Abstract
The present work calculated the rate of inactivation of Cryptosporidium parvum oocysts attributable to daily oscillations of low ambient temperatures. The relationship between air temperature and the internal temperature of bovine feces on commercial operations was measured, and three representative 24-h thermal regimens in the ∼15°C, ∼25°C, and ∼35°C ranges were chosen and emulated using a thermocycler. C. parvum oocysts suspended in deionized water were exposed to the temperature cycles, and their infectivity in mice was tested. Oral inoculation of 103 treated oocysts per neonatal BALB/c mouse (∼14 times the 50% infective dose) resulted in time- and temperature-dependent reductions in the proportion of infected mice. Oocysts were completely noninfectious after 14 24-h cycles with the 30°C regimen and after 70 24-h cycles with the 20°C regimen. In contrast, oocysts remained infectious after 90 24-h cycles with the 10°C regimens. The estimated numbers of days needed for a 1-log10 reduction in C. parvum oocyst infectivity were 4.9, 28.7, and 71.5 days for the 30, 20, and 10°C thermal regimens, respectively. The loss of infectivity of oocysts induced by these thermal regimens was due in part to partial or complete in vitro excystation.
It is well recognized that the protozoan parasite Cryptosporidium parvum causes waterborne enteric disease and poses a significant threat to public health. Fecal contamination from infected hosts, such as humans and some species of livestock and wildlife (17), can lead to elevated concentrations of C. parvum oocysts in drinking, recreational, and irrigation water supplies (6, 8). Once excreted, C. parvum oocysts can be eluted from fresh fecal matrices during precipitation events that generate surface flow or runoff conditions (4, 5, 12, 21, 32). During cool moist conditions oocysts can persist for months in the environment (10, 11, 25, 30), but factors such as extremes of temperature, exposure to UV radiation, and desiccation can substantially reduce the number of infective oocysts prior to waterborne transport (2, 7, 9, 11, 19, 24, 25, 29, 30).
To examine thermal stress, most studies have used constant thermal regimens to investigate the effect of temperature on the viability or infectivity of Cryptosporidium oocysts (11, 14, 20, 28, 30). To complement this work, we previously investigated the impact of large daily changes in the ambient temperature on C. parvum oocyst infectivity, using spring through autumn thermal regimens and temperatures measured inside bovine fecal pats that were exposed to solar radiation at cow-calf and dairy production facilities (23). Under California's summer climatic conditions, internal fecal pat temperatures range from 45°C to 75°C during the day and decrease 10 to 60°C during the night. Exposing oocysts to these large thermal fluctuations results in >3.3-log10 reductions in oocyst infectivity in each 24-h cycle (23). The present study was conducted in order to measure the effect of exposure to oocysts to cool-season daily temperatures (with peaks at temperatures greater than 10°C, 20°C, and 30°C) on the rate of inactivation of C. parvum oocysts. Determining the temperature-dependent rate of C. parvum oocyst inactivation for these lower temperatures would allow grazing management and source water assessment plans to more properly predict the amount of time needed for exclusion of cattle prior to the onset of winter precipitation in order to inactivate sufficient numbers of oocysts in critical watersheds.
MATERIALS AND METHODS
Acquiring ambient and fecal matrix temperatures.
As described previously (23), air temperature and internal temperature data for bovine fecal pats that were exposed to sunlight were collected for 12 months using an Optic StowAway Temp Logger system (Onset Computer Corporation, Bourne, MA) at 11 commercial diary and cow-calf operations throughout California.
Simulating fecal matrix temperatures.
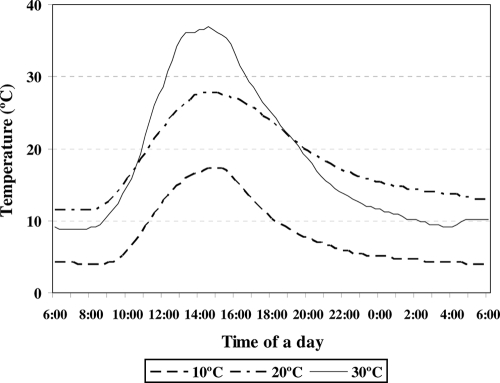
From our database of air and fecal matrix temperatures for lower- to middle-elevation regions (100 to 2,500 ft) in California, we selected three typical 24-h profiles of fecal matrix temperatures with maximum midday values of 17.3°C, 27.6°C, and 36.4°C (Fig. 1). Using a custom-made UNIX fitting algorithm, we constructed a time-by-temperature 24-h step function that emulated the three daily thermal profiles. The step functions were then programmed into a 96-well automated thermocycler (GeneAmp 2700 PCR system; Applied Biosystems, Foster City, CA).
