Abstract
Shear-enhanced adhesion, although not observed for fimbria-mediated adhesion of oral Actinomyces spp., was noted for Hsa-mediated adhesion of Streptococcus gordonii to sialic acid-containing receptors, an interaction implicated in the pathogenesis of infective endocarditis.
Colonization of the tooth surface involves adhesin-mediated interactions between different species of bacteria and between bacteria and salivary components adsorbed onto the acquired enamel pellicle (11, 16). Examples include Hsa-mediated attachment of Streptococcus gordonii to α2-3-linked sialic acid termini of mucinous glycoproteins (18, 21, 23), type 1 fimbria-mediated attachment of Actinomyces oris (formerly Actinomyces naeslundii) to proline-rich proteins (PRPs) (9, 15), and type 2 fimbria-mediated attachment of A. oris and of A. naeslundii to Galβ1-3GalNAc motifs in mucins (15). Type 2 fimbriae also recognize Galβ1-3GalNAc motifs in cell surface receptor polysaccharides (RPS) of certain initial colonizing streptococci (4). Importantly, all these interactions occur in the presence of soluble salivary components that are potential inhibitors of adhesion. Thus, to fulfill their biological role, the corresponding adhesins must be selective for surface-associated receptor molecules rather than for their soluble counterparts. For example, type 1 fimbria-mediated adhesion of actinomyces to surface-associated PRP is not inhibited by soluble PRP (8); exposure of a cryptic receptor on PRP molecules upon adsorption to the tooth surface was postulated. A different mechanism was suggested for type 2 fimbria-mediated adhesion of Actinomyces spp. in the presence of soluble mucins. In this case, heavily fimbriated cells were thought to bind with greater avidity to surface-associated receptors (5).
Another explanation for bacterial adhesion in the presence of soluble receptors has emerged from recent studies on the effect of shear force on fimbria-mediated adhesion of Escherichia coli to mannose-containing receptors (17, 24, 25). At low shear, cells attached weakly and rolled along the receptor-coated substratum. As shear was increased, cells became stationary; subsequent reduction in shear caused the cells to resume rolling. These reversible changes in adhesion strength were postulated to arise from shear-dependent drag force on bacteria when bound to surface-associated ligands. Structural data suggested that a shear-dependent conformational change in the mannose-binding fimbrial adhesin FimH resulted from the formation of a so-called catch bond that increased the strength of the adhesin-ligand interaction. This increase may favor recognition of surface-associated receptors over soluble receptors because soluble receptors are not subject to shear stress and thus cannot induce catch bond formation.
Examples of shear-enhanced adhesion also include the interaction between von Willebrand factor and GPIbα on platelets (7) and recognition of P-selectin by P-selectin glycoprotein ligand on leukocytes (12). The present study addresses whether fimbria-mediated adhesion of Actinomyces spp. and Hsa-mediated adhesion of S. gordonii are shear enhanced.
Type 1 and type 2 fimbria-mediated adhesion of Actinomyces spp.
Wild-type and mutant strains of the recently separated species A. oris (10) and A. naeslundii that bear different fimbriae (2, 6) were grown overnight at 37°C in static brain heart infusion broth (Oxoid, Hampshire, England) and then recultured for roughly 4 h prior to experiments. The Bioflux 100 (Fluxion Biosciences, South San Francisco, CA) uses pneumatic pressure to drive liquid through channels 400 μm wide by 70 μm deep; wall shear force (pascals) is controlled by associated software. Channels were conditioned for 75 min at 37°C with 150 μl of 25% saliva (13), 25 μg/ml asialofetuin (Sigma-Aldrich, St. Louis, MO) in 20 mM sodium bicarbonate buffer (pH 8.0), 5 μg/ml PRP-1 (gift from D. Hay) in 20 mM sodium bicarbonate buffer or, as a control, phosphate-buffered saline (PBS; pH 7.4) and then washed with 3 volumes of PBS containing 1% bovine serum albumin (Sigma-Aldrich) (PBS-BSA). Experiments were performed at room temperature using PBS-washed cells resuspended to an optical density at 600 nm (OD600) of 0.1 in PBS-BSA. In dynamic adhesion experiments, cell suspensions were passed through channels at a shear force of 5 Pa. Shear was then reduced stepwise to 2.5, 1, 0.5, 0.1, 0.05, and 0 Pa (i.e., no flow). Adherent bacteria (i.e., rolling cells and stationary cells) were recorded over a period of 5 min as digital video using a Hamamatsu (Hamamatsu Corp., Bridgewater, NJ) C8484-05C camera mounted on a Leica DM IRB inverted microscope (Leica Microsystems, Bannockburn, IL) and controlled by SimplePCI acquisition software (Hamamatsu Corp., Sewickley, PA). Counting was done with MetaMorph software (Molecular Devices, Downingtown, PA) (1). The shutter speed of the digital camera was set such that adherent bacteria (either stationary or rolling) were visible but unattached bacteria (i.e., those moving at the same velocity as the bulk liquid phase) were blurred out. Bacteria were defined as rolling if they moved more than one-half cell diameter over a period of 10 s. The number of bacteria observed within a field of view (375 μm by 675 μm) was never greater than 180, which represented <1% surface coverage. Therefore, interactions between bacteria were discounted. Experiments were repeated six times. Shear-enhanced experiments were subsequently performed in which cell suspension was flowed through the channels at low shear (0.01 Pa) and, after a sufficient number of cells had accumulated, shear was increased stepwise. The number of stationary and of rolling bacteria was determined as described above. In control experiments, the expected shear-enhanced adherence of E. coli to mannose-containing RNase B (1) was replicated.
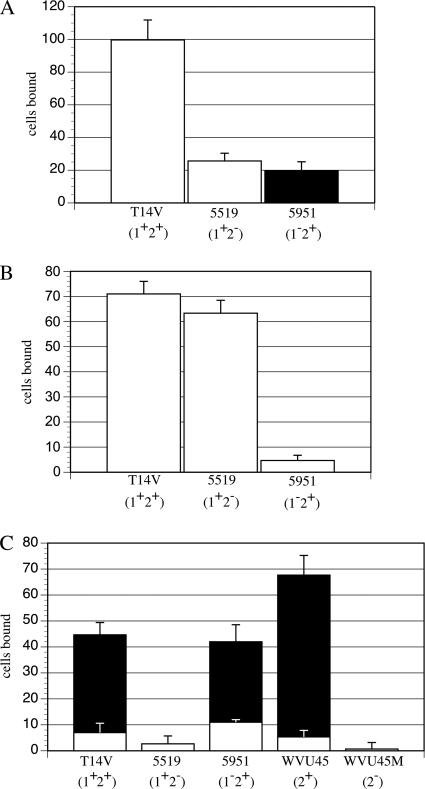
In dynamic adhesion experiments, Actinomyces cells were not seen on buffer-treated control surfaces or on receptor-coated surfaces. However, following resumption of flow after the 5-min step at 0 Pa, adherence of these bacteria to saliva-, PRP-1-, or asialofetuin-conditioned surfaces was noted. Results shown in Fig. 1 were obtained at a shear force of 5 Pa; results obtained at lower shear forces (not shown) were similar. On saliva-conditioned glass (Fig. 1A), A. oris wild-type strain T14V, which bears type 1 and type 2 fimbriae (1+2+), adhered in greater numbers than either of the fimbria-deficient mutant strains 5519 (1+2−) and 5951 (1−2+). The strains bearing type 1 fimbriae (T14V and 5519) were attached as stationary cells across the range of shear force, whereas the strain bearing only type 2 fimbriae (5951) rolled across the surface.
FIG. 1.
Dynamic adhesion experiment with A. oris T14V bearing type 1 and type 2 fimbriae (1+2+), A. naeslundii WVU45 bearing type 2 fimbriae (2+), or different fimbria-deficient mutants (fimbria production in parentheses) on glass coated with 25% saliva (A), 5 μg/ml PRP-1 (B), or 25 μg/ml asialofetuin (C). Cell suspensions were pumped into channels of a Bioflux 100 plate, and after a 5-min adherence period at 5 Pa, the shear force was decreased over sequential 5-min intervals to 2.5, 1, 0.5, 0.1, 0.05, and 0 Pa (i.e., no flow). Mean values for total cells bound (rolling plus stationary; filled bars, rolling cells; open bars, stationary cells) and exact 95% confidence intervals for a Poisson variable were calculated from six repetitions. Adherence was recorded only upon application of shear force after flow had reached 0, after which shear was increased stepwise as in shear-enhanced experiments. The values shown are those recorded at the system's highest shear force (5 Pa); the number of cells bound as well as the fraction of stationary cells remained essentially the same for each strain regardless of shear force, i.e., neither typical dynamic adhesion nor shear-enhanced adhesion was seen.
All strains were stationary on glass conditioned with PRP-1, the receptor for type 1 fimbriae (Fig. 1B), but most cells were rolling on the asialofetuin film, which supports type 2 fimbria-mediated adhesion (Fig. 1C). The involvement of type 1 fimbriae in adhesion to adsorbed PRP-1 was evident from much greater binding of strains T14V and 5519 than of the type 1-deficient strain 5951. Likewise, the role of type 2 fimbriae in adhesion to adsorbed asialofetuin was clear from much greater binding of strains T14V and 5951 than of the type 2-deficient strain 5519. A. naeslundii strain WVU45, which bears only type 2 fimbriae, also rolled on asialofetuin-coated glass, but its fimbria-deficient mutant strain WVU45M was nonadherent. Importantly, the proportions of rolling and stationary cells of all Actinomyces strains remained essentially the same as shear force was increased from 0.05 to 5 Pa (data not shown).
Although shear-enhanced adhesion of Actinomyces was not observed, it might be detected under different experimental conditions. In this regard, type 2 fimbria-mediated coaggregations of actinomyces with RPS-bearing streptococci are enhanced by vortex mixing (3); these coaggregates dissipate on standing and reform upon subsequent mixing. Likewise, shear from on-slide rocking promotes agglutination of guinea pig red blood cells by E. coli (25). Whether the ability of shear to promote bacterial coaggregation depends on increased contact between complementary cell types or, alternatively, on the formation of a catch bond remains to be determined.
Hsa-mediated adhesion of S. gordonii.
S. gordonii DL1 and its Hsa-deficient mutant strain D102 (19, 21), both of which typically appear as single cells or short chains, were prepared as described above for actinomyces strains. In control experiments, these streptococci did not adhere to PBS- or asialofetuin-conditioned substrata (results not shown). In addition, dynamic adhesion of Hsa-deficient strain D102 to fetuin, an Hsa receptor, was minimal over the range of shear stress (Fig. 2A). Adhesion of Hsa-bearing strain DL1 to saliva- or fetuin-conditioned glass was negligible at high flow rates (5 or 3 Pa) but increased as flow rate decreased and reached maximum at 0.01 Pa (Fig. 2A). In further experiments (10 repetitions) bacteria were allowed to adhere at 0.01 Pa for approximately 5 min to permit sufficient accumulation of bound bacteria. Flow rate was then increased through a series of sequential 5-min steps. The initial fraction (at 0.01 Pa) of stationary cells bound to glass conditioned with 5 μg/ml fetuin, 25 μg/ml fetuin, or 25% saliva was, respectively, approximately 0.1, 0.4, and 0.6 (Fig. 2B). As shear was increased to 5 Pa, the fraction of stationary cells increased to 1 on all films with no apparent loss of adherent bacteria (i.e., rolling plus stationary cells). Thus, a shear-dependent increase in the strength of Hsa-mediated adhesion occurred. The reversible nature of this effect was demonstrated by increasing the shear on DL1 cells adherent to fetuin from 0.05 to 2.5 Pa for 1 min and then returning to the starting value. In response, a sizeable fraction of the rolling cells became stationary at the higher shear and resumed rolling when shear was reduced (Fig. 3). Video S1 in the supplemental material (accelerated recording of the period 45 to 77 s) shows the conversion of weakly attached rolling cells to firmly attached stationary cells.
FIG. 2.
(A) Dynamic adhesion of wild-type S. gordonii DL1 to glass coated with different concentrations of fetuin or 25% saliva. Cell suspensions of this strain or the Hsa-deficient mutant strain D102 were pumped through channels of a Bioflux 100 plate at flow rates corresponding to shear forces ranging from 5 to 0.01 Pa. Total cells (i.e., rolling plus stationary) bound per field of view were determined after 5 min at each shear force. Mean values for cells bound per field of view and exact 95% confidence intervals for a Poisson variable were calculated from six repetitions. (B) Shear-enhanced adhesion of S. gordonii DL1. Cell suspensions were pumped through channels of a Bioflux 100 plate at a shear force of 0.01 Pa for 5 min to allow accumulation of bound cells as in panel A. Shear force was then set at a value ranging from 0.01 to 5 Pa, as indicated on the logarithmic x axis. After 5 min at each shear force, the fraction of stationary cells was determined by counting stationary and rolling cells. Mean values for the fraction of stationary cells and exact 95% confidence intervals for a binomial distribution were calculated from 10 repetitions.
FIG. 3.
Reversible nature of S. gordonii DL1 shear-enhanced adhesion to glass coated with 25 μg/ml fetuin. Cell suspensions were pumped through channels of a Bioflux 100 plate at a shear force of 0.01 Pa for 5 min to allow accumulation of approximately 150 total cells per field of view. Shear force was then increased to 0.05 Pa for 60 s, increased to 2.5 Pa for another 60 s, and then decreased to 0.05 Pa for 60 s. During each time, the number of total cells bound remained constant. The fraction of stationary cells was determined by continuous counting of both stationary and rolling cells.
Shear enhancement of binding of S. gordonii to adsorbed sialic acid-containing receptors raises the possibility that Hsa is a catch bond adhesin. While initial studies of Hsa failed to detect binding of the native antigen (i.e., Hs antigen) to surface-associated sialic acid-containing receptors (19), binding of a glutathione S-transferase (GST) fusion protein containing the amino-terminal sialic acid binding domain was readily detected (22). These observations suggest that Hsa, like FimH of E. coli (17), may exist in low- and high-affinity conformations. In addition to a possible role for catch bond formation in oral colonization, Hsa (of S. gordonii DL1) and the closely related GspB (of S. gordonii M99) are virulence determinants in a rat model of infective endocarditis (20, 27). While the precise role of these adhesins in pathogenesis remains to be established, they do mediate binding of streptococci to cell surface mucins on platelets (22, 28) and host phagocytes (14, 26) and thereby may contribute to the tropism of circulating bacteria for damaged heart valves. The possibility that such interactions are shear enhanced may contribute to the persistence of streptococci in a turbulent environment.
Supplementary Material
Acknowledgments
We thank W. Thomas for the gift of E. coli.
This research was supported by the intramural research program at the National Institute of Dental and Craniofacial Research, which included a postbaccalaureate fellowship for A. M. Ding.
Footnotes
Published ahead of print on 18 December 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Anderson, B. N., A. M. Ding, L. M. Nilsson, K. Kusuma, V. Tchesnokova, V. Vogel, E. V. Sokurenko, and W. E. Thomas. 2007. Weak rolling adhesion enhances bacterial surface colonization. J. Bacteriol. 189:1794-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cisar, J. O., V. A. David, S. H. Curl, and A. E. Vatter. 1984. Exclusive presence of lactose-sensitive fimbriae on a typical strain (WVU45) of Actinomyces naeslundii. Infect. Immun. 46:453-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cisar, J. O., P. E. Kolenbrander, and F. C. McIntire. 1979. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect. Immun. 24:742-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cisar, J. O., A. L. Sandberg, C. Abeygunawardana, G. P. Reddy, and C. A. Bush. 1995. Lectin recognition of host-like saccharide motifs in streptococcal cell wall polysaccharides. Glycobiology 5:655-662. [DOI] [PubMed] [Google Scholar]
- 5.Cisar, J. O., Y. Takahashi, S. Ruhl, J. A. Donkersloot, and A. L. Sandberg. 1997. Specific inhibitors of bacterial adhesion: observations from the study of gram-positive bacteria that initiate biofilm formation on the tooth surface. Adv. Dent. Res. 11:168-175. [DOI] [PubMed] [Google Scholar]
- 6.Cisar, J. O., A. E. Vatter, W. B. Clark, S. H. Curl, S. Hurst-Calderone, and A. L. Sandberg. 1988. Mutants of Actinomyces viscosus T14V lacking type 1, type 2, or both types of fimbriae. Infect. Immun. 56:2984-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doggett, T. A., G. Girdhar, A. Lawshe, D. W. Schmidtke, I. J. Laurenzi, S. L. Diamond, and T. G. Diacovo. 2002. Selectin-like kinetics and biomechanics promote rapid platelet adhesion in flow: the GPIbα-vWF tether bond. Biophys. J. 83:194-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons, R. J. 1989. Bacterial adhesion to oral tissues: a model for infectious diseases. J. Dent. Res. 68:750-760. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons, R. J., D. I. Hay, J. O. Cisar, and W. B. Clark. 1988. Adsorbed salivary proline-rich protein 1 and statherin: receptors for type 1 fimbriae of Actinomyces viscosus T14V-J1 on apatitic surfaces. Infect. Immun. 56:2990-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henssge, U., T. Do, D. R. Radford, S. C. Gilbert, D. Clark, and D. Beighton. 2009. Emended description of Actinomyces naeslundii and descriptions of Actinomyces oris sp. nov. and Actinomyces johnsonii sp. nov., previously identified as Actinomyces naeslundii genospecies 1, 2 and WVA 963. Int. J. Syst. Evol. Microbiol. 59:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolenbrander, P. E., and R. J. Palmer, Jr. 2004. Human oral bacterial biofilms, p. 85-117. In M. Ghannoum and G. A. O'Toole (ed.), Microbial biofilms. ASM Press, Washington, DC.
- 12.Marshall, B. T., M. Long, J. W. Piper, T. Yago, R. P. McEver, and C. Zhu. 2003. Direct observation of catch bonds involving cell-adhesion molecules. Nature 423:190-193. [DOI] [PubMed] [Google Scholar]
- 13.Palmer, R. J., Jr., K. Kazmerzak, M. C. Hansen, and P. E. Kolenbrander. 2001. Mutualism versus independence: strategies of mixed-species oral biofilms in vitro using saliva as the sole nutrient source. J. Bacteriol. 69:5794-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruhl, S., J. O. Cisar, and A. L. Sandberg. 2000. Identification of polymorphonuclear leukocyte and HL-60 cell receptors for adhesins of Streptococcus gordonii and Actinomyces naeslundii. Infect. Immun. 68:6346-6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruhl, S., A. L. Sandberg, and J. O. Cisar. 2004. Salivary receptors for the proline-rich protein-binding and lectin-like adhesins of oral actinomyces and streptococci. J. Dent. Res. 83:505-510. [DOI] [PubMed] [Google Scholar]
- 16.Scannapieco, F. A. 1994. Saliva-bacterium interactions in oral microbial ecology. Crit. Rev. Oral Biol. Med. 5:203-248. [DOI] [PubMed] [Google Scholar]
- 17.Sokurenko, E. V., V. Vogel, and W. E. Thomas. 2008. Catch-bond mechanism of force-enhanced adhesion: counterintuitive, elusive, but widespread? Cell Host Microbe 4:314-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi, Y., K. Konishi, J. O. Cisar, and M. Yoshikawa. 2002. Identification and characterization of hsa, the gene encoding the sialic acid-binding adhesin of Streptococcus gordonii DL1. Infect. Immun. 70:1209-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi, Y., A. L. Sandberg, S. Ruhl, J. Muller, and J. O. Cisar. 1997. A specific cell surface antigen of Streptococcus gordonii is associated with bacterial hemagglutination and adhesion to α2-3-linked sialic acid-containing receptors. Infect. Immun. 65:5042-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi, Y., E. Takashima, K. Shimazu, H. Yagishita, T. Aoba, and K. Konishi. 2006. Contribution of sialic acid-binding adhesin to pathogenesis of experimental endocarditis caused by Streptococcus gordonii DL1. Infect. Immun. 74:740-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi, Y., A. Yajima, J. O. Cisar, and K. Konishi. 2004. Functional analysis of the Streptococcus gordonii DL1 sialic acid-binding adhesin and its essential role in bacterial binding to platelets. Infect. Immun. 72:3876-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takamatsu, D., B. A. Bensing, H. Cheng, G. A. Jarvis, I. R. Siboo, J. A. Lopez, J. M. Griffiss, and P. M. Sullam. 2005. Binding of the Streptococcus gordonii surface glycoproteins GspB and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ib∝. Mol. Microbiol. 58:380-392. [DOI] [PubMed] [Google Scholar]
- 23.Takamatsu, D., B. A. Bensing, A. Prakobphol, S. J. Fisher, and P. M. Sullam. 2006. Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins. Infect. Immun. 74:1933-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas, W. E., L. M. Nilsson, M. Forero, E. V. Sokurenko, and V. Vogel. 2004. Shear-dependent ‘stick-and-roll’ adhesion of type 1 fimbriated Escherichia coli. Mol. Microbiol. 53:1545-1557. [DOI] [PubMed] [Google Scholar]
- 25.Thomas, W. E., E. Trintchina, M. Forero, V. Vogel, and E. V. Sokurenko. 2002. Bacterial adhesion to target cells enhanced by shear force. Cell 109:913-923. [DOI] [PubMed] [Google Scholar]
- 26.Urano-Tashiro, Y., A. Yajima, E. Takashima, Y. Takahashi, and K. Konishi. 2008. Binding of the Streptococcus gordonii DL1 surface protein Hsa to the host cell membrane glycoproteins CD11b, CD43, and CD50. Infect. Immun. 76:4686-4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong, Y. Q., B. A. Bensing, A. S. Bayer, H. F. Chambers, and P. M. Sullam. 2008. Role of the serine-rich surface glycoprotein GspB of Streptococcus gordonii in the pathogenesis of infective endocarditis. Microb. Pathog. 45:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yajima, A., Y. Takahashi, and K. Konishi. 2005. Identification of platelet receptors for the Streptococcus gordonii DL1 sialic acid-binding adhesin. Microbiol. Immunol. 49:795-800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.