buffer, 1 μl each forward and reverse primer (10 μM), and 1 μl genomic DNA, yielding a total volume of 20 μl. The primers PCβf and PCαr were used to amplify the intergenic spacer and flanking regions of the cpcB and cpcA genes of the phycocyanin operon (PC-IGS) (27). PCR was also used to check whether the isolated strains were potential producers of either CYN or PSP toxins. The peptide synthetase (PS) gene of the cylindrospermopsin gene cluster was detected according to the PRC method of Schembri et al. (36) using M13 and M14 as primers. To simplify and accelerate detection of the saxitoxin gene cluster, we designed two primers, sxtaf (GCGTACATCCAAGCTGGACTCG) and sxtar (GTAGTCCAGCTAAGGCACTTGC), which amplify a part of the sxtA gene of the saxitoxin gene cluster. The sxtA gene encodes a polyketide synthase (PKS)-like structure (16).
The cycling protocol for the PC-IGS fragment was one cycle of 5 min at 94°C and then 30 cycles of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C with a final elongation step of 72°C for 10 min. The protocol for sxtA gene fragments was one cycle of 4 min at 94°C and then 30 cycles of 10 s at 94°C, 20 s at 55°C, and 1 min at 72°C. PCR products were visualized by 1.5% agarose gel electrophoresis with ethidium bromide staining and UV illumination.
Amplified PC-IGS and sxtA products were purified through Qiaquick PCR purification columns (Qiagen, Hilden, Germany), and the DNA was eluted in elution buffer according to the manufacturer's protocol. Sequencing of the purified PC-IGS and sxtA products was performed using the same primers as for PCR. For each PCR product, both strands were sequenced on an ABI 3100 Avant genetic analyzer using the BigDye terminator V.3.1 cycle sequencing kit (Applied Biosystems, Applera Deutschland GmbH, Darmstadt, Germany) according to the manufacturer's instructions.
Phylogenetic analysis.
Sequences of the PC-IGS locus in all Anabaena, Aphanizomenon, and Anabaenopsis strains were analyzed using Seqassem version 04/2008 (14). The Align (version 03/2007) MS Windows-based manual sequence alignment editor (14) was used to obtain DNA sequence alignments, which were then corrected manually. Segments with highly variable and ambiguous regions and gaps making proper alignment impossible were excluded from the analyses. A PC-IGS set containing 544 positions was used. Nostocaceae Cyanobiont (AY181211) was employed as the outgroup in the PC-IGS tree. Sixteen additional Aphanizomenon and Anabaena sequences derived from GenBank were included in the PC-IGS analyses. A set containing 555 positions was used for sxtA analysis.
Phylogenetic trees for PC-IGS and sxtA were constructed using the maximum likelihood (ML) algorithm in PAUP* v.10b (39). In the ML analyses, evolutionary substitution models were evaluated using the AIC criterion in Modeltest v.3.06 (32). GTR+I+G was found to be the best-fitting evolutionary model for the PC-IGS gene. The K81uf evolutionary model was used for sxtA. Due to limited computer capacity, ML analyses of all trees were performed with 100 bootstrap replicates using PAUP* v.10b (39).
Cyanotoxin analysis. (i) ELISA.
All 92 Aphanizomenon, Anabaena, and Anabaenopsis strains were tested for PSP toxins and CYN by using the Abraxis saxitoxin ELISA and Abraxis cylindrospermopsin ELISA kits (Abraxis LLC, Warminister, PA) following the manufacturer's instructions. The test is an indirect competitive ELISA designed to detect saxitoxin or cylindrospermopsin based on specific antibody recognition. Before analysis, 5 ml of culture material from each cyanobacterial strain was frozen and thawed three times to extract the toxins. The ELISA results do not distinguish between dissolved and cell-bound toxins. The color reaction of the ELISA test was evaluated at 450 nm using a Biotek Synergi 2 microtiter plate reader (Biotek, Bad Friedrichshall, Germany).
(ii) LC-MS/MS.
Between 10 and 20 ml of culture material of each strain was filtered through 0.45-μm-pore RC 55 membrane filters (Whatman, Dassel, Germany). Filters were extracted twice with 1.5 ml of acetonitrile-water-formic acid (75:14.9:0.1) at room temperature. Each extraction step included 10 min ultrasonication followed by shaking for 1 h and centrifugation. The supernatants were combined and dried by vacuum centrifugation.
LC-MS/MS sample analyses were carried out on an Agilent 1100 series high-pressure liquid chromatography (HPLC) system (Agilent Technologies, Waldbronn, Germany) coupled to a API 4000 triple-quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Framingham, MA) equipped with a turbo ion spray interface. The extracts were separated using a 5-μm, 2- by 250-mm TSKgel Amide-80 column (Tosohaas, PA) at 30°C. The mobile phase consisted of water (A) and acetonitrile-water (95:5) (B), both containing 2.0 mM ammonium formate and 3.6 mM formic acid (pH 3.5); the flow rate was 0.2 ml min−1. The following gradient program was used for analysis of multiple toxins (cylindrospermopsin, anatoxin-a, and paralytic shellfish poisons): 75% B for 5 min, 75% to 65% B over 1 min, hold for 13 min, 65 to 45% B over 4 min, and hold for 10 min. (5). The injection volume was 10 μl.
The mass spectrometer was operated in the selected reaction monitoring mode for detection and quantification of the following toxins as described by Dell'Aversano et al. (5): cylindrospermopsin (CYN); anatoxin-a (ATX); saxitoxin (STX); neosaxitoxin (NEO); decarbamoylsaxitoxin (dcSTX); decarbamoylneosaxitoxin (dcNEO); gonyautoxins 1 (GTX1), 2, 3, 4, and 5; decarbamoylgonyautoxin 3 (dcGTX-3); and N-sulfogonyautoxins 1 (C1) and 2. Standard curves were established for all the toxins. CYN and PSP toxin standards obtained from the National Research Council (Halifax, Canada) and anatoxin-a standards from Tocris (United Kingdom) were analyzed in line with the unknowns (one calibration curve after 10 unknowns).
Nucleotide sequence accession numbers.
The sequence data were submitted to the EMBL Nucleotide Sequence Database under the accession numbers listed in Table 2 (see also Table S1 in the supplemental material).
TABLE 2.
Aphanizomenon, Anabaena and Anabaenopsis strains from different freshwater lakes evaluated in this study by ELISA, LC-MS/MS, and genetic properties
| Species and strain | Lake | Detection ofa: |
Accession no. |
|||||
|---|---|---|---|---|---|---|---|---|
| CYN |
PSP toxins |
ATX (LC-MS/MS) | sxtA | PC-IGS | ||||
| ELISA | LC-MS/MS | ELISA | LC-MS/MS | |||||
| Aphanizomenon | ||||||||
| A. gracile | ||||||||
| AB200816 | Scharmützel | − | − | + | + | − | FN552384 | FN552296 |
| AB2008/18 | Scharmützel | − | − | + | + | − | FN552385 | FN552297 |
| AB2008/19 | Scharmützel | − | − | + | + | − | FN552386 | FN552298 |
| AB2008/21 | Scharmützel | − | − | + | + | − | FN552387 | FN552299 |
| AB2008/23 | Scharmützel | − | − | + | + | − | FN552388 | FN552300 |
| AB2008/29 | Scharmützel | − | − | + | + | − | FN552389 | FN552304 |
| AB2008/31 | Scharmützel | − | − | + | + | − | FN552390 | FN552305 |
| AB2008/47 | Melang | − | − | + | + | − | FN552391 | FN552312 |
| AB2008/48 | Melang | − | − | + | + | − | FN552392 | FN552313 |
| AB2008/49 | Melang | − | − | + | + | − | FN552393 | FN552314 |
| AB2008/50 | Melang | − | − | + | + | − | FN552394 | FN552315 |
| AB2008/51 | Melang | − | − | + | + | − | FN552395 | FN552316 |
| AB2008/59 | Melang | − | − | + | + | − | FN552396 | FN552318 |
| AB2008/65 | Melang | − | − | + | + | − | FN552397 | FN552320 |
| A. flos-aquae | ||||||||
| AB2008/63 | Stechlin | − | − | − | − | − | FN552398 | FN552366 |
| ST122 | Stechlin | − | − | − | − | − | FN552399 | FN552361 |
| ST128 | Stechlin | − | − | − | − | − | FN552400 | FN552362 |
| ST130 | Stechlin | − | − | − | − | − | FN552401 | FN552363 |
| A. issatschenkoi | ||||||||
| AB2008/08 | Langer | − | − | − | − | − | FN552405 | FN552374 |
| AB2008/09 | Langer | − | − | − | − | − | FN552406 | FN552375 |
| AB2008/11 | Langer | − | − | − | − | − | FN552407 | FN552376 |
| Anabaena planktonica | ||||||||
| ST16 | Stechlin | − | − | − | − | − | FN552402 | FN552326 |
| ST182 | Stechlin | − | − | − | − | − | FN552403 | FN552328 |
| ST195 | Stechlin | − | − | − | − | − | FN552404 | FN552331 |
| Anabaenopsis elenkinii | ||||||||
| AB2008/61 | Langer | − | − | − | − | − | FN552408 | FN552382 |
+, detected; −, not detected.
RESULTS
Identification of toxin producers.
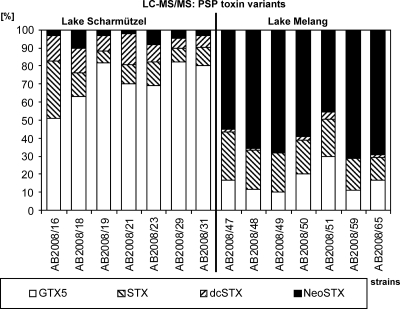
As determined by ELISA and LC-MS/MS, 14 A. gracile strains from Lakes Melang and Scharmützel were able to produce PSP toxins (Table 2). The other 78 Anabaena, Aphanizomenon, and Anabaenopsis strains tested negative for PSP toxins by ELISA and LC-MS/MS (Table 2; see Table S1 in the supplemental material). LC-MS/MS revealed that each of the 14 A. gracile strains detected in Lakes Scharmützel and Melang produced four PSP toxin variants—gonyautoxin 5 (GTX5), saxitoxin (STX), decarbamoyl-saxitoxin (dcSTX), and neosaxitoxin (NEO)—but at different ratios (Fig. 1). Of the 7 strains from Lake Scharmützel, GTX5 was the most prevalent PSP toxin variant (50.8 to 82.3%), while NEO was the most prevalent PSP toxin variant (45.6 to 70.5%) of the 7 strains from Lake Melang (Fig. 1). The GTX5 fraction was only 11.4 to 29.8% in Lake Melang strains, and the NEO fraction ranged from 2.3 to 10.3% in Lake Scharmützel strains. STX and dcSTX were found in all 14 strains, but at lower ratios of 7.4 to 31.9% and 0.7 to 14.3%, respectively (Fig. 1). According to ELISA and LC-MS/MS, all 92 Anabaena and Aphanizomenon strains tested negative for CYN and ATX.
FIG. 1.
Ratio (%) of PSP toxin variants in 14 A. gracile strains from Lakes Scharmützel and Melang, as determined with LC-MS/MS.
Amplification of sxtA and the PS gene.
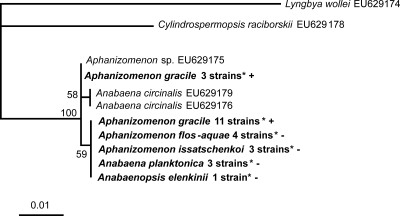
All 92 Aphanizomenon, Anabaena, and Anabaenopsis strains were investigated for the presence of the saxitoxin gene cluster. Amplification of the sxtA gene was observed in all 14 PSP toxin-producing A. gracile strains and in certain non-PSP toxin-producing strains of A. flos-aquae (n = 4), A. planktonica (n = 3), A. issatschenkoi (n = 3), and A. elenkinii (n = 1) from Lakes Melang and Scharmützel. All sxtA sequences were aligned with sequences taken from GenBank (NCBI), and a phylogenetic tree was constructed to demonstrate the similarity of our sequences to sxtA sequences from known PSP toxin-producing strains (Fig. 2). No amplification of the sxtA gene was detected in the other 67 Aphanizomenon, Anabaena, and Anabaenopsis strains. None of the 92 strains exhibited amplification of the PS gene of the CYN gene cluster.
FIG. 2.
Maximum likelihood tree based on partial sxtA sequences of 30 cyanobacterial strains. Strains from this study are marked in bold. Bootstrap values above 50 are included. The bar indicates 1% sequence divergence. +, PSP toxin producer; −, PSP toxin not detected; *, accession number listed in Table 2.
Morphological and phylogenetic characterization.
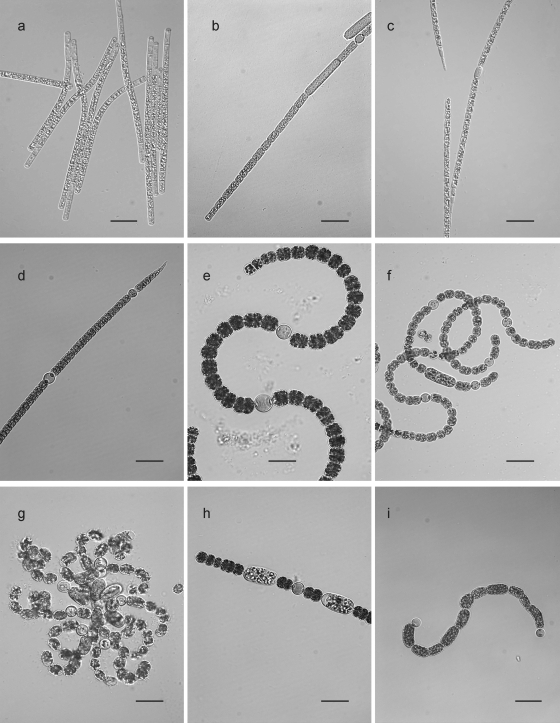
Based on their morphological features, the isolated strains were identified as A. gracile, A. flos-aquae, A. issatschenkoi, A. bergii, Anabaena crassa (Lemm.) Kom.-Legn. et Cronb., Anabaena flos-aquae Breb. ex Born. et Flah., Anabaena lemmermanii Richt., Anabaena planktonica Brunnthaler, and Anabaenopsis elenkinii Miller (Fig. 3).
FIG. 3.
Micrographs of Nostocales strains investigated in this study. (a) Aphanizomenon flos-aquae; (b) Aphanizomenon gracile; (c) Aphanizomenon issatschenkoi; (d) Anabaena bergii; (e) Anabaena crassa; (f) Anabaena flos-aquae; (g) Anabaena lemmermanii; (h) Anabaena planktonica; and (i) Anabaenopsis elenkinii. Scale bars indicate 25 μm.
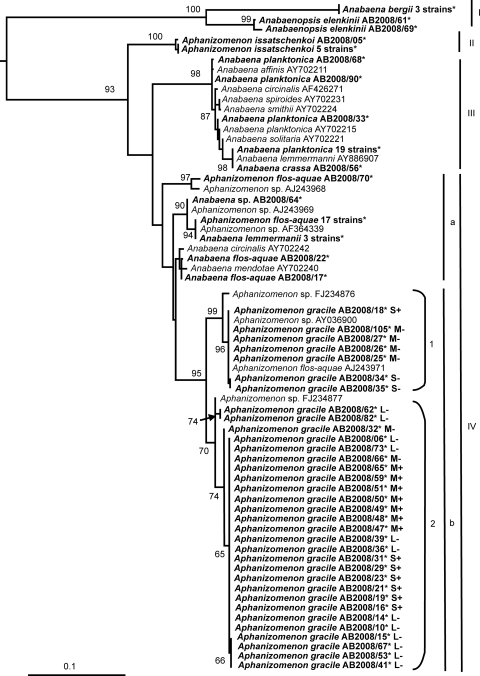
Phylogenetic relationships of the investigated strains are presented in the maximum likelihood tree of the PC-IGS region (Fig. 4). Four main clusters were found. Cluster I contained A. bergii and Anabaenopsis elenkinii strains, and cluster II contained A. issatschenkoi strains. Anabaena spp. were grouped together in cluster III. Cluster IV contained sequences of Anabaena spp. and Aphanizomenon spp. (Fig. 4). Subcluster IVa included Anabaena spp. and A. flos-aquae strains, and subcluster IVb contained A. gracile and A. flos-aquae strains but no Anabaena sequences. The 34 A. gracile strains investigated were distributed in subclusters 1 and 2 of subcluster IVb. Subcluster IVb1 comprised seven A. gracile strains from Lakes Melang and Scharmützel, including one PSP toxin-producing strain from Lake Scharmützel. Sequences of A. flos-aquae and Aphanizomenon obtained from GenBank were also located here. Subcluster IVb2 comprised one Aphanizomenon sp. sequence obtained from GenBank and 27 A. gracile strains from Lakes Melang, Langer, and Scharmützel, including 13 PSP toxin-producing strains from Lakes Melang and Scharmützel.
FIG. 4.
Maximum likelihood tree based on partial PC-IGS sequences of 107 cyanobacterial strains. Strains from this study are marked in bold. Bootstrap values above 50 are included. The scale bar indicates 10% sequence divergence. L, Lake Langer; M, Lake Melang; S, Lake Scharmützel. +, PSP toxin producer; −, PSP toxin not detected; *, accession number listed in Table 2 (and see Table S1 in the supplemental material).
DISCUSSION
This study demonstrated that 14 A. gracile strains from two northeast German lakes (Melang and Scharmützel) are PSP toxin producers. This is the first evidence implicating A. gracile as a PSP toxin producer in German water bodies. The only previously known PSP toxin-producing A. gracile strain was isolated from Lake Crato in Portugal (31). PSP toxin production has also been detected in several A. flos-aquae strains (25, 30). However, after morphological and genetic reevaluation, two PSP toxin-producing A. flos-aquae strains (NH5 and LMECYA 31) were reclassified as Aphanizomenon sp. and A. issatschenkoi, respectively (21, 22, 25). PSP toxin-producing A. flos-aquae strains have also been isolated from a Chinese lake (Dianchi) and a Portuguese reservoir (Crestuma-Lever) (9, 23). In both cases, classification as A. flos-aquae is not unequivocal because of the lack of morphological descriptions. The 18 A. flos-aquae strains investigated in our study were unable to produce PSP toxins.
LC-MS/MS confirmed the presence of the same four PSP toxin variants (STX, NEO, GTX5, and dcSTX) in all 14 PSP toxin-producing A. gracile strains. However, the strains from Lake Melang mainly produced NEO, one of the most toxic PSP toxin variants (15, 38). Mouse bioassays have shown that this toxin is 14 times more toxic than GTX5, the variant mainly produced in the strains from Lake Scharmützel (15, 38).
The same four PSP toxin variants were found by Dias et al. (6) in A. issatschenkoi strain LMECYA 31. At 22°C, which corresponded to our culture conditions, Dias et al. (6) observed predominant production of GTX5 in this strain. The Portuguese A. gracile strain LMECYA 40 only produced two PSP toxin variants, STX and NEO, and A. flos-aquae strains isolated from Chinese Lake Dianchi produced three PSP toxin variants, STX, NEO, and GTX5 (23, 31). Australian Anabaena circinalis and American Lyngbya wollei strains exhibited a higher number (up to 9) of PSP toxin variants (29, 41).
Amplification of parts of the sxtA gene in our 14 PSP toxin-producing Aphanizomenon gracile strains confirmed the presence of a saxitoxin gene cluster in these strains. Interestingly, we also found the sxtA gene in 11 non-PSP toxin-producing A. issatschenkoi, A. flos-aquae, A. elenkinii, and A. planktonica strains. According to Li et al. (22) and Liu et al. (23), strains of A. issatschenkoi and A. flos-aquae have been confirmed as PSP toxin producers.
The sxtA gene encodes a polyketide synthase (PKS)-like structure (16). PKS is involved in the synthesis of secondary metabolites like microcystin, cylindrospermopsin, and saxitoxin (16, 28, 36). Moustafa et al. (26) demonstrated that assembly of the saxitoxin gene cluster in the cyanobacterium A. circinalis involved multiple horizontal gene transfers from different bacterial and cyanobacterial sources. The same authors hypothesize that various former PSP toxin-producing A. circinalis strains most likely lost the ability to produce PSP toxins over time, leading to a coexistence of PSP toxin-producing and non-PSP toxin-producing strains (26). This mechanism implies that the non-PSP toxin-producing A. gracile strains in our study have lost all or part of the saxitoxin gene cluster.
Previous 16S rRNA gene, internal transcribed spacer 1 (ITS1), and rbcLX sequence studies by Gugger et al. (10), Lyra et al. (24), and Rajaniemi et al. (34) have demonstrated that planktonic Aphanizomenon spp. and Anabaena spp. are genetically heterogeneous and form intermixed clusters in phylogenetic trees. Our phylogenetic study using PC-IGS sequences supports these findings in the case of Anabaena spp. and A. flos-aquae. Clusters III and IVa of our phylogenetic tree comprise Anabaena and A. flos-aquae sequences. In cluster IVb, our A. gracile strains are clearly separated from the Anabaena spp. and Aphanizomenon flos-aquae strains. Considering that A. flos aquae CCAP 1401 (accession no. AJ243971), which is included in cluster IVb, was renamed A. gracile in the Czech Culture Collection of Autotrophic Organisms (CCALA), morphological classification of our strains as A. gracile seems well supported.
Our PSP toxin-producing and non-PSP toxin-producing A. gracile strains could not be distinguished using the phylogenetic tree of PC-IGS. In cluster IVb, toxic strains are grouped together with nontoxic strains. Although toxic strains from Lakes Melang and Scharmützel were characterized by different ratios of GTX5 and NEO, no phylogenetically similar pattern was found in the PC-IGS tree.
Identification of the PSP toxin-producing A. gracile strains isolated from Lake Melang explains the presence of paralytic shellfish poisoning toxins there (3). However, PSP toxin-producing A. gracile strains were also detected in Lake Scharmützel, although no PSP toxins were detected there in a survey conducted in 1995 to 1997 (3). According to Rücker et al. (35), A. gracile biovolumes in Lakes Melang and Langer are much higher than those in Lake Scharmützel. When the proportion of PSP toxin-producing A. gracile in the phytoplankton community is as low as that in Lake Scharmützel, the PSP toxin concentration in water samples is probably below the detection limit. Although paralytic shellfish poisoning toxins were detected in Lake Langer in 2007 (J. Fastner, unpublished data), we did not find any PSP toxin-producing cyanobacterial strains there. Therefore, an extensive survey would be needed in order to detect PSP toxin-producing cyanobacteria in Lake Langer.
The same applies to ATX and CYN. In our study, PCR, ELISA, and LC-MS/MS did not reveal the presence of ATX or CYN producers among the investigated strains, although ATX and CYN had been detected in Lakes Langer and Melang in the 1990s (3, 7). ATX producers have yet to be detected in German water bodies. The only known CYN producer in northeast German water bodies is A. flos-aquae (33). Because A. gracile exhibited the highest correlation coefficients between biovolume and CYN concentration in a broad range of German water bodies, it is viewed as a prime candidate (35, 43).
In conclusion, this is the first study identifying A. gracile as a PSP toxin producer in German water bodies. As non-PSP toxin-producing A. issatschenkoi, A. flos-aquae, A. planktonica, and Anabaenopsis sp. strains also possessed the sxtA gene of the saxitoxin gene cluster, it is very likely that other nostocalean species in German water bodies can also produce paralytic shellfish poisoning toxins.
Supplementary Material
Acknowledgments
We thank Monika Degebrodt and Marén Lentz for excellent technical assistance. The help of Gabriele Gericke and Christa Woodgett with toxin analyses is also greatly appreciated.
This study was funded by the German Ministry of Education and Research (BMBF, 0330792A) and Kompetenzzentrum Wasser Berlin with financial support from Veolia Water and Berliner Wasserbetriebe.
Footnotes
Published ahead of print on 4 January 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Carmichael, W. W. 1988. Toxins of freshwater algae, p. 121-147. In A. T. Tu (ed.), Handbook of natural toxins. Volume 3. Marine toxins and venoms. Marcel Dekker, Inc., New York, NY.
- 2.Carmichael, W. W. 1992. Cyanobacteria and secondary metabolites—the cyanotoxins. J. Appl. Bacteriol. 72:445-459. [DOI] [PubMed] [Google Scholar]
- 3.Chorus, I. 2001. Cyanotoxins, occurrence, causes, consequences. Springer, Berlin, Germany.
- 4.Codd, G. A., S. G. Bell, K. Kaya, C. J. Ward, K. A. Beattie, and J. S. Metcalf. 1999. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 34:405-415. [Google Scholar]
- 5.Dell'Aversano, C., G. K. Eaglesham, and M. A. Quilliam. 2004. Analysis of cyanobacterial toxins by hydrophilic interaction liquid chromatography-mass spectrometry. J. Chromatogr. A 1028:155-164. [DOI] [PubMed] [Google Scholar]
- 6.Dias, E., P. Pereira, and S. Franca. 2002. Production of paralytic shellfish toxins by Aphanizomenon sp. LMECYA 31 (cyanobacteria). J. Phycol. 38:705-712. [Google Scholar]
- 7.Fastner, J., R. Heinze, A. R. Humpage, U. Mischke, G. K. Eaglesham, and I. Chorus. 2003. Cylindrospermopsin occurrence in two German lakes and preliminary assessment of toxicity and toxin production of Cylindrospermopsis raciborskii (Cyanobacteria) isolates. Toxicon 42:313-321. [DOI] [PubMed] [Google Scholar]
- 8.Fastner, J., J. Rücker, A. Stüken, K. Preußel, B. Nixdorf, I. Chorus, A. Köhler, and C. Wiedner. 2007. Occurrence of the cyanobacterial toxin cylindrospermopsin in Northeast Germany. Environ. Toxicol. 22:26-32. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira, F. M. B., J. M. F. Soler, M. L. Fidalgoa, and P. Fernández-Vila. 2001. PSP toxins from Aphanizomenon flos-aquae (cyanobacteria) collected in the Crestuma-Lever reservoir (Douro River, northern Portugal). Toxicon 39:757-761. [DOI] [PubMed] [Google Scholar]
- 10.Gugger, M., C. Lyra, P. Henriksen, A. Couté, J.-F. Humbert, and K. Sivonen. 2002. Phylogenetic comparison of the cyanobacterial genera Anabaena and Aphanizomenon. Int. J. Syst. Evol. Microbiol. 52:1867-1880. [DOI] [PubMed] [Google Scholar]
- 11.Harada, K., I. Ohtani, K. Iwamoto, M. Suzuki, M. F. Watanabe, M. Watanabe, and K. Terao. 1994. Isolation of cylindrospermopsin from a cyanobacterium Umezakia natans and its screening method. Toxicon 32:73-84. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins, P. R., M. T. C. Runnegar, A. R. B. Jackson, and I. R. Falconer. 1985. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Appl. Environ. Microbiol. 50:1292-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins, P. R., N. R. Chandrasena, G. J. Jones, A. R. Humpage, and I. R. Falconer. 1997. Isolation and toxicity of Cylindrospermopsis raciborskii from an ornamental lake. Toxicon 35:341-346. [DOI] [PubMed] [Google Scholar]
- 14.Hepperle, D. 2008. Align vers. 03/2007, multisequence alignment-editor and preparation/manipulation of phylogenetic datasets and Seq Assem Vers. 04/2008, contig sequence assembly software. http://www.sequentix.de.
- 15.Jaime, E. 2003. Analytik und Vorkommen von Paralytic Shellfish Poisoning (PSP)-Toxinen in marinen Organismen. Ph.D. thesis. University of Jena, Jena, Germany.
- 16.Kellmann, R., T. K. Michali, Y. J. Jeon, R. Pickford, F. Pomati, and B. A. Neilan. 2008. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 74:4044-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komárek, J. 2005. Phenotypic diversity of the heterocytous cyanoprokaryotic genus Anabaenopsis. Czech Phycol., Olomouc. 5:1-35. [Google Scholar]
- 18.Komárek, J., and J. Komárkova. 2006. Diversity of Aphanizomenon-like cyanobacteria. Czech Phycol. 6:1-32. [Google Scholar]
- 19.Komárková-Legnerová J., and P. Eloranta. 1992. Planktic blue-green algae (Cyanophyta) from central Finland (Jyväskylä region) with special reference to the genus Anabaena. Arch. Hydrobiol./Algol. Stud. 67:103-133. [Google Scholar]
- 20.Kotai, J. 1972. Instructions for preparation of modified nutrient solution Z8 for algae. Publication B-11/69. Norwegian Institute for Water Research, Oslo, Norway.
- 21.Li, R., W. W. Carmichael, Y. Liu, and M. W. Watanabe. 2000. Taxonomic re-evaluation of Aphanizomenon flos-aquae NH-5 based on morphology and 16S rRNA gene sequences. Hydrobiologia 438:99-105. [Google Scholar]
- 22.Li, R., W. W. Carmichael, and P. Pereira. 2003. Morphological and 16S rRNA gene evidence for reclassification of the paralytic shellfish toxin producing Aphanizomenon flos-aquae LMECYA31 as Aphanizomenon issatschenkoi (Cyanophyceae). J. Phycol. 39:814-818. [Google Scholar]
- 23.Liu, Y., W. Chen, D. Li, Y. Shen, Y. Liu, and L. Song. 2006. Analysis of paralytic shellfish toxins in Aphanizomenon DC-1 from Lake Dianchi, China. Environ. Toxicol. 21:289-295. [DOI] [PubMed] [Google Scholar]
- 24.Lyra, C., S. Suomalainen, M. Gugger, C. Vezie, P. Sundman, L. Paulin, and K. Sivonen. 2001. Molecular characterization of planktic cyanobacteria of Anabaena, Aphanizomenon, Microcystis and Planktothrix genera. Int. J. Syst. Evol. Microbiol. 51:513-526. [DOI] [PubMed] [Google Scholar]
- 25.Mahmood, N. A., and W. W. Carmichael. 1986. Paralytic shellfish poisons produced by the freshwater cyanobacterium Aphanizomenon flos-aquae NH-5. Toxicon 24:175-186. [DOI] [PubMed] [Google Scholar]
- 26.Moustafa, A., J. E. Loram, J. D. Hackett, D. M. Anderson, F. G. Plumley, and D. Bhattacharya. 2009. Origin of saxitoxin biosynthetic genes in cyanobacteria. PLoS One 4:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neilan, B. A., D. Jacobs, and A. Goodman. 1995. Genetic diversity and phylogeny of toxic cyanobacteria determined by DNA polymorphisms within the phycocyanin locus. Appl. Environ. Microbiol. 6:3875-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishizawa, T., M. Asayama, and M. Shirai. 2001. Cyclic heptapeptide microcystin biosynthesis requires the glutamate racemase gene. Microbiology 147:1235-1241. [DOI] [PubMed] [Google Scholar]
- 29.Onodera, H., M. Satake, Y. Oshima, T. Yasumoto, and W. W. Carmichael. 1997. New saxitoxin analogues from the freshwater filamentous cyanobacterium Lyngbya wollei. Nat. Toxins 5:146-151. [DOI] [PubMed] [Google Scholar]
- 30.Pereira, P., H. Onodera, D. Andrinolo, S. Franca, F. Araújo, N. Lagos, and Y. Oshima. 2000. Paralytic shellfish toxins in the freshwater cyanobacterium Aphanizomenon flos-aquae isolated from Montargil reservoir, Portugal. Toxicon 38:1689-1702. [DOI] [PubMed] [Google Scholar]
- 31.Pereira, P., R. Li, W. W. Carmichael, E. Dias, and S. Franca. 2004. Taxonomy and production of paralytic shellfish toxins by the freshwater cyanobacterium Aphanizomenon gracile LMECYA40. Eur. J. Phycol. 39:361-368. [Google Scholar]
- 32.Posada, D., and K. A. Crandal. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 33.Preußel, K., A. Stüken, C. Wiedner, I. Chorus, and J. Fastner. 2006. First report on cylindrospermopsin producing Aphanizomenon flos-aquae (Cyanobacteria) isolated from two German lakes. Toxicon 47:156-162. [DOI] [PubMed] [Google Scholar]
- 34.Rajaniemi, P., P. Hrouzek, K. Kastovská, R. Willame, A. Rantala, L. Hoffmann, J. Komárek, and K. Sivonen. 2005. Phylogenetic and morphological evaluation of the genera Anabaena, Aphanizomenon, Trichormus and Nostoc (Nostocales, Cyanobacteria). Int. J. Syst. Evol. Microbiol. 55:11-26. [DOI] [PubMed] [Google Scholar]
- 35.Rücker, J., A. Stüken, B. Nixdorf, J. Fastner, I. Chorus, and C. Wiedner. 2007. Concentrations of particulate and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon 50:800-809. [DOI] [PubMed] [Google Scholar]
- 36.Schembri, M. A., B. A. Neilan, and C. P. Saint. 2001.Identification of genes implicated in toxin production in the cyanobacterium Cylindrospermopsis raciborskii. Environ. Toxicol. 16:413-421. [DOI] [PubMed] [Google Scholar]
- 37.Shaw, G. R., A. Sukenik, A. Livne, R. K. Chiswell, M. J. Smith, A. A. Seawright, R. L. Norris, G. K. Eaglesham, and M. R. Moore. 1999. Blooms of the cylindrospermopsin containing cyanobacterium, Aphanizomenon ovalisporum (Forti), in newly constructed lakes, Queensland, Australia. Environ. Toxicol. 14:167-177. [Google Scholar]
- 38.Sullivan, J. J., M. M. Wekell, and L. L. Kentala. 1985. Application of HPLC for the determination of PSP toxins in shellfish. J. Food Sci. 50:26-29. [Google Scholar]
- 39.Swofford, D. L. 2002. PAUP*: phylogenetic analysis using parsimony (* and other methods), version 4.0 b10. Sinauer, Sunderland, MA.
- 40.Terao, K., S. Ohmori, K. Igarashi, I. Ohtani, M. F. Watanabe, K. I. Harada, E. Ito, and M. Watanabe. 1994. Electron-microscopic studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue green alga Umezakia natans. Toxicon 32:833-843. [DOI] [PubMed] [Google Scholar]
- 41.Testé, V., J.-F. Briand, B. C. Nicholson, and S. Puiseux-Dao. 2002. Comparison of changes in toxicity during growth of Anabaena circinalis (cyanobacteria) determined by mouse neuroblastoma bioassay and HPLC. J. Appl. Phycol. 14:399-407. [Google Scholar]
- 42.Wang, D.-Z. 2008. Neurotoxins from marine dinoflagellates: a brief review. Mar. Drugs 6:349-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiedner, C., J. Rücker, J. Fastner, I. Chorus, and B. Nixdorf. 2008. Seasonal dynamics of cylindrospermopsin and cyanobacteria in two German lakes. Toxicon 52:677-686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.