FIG. 1.
Representative 24-h 10°C, 20°C, and 30°C thermal profiles for bovine fecal pats located on grazed rangeland throughout California from spring to autumn in 2000.
Source and purification of C. parvum oocysts.
Feces were collected from naturally infected calves that were 9 to 21 days old from a commercial dairy in Tulare, CA. Oocysts isolated from the same location and a similar age group were classified as C. parvum using a molecular procedure (33). Using an acid-fast protocol with direct fecal smears, samples having more than 25 oocysts per microscopic field (magnification, ×400) were washed using a series of 40-, 100-, 200-, and 270-mesh sieves with Tween water (0.2% [vol/vol] Tween 20 in deionized water). Each resulting suspension was centrifuged at 1,500 × g for 20 min in a 250-ml centrifuge tube, the supernatant was discarded, and the pellet was resuspended in Tween water. Oocysts were purified using a discontinuous sucrose gradient (3) and suspended in deionized water. The concentration of purified oocysts was determined by determining the arithmetic mean for six separate counts using a phase-contrast hemacytometer. Stock solutions were prepared by diluting oocysts in deionized water to obtain concentrations of 104 and 106 oocysts/ml, stored at 4°C, and used within 1 week.
Treatment of C. parvum oocysts with thermal profiles.
For each thermal regimen, 96 100-μl MicroAmp reaction tubes (Applied Biosystems, Foster City, CA) were filled with an oocyst stock solution (103 oocysts/tube). After a 24-h thermal cycle was completed, a set of 10 tubes was removed from the thermocycler. The remaining tubes were subjected to a replicate 24-h thermal cycle, another set of 10 tubes was removed, and the procedure was repeated until the oocysts were noninfectious. The process of removing tubes and restarting the thermocycler was completed within 1 min so that the break in the continuous temperature was minimized. Oocyst suspensions from replicate temperature-duration tubes were combined, and the percentages of intact, partial, and ghost oocysts were determined using differential interference contrast microscopy at a magnification of ×400 (Olympus BX 60; Olympus America, Inc., New York, NY). One hundred microliters per neonatal mouse was used as an oral inoculum to test the infectivity of the original preparation containing 103 oocysts/tube, using the assay described below. The numbers of experiments conducted for each thermal regimen are shown in Table 1. For positive controls, neonatal mice were given 103 fresh oocysts; for negative controls, neonatal mice were inoculated with 105 heat-inactivated oocysts (exposed to 70°C for 2 h) to monitor possible detection of oocysts in the intestine directly from inocula.
TABLE 1.
Infectivity of C. parvum oocysts in neonatal BALB/c mice after oocysts suspended in water were exposed to 24-h temperature cycles
| Temp cyclea | Length of exposure (days)b | % of infected mice (no. of infected mice/no. of inoculated mice) |
||
|---|---|---|---|---|
| Treated oocysts | Fresh oocysts | Inactivated oocysts | ||
| 10°C | 1, 3, 5, 7c | 100 (7/7)c | 100 (7/7)c | 0 (0/6)c |
| 15, 30, 45, 60d | 100 (16/16)d | 100 (10/10)d | 0 (0/11)d | |
| 75 | 92.9 (13/14) | 100 (11/11) | 0 (0/11) | |
| 90 | 61.5 (8/13) | 100 (12/12) | 0 (0/11) | |
| 20°C | 1, 2, 3, 4, 5, 6, 7c | 100 (7/7)c | 100 (7/7)c | 0 (0/7)c |
| 8, 9, 10, 14, 21, 28c | 100 (7/7)c | 100 (7/7)c | 0 (0/7)c | |
| 35 | 53.8 (7/13) | 100 (13/13) | 0 (0/10) | |
| 42 | 42.9 (3/7) | 100 (7/7) | 0 (0/6) | |
| 49 | 28.6 (4/14) | 100 (13/13) | 0 (0/10) | |
| 56 | 33.3 (2/6) | 100 (6/6) | 0 (0/6) | |
| 63 | 23.1 (3/13) | 100 (13/13) | 0 (0/12) | |
| 70 | 0 (0/14) | 100 (12/12) | 0 (0/11) | |
| 71, 72 | 0 (0/6)c | 100 (6/6)c | 0 (0/5)c | |
| 30°C | 1, 2, 3, 4, 5e | 100 (10/10)e | 100 (6/6)e | 0 (0/6)e |
| 6 | 64.7 (11/17) | 100 (11/11) | 0 (0/11) | |
| 7 | 26.7 (4/15) | 100 (12/12) | 0 (0/11) | |
| 8 | 33.3 (2/6) | 100 (6/6) | 0 (0/6) | |
| 9 | 28.6 (2/7) | 100 (6/6) | 0 (0/6) | |
| 10 | 14.3 (1/7) | 100 (6/6) | 0 (0/6) | |
| 11 | 16.7 (1/6) | 100 (6/6) | 0 (0/6) | |
| 12 | 20.0 (1/5) | 100 (6/6) | 0 (0/6) | |
| 13 | 16.7 (1/6) | 100 (6/6) | 0 (0/6) | |
| 14 | 0 (0/21) | 100 (16/16) | 0 (0/14) | |
| 15 | 0 (0/14) | 100 (10/10) | 0 (0/9) | |
The 24-h thermal profiles mimicked the temperatures in bovine fecal pats shown in Fig. 1.
One day was equivalent to a single 24-h temperature cycle shown in Fig. 1.
The infectivity data were equivalent for the different lengths of exposure indicated. The number of mouse pups typically was six or seven.
The infectivity data were equivalent for the different lengths of exposure. The number of mouse pups typically ranged from 11 to 16.
The infectivity data were equivalent for the different lengths of exposure. The number of mouse pups ranged from 5 to 17.
In vivo infectivity assay for C. parvum oocysts.
A neonatal BALB/c mouse model, modified from the model of Finch et al. (13), was used to test oocyst infectivity (16). Female BALB/c mice with neonatal pups were purchased from Harlan Company (San Diego, CA), housed in cages fitted with air filters, and given food and water ad libitum. Intragastric oocyst inocula were delivered in 100 μl of deionized water to 4-day-old neonatal mice, using a 24-gauge ballpoint feeding needle. One hour prior to infection, the neonatal mice were removed from the dam to ensure that their stomachs were empty; following inoculation, the dam was returned to the pups. Litters of mice were randomly assigned to treatment groups, including positive and negative controls as described above.
To determine the Cryptosporidium infection status of neonatal mouse pups 7 days postinoculation, the entire intestine was collected and suspended in 5 ml deionized water in a 50-ml tube and homogenized with a tissue homogenizer (Kika Werke Gmbh & Co. KG, Staufen, Germany). The homogenate was centrifuged at 1,500 × g for 10 min, the supernatant was discarded, and pellet was resuspended in deionized water and filtered through a 20-μm nylon net filter (Millipore Co., Bedford, MA) that had been fixed on a Swinnex holder (Millipore Co., Bedford, MA). The filtrate was concentrated to 1 ml by centrifugation at 1,500 × g for 10 min. Fifty microliters of the final suspension was mixed with 50 μl of fluorescent isothiocyanate-labeled anti-Cryptosporidium immunoglobulin M antibodies (Meridian, Cincinnati, OH) and 2 μl of 0.5% Evans blue in phosphate-buffered saline and incubated at room temperature for 45 min in a dark box. Three duplicate wet mount slides were prepared from each sample using 20 μl of reaction mixture per slide. Slides were examined by epifluorescence microscopy (Olympus America, Inc., New York, NY). A mouse was considered infected if one or more oocysts were detected in any of the three slides. Tissue homogenates were shown previously to be twice as sensitive for detecting C. parvum infections in neonatal mice as histopathology (16).
Statistical modeling.
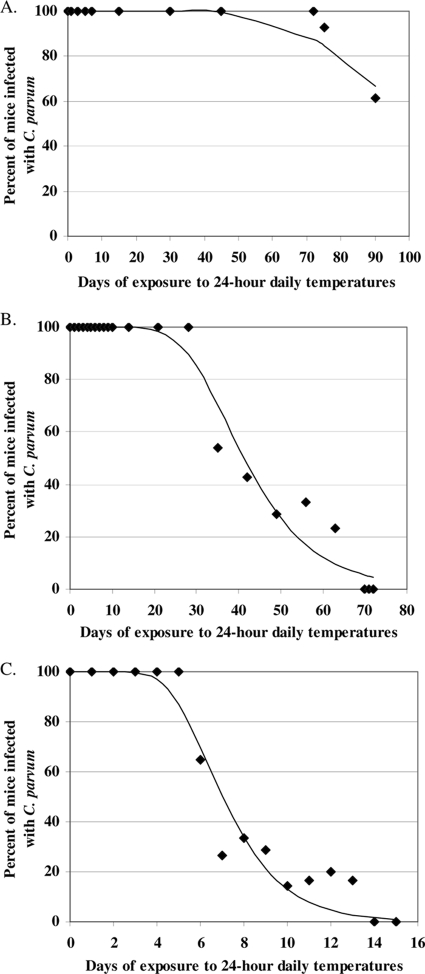
The infection status of each mouse pup was coded as either infected (y = 1) or uninfected (y = 0) using the in vivo neonatal mouse assay described above. The data resulted in a percentage of infection for litters of mice for each time point (number of days) for each of three 24-h thermal profiles (10, 20, and 30°C). Given the asymmetry (steep decline and then tailing) of the raw data for the percentage of mice infected as a function of time (Fig. 2), we used complementary log-log regression (1) instead of logistic regression to model the effect of daily thermal exposure on the infectivity of C. parvum oocysts for neonatal mice. For each 24-h thermal profile (10, 20, or 30°C), this model was formulated using equation 1:
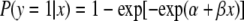 |
(1) |
where P(y = 1| x) was the fitted or expected probability that a BALB/c mouse pup was infected with C. parvum given that the oocysts had been exposed to x 24-h thermal profiles (e.g., days) and α and β were the maximum likelihood estimates obtained from the complementary log-log regression. Because we used litters of mice for each time point, we adjusted the standard errors for potential clustering of infection status within litters (e.g., lack of independence of infection status for littermates) (15).
FIG. 2.
(A) Effect of daily temperature fluctuations up to ∼15°C on the infectivity of C. parvum oocysts. (B) Effect of daily temperature fluctuations up to ∼25°C on the infectivity of C. parvum oocysts. (C) Effect of daily temperature fluctuations up to ∼35°C on the infectivity of C. parvum oocysts.
To calculate the number of days with each thermal profile (10, 20, or 30°C) needed for a 1-log10 reduction in the number of infective oocysts, the following calculations were used. We established previously the infectious dose curve for C. parvum oocysts in neonatal BALB/c mice, defined using equation 2:
 |
(2) |
where P(y = 1| x oocysts) is the fitted or expected probability that a BALB/c mouse pup was infected with C. parvum if it was given an oral dose of x oocysts, α is −24.244, and β is 5.695. Given that we used an inoculum of 1,000 oocysts per pup, a 1-log10 reduction in the number of infective oocysts was 100 oocysts. Using the dose-response curve described above, a dose of 100 infective oocysts generates a probability of mouse infection of 0.879 (1/{1 + exp[24.244 − 5.695×ln 100]} = 0.879). Hence, we calculated the number of days with each thermal profile (10, 20, or 30°C) needed to generate a probability (P) of neonatal mouse infection of 0.879. Using the complementary log-log regression fitted as described above for each thermal profile (10, 20, or 30°C), we then solved for x (days) using equation 3:
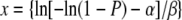 |
(3) |
where P is 0.879 and α and β are the maximum likelihood estimates obtained from the complementary log-log regression (equation 1) that was fitted to the data shown in Table 1.
Nucleotide sequence accession number.
The DNA sequence of oocysts has been deposited in the NCBI GenBank under accession no. FJ752165.
RESULTS AND DISCUSSION
Complementary log-log regression models (equation 1) were fitted to the data for each 24-h thermal regimen and are shown in Fig. 2. For the 30°C thermal regimen, the α and β maximum likelihood estimates were 3.37 (95% confidence interval [CI], 2.44 to 4.30) and −0.533 (95% CI, −0.677 to −0.388); for the 20°C thermal regimen, the α and β estimates were 3.30 (95% CI, 2.16 to 4.45) and −0.089 (95% CI, −0.116 to −0.062); and for the 10°C thermal regimen, the α and β estimates were 3.28 (95% CI, 2.18 to 4.37) and −0.035 (95% CI, −0.049 to −0.022). A 1-log10 reduction in C. parvum oocyst infectivity would have reduced the ingested dose of infective oocysts from 1,000 to 100 oocysts, resulting in infection of about 88% of the mouse pups based on predictions from equation 2. Therefore, using the values for the α and β coefficients from equation 1 to determine how many days of thermal exposure would result in infection of 88% of the mice (solving equation 3), the estimated numbers of days of each 24-h thermal regimen needed for a 1-log10 reduction in C. parvum oocyst infectivity were 5, 29, and 72 days for the 30, 20, and 10°C thermal regimens, respectively.
Using an inoculum containing 103 oocysts, which is ∼14 times larger than the 50% infective dose (ID50) dose, which is 70.6 oocysts (23), complete loss of infectivity occurred after 70 daily cycles of the 20°C thermal regimen and after 14 daily cycles of the 30°C thermal regimen. These lengths of time for complete loss of infectivity may provide a general guideline for the minimal amount of time needed for oocyst inactivation following exclusion of livestock before the onset of late fall or early winter rains on California rangeland (22). In contrast, despite 90 daily cycles of the 10°C thermal regimen, cryptosporidial infections still occurred in 61.5% of inoculated mice. This period of time exceeds not only the interval between runoff-generating precipitation events for western United States grazed rangelands but also the entire duration of seasonal cooler temperatures (winter) for many grazed locations in California. This work on oocyst inactivation with cooler-season temperatures complements our previous work for warmer seasons, where we demonstrated that exposure to a singe daily cycle of 40°C, 50°C, 60°C, or 70°C thermal regimens (mid-spring through mid-fall fecal pat temperatures) resulted in complete loss of infectivity for an oral dose of 105 oocysts in BALB/c mice, or about 1,400 times the ID50 (23). Although we did not investigate the effects of winter freezing on oocyst infectivity, data from previous work suggest that Cryptosporidium oocysts do not persist over winter under freezing conditions (31) and that 99% of oocysts exposed to soils frozen at −10°C become inactivated within 50 days whether or not freeze-thaw cycles occur (18). Prolonged freezing or repeated freeze-thaw conditions are uncommon in many locations in California utilized for livestock grazing. Hence, the cooler but above-freezing conditions common to much of grazed California rangeland dramatically extend oocyst survival during the season when there are peak runoff conditions, which is typically December through March (22).
The number of days needed for complete loss of infectivity likely depends on the oocyst dose given to mouse pups, so that for larger and smaller doses longer and shorter exposures are required to achieve total loss of infectivity, respectively. This finding underscores the need to describe oocyst survival at specific temperature maxima not only as a function of time but also as a function of the oocyst dose if we are to compare results of different studies and different infectivity or viability methods (vital dyes, cell culture, etc.). Alternatively, comparing data for duration of survival obtained in different studies for similar environmental conditions might be facilitated by calculation of decimal reduction times (DRT) (the time required to reduce survival or infectivity by 1 log10 or 90%) if the inactivation rate follows a first-order decay function and DRT values are independent of dose for assays estimating Cryptosporidium infectivity.
The temperature exposure regimens used in this study mimicked the naturally occurring daily fluctuations of temperature experienced by oocysts in bovine fecal material exposed to solar radiation (Fig. 1). Our results were relatively consistent with the results of previous work that used constant temperatures to study thermal inactivation of C. parvum oocysts. For example, approximately 30% of oocysts in natural mineral waters remained viable after 12 weeks of incubation at 20°C, as assessed by using inclusion or exclusion of fluorogenic vital dyes (26). Oocysts in reservoir water remained infective for at least 3 months when they were stored between 4°C and 15°C (20), as assessed by a cell culture-TaqMan PCR assay. In sterile distilled water oocysts survived for 10 weeks at 25°C, as assessed using a mouse infectivity assay (27).
Consistent with our previous work (23), the primary mechanism of oocyst inactivation was partial or complete excystation in the environment (Table 2) and presumