Abstract
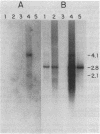
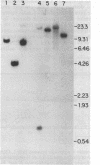
We have isolated and sequenced four overlapping cDNA clones from a normal adult human colon library, which together gave the entire nucleotide sequence for biliary glycoprotein I (BGP I). BGP I is a member of the carcinoembryonic antigen (CEA) gene family, which is a subfamily in the immunoglobulin gene superfamily. The deduced amino acid sequence of the combined clones for BGP I revealed a 34-residue leader sequence followed by a 108-residue N-terminal domain, a 178-residue immunoglobulin-like domain, a 108-residue region specific to BGP I, a 24-residue transmembrane domain, and a 35-residue cytoplasmic domain. The nucleotide sequence of BGP I exhibited greater than 80% identity with CEA and nonspecific crossreacting antigen (NCA) in the leader peptide, N-terminal domain, and immunoglobulin-like domain. The BGP I-specific domain, designated A', was 56.7% and 55.8% identical at the nucleotide level and 42.6% and 39.6% identical at the amino acid level to the immunoglobulin-like domain of NCA and the first immunoglobulin-like domain of CEA, respectively. Beyond nucleotide position 1375 the 3' region of the BGP I cDNA was found to be specific for BGP I. Hybridization of a probe from this region to electrophoretic blots of RNAs from different human tissues showed a predominant 2.8-kilobase (kb) message accompanied by weaker bands 4.1 and 2.1 kb in size. The same probe gave a single band in Southern blot analysis of restricted total human DNA. Using a coding region probe from the BGP I domain A', we observed 4.1- and 2.1-kb messages. Lack of the 2.8-kb band suggested that different forms of BGP I may be generated by posttranscriptional modification of the same gene. We propose that BGP I diverged from NCA by acquiring an immunoglobulin-like domain substantially different from the domains found in NCA or CEA and also a new cytoplasmic domain. The latter feature should result in a substantially different membrane anchorage mechanism of BGP I compared to CEA, which lacks the cytoplasmic domain and is anchored via a phosphatidylinositol-glycan structure. Protein structural analysis of BGP I isolated from human bile revealed a blocked N terminus, 129 amino acids of internal sequence that are in agreement with the translated cDNA sequence, and five glycosylation sites in the peptides sequenced.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hefta S. A., Hefta L. J., Lee T. D., Paxton R. J., Shively J. E. Carcinoembryonic antigen is anchored to membranes by covalent attachment to a glycosylphosphatidylinositol moiety: identification of the ethanolamine linkage site. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4648–4652. doi: 10.1073/pnas.85.13.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M., Trecartin R., Todd D. Identification of a nondeletion defect in alpha-thalassemia. N Engl J Med. 1977 Nov 17;297(20):1081–1084. doi: 10.1056/NEJM197711172972002. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Meese E., Blin N. Simultaneous isolation of high molecular weight RNA and DNA from limited amounts of tissues and cells. Gene Anal Tech. 1987 May-Jun;4(3):45–49. doi: 10.1016/0735-0651(87)90017-3. [DOI] [PubMed] [Google Scholar]
- Neumaier M., Fenger U., Wagener C. Monoclonal antibodies for carcinoembryonic antigen (CEA) as a model system: identification of two novel CEA-related antigens in meconium and colorectal carcinoma tissue by Western blots and differential immunoaffinity chromatography. J Immunol. 1985 Nov;135(5):3604–3609. [PubMed] [Google Scholar]
- Neumaier M., Zimmermann W., Shively L., Hinoda Y., Riggs A. D., Shively J. E. Characterization of a cDNA clone for the nonspecific cross-reacting antigen (NCA) and a comparison of NCA and carcinoembryonic antigen. J Biol Chem. 1988 Mar 5;263(7):3202–3207. [PubMed] [Google Scholar]
- Oikawa S., Nakazato H., Kosaki G. Primary structure of human carcinoembryonic antigen (CEA) deduced from cDNA sequence. Biochem Biophys Res Commun. 1987 Jan 30;142(2):511–518. doi: 10.1016/0006-291x(87)90304-4. [DOI] [PubMed] [Google Scholar]
- Paxton R. J., Mooser G., Pande H., Lee T. D., Shively J. E. Sequence analysis of carcinoembryonic antigen: identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc Natl Acad Sci U S A. 1987 Feb;84(4):920–924. doi: 10.1073/pnas.84.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenberg T. Carcinoembryonic antigen-like substances of human bile. Isolation and partial characterization. Int J Cancer. 1976 May 15;17(5):588–596. doi: 10.1002/ijc.2910170506. [DOI] [PubMed] [Google Scholar]
- Svenberg T., Hammarström S., Hedin A. Purification and properties of biliary glycoprotein I (BGP I). Immunochemical relationship to carcinoembryonic antigen. Mol Immunol. 1979 Apr;16(4):245–252. doi: 10.1016/0161-5890(79)90063-4. [DOI] [PubMed] [Google Scholar]
- Svenberg T., Wahren B., Hammarström S. Elevated serum levels of a biliary glycoprotein (BGP I) in patients with liver or biliary tract disease. Clin Exp Immunol. 1979 May;36(2):317–325. [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. A., Pande H., Paxton R. J., Shively L., Padma A., Simmer R. L., Todd C. W., Riggs A. D., Shively J. E. Molecular cloning of a gene belonging to the carcinoembryonic antigen gene family and discussion of a domain model. Proc Natl Acad Sci U S A. 1987 May;84(9):2965–2969. doi: 10.1073/pnas.84.9.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Ortlieb B., Friedrich R., von Kleist S. Isolation and characterization of cDNA clones encoding the human carcinoembryonic antigen reveal a highly conserved repeating structure. Proc Natl Acad Sci U S A. 1987 May;84(9):2960–2964. doi: 10.1073/pnas.84.9.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Weber B., Ortlieb B., Rudert F., Schempp W., Fiebig H. H., Shively J. E., von Kleist S., Thompson J. A. Chromosomal localization of the carcinoembryonic antigen gene family and differential expression in various tumors. Cancer Res. 1988 May 1;48(9):2550–2554. [PubMed] [Google Scholar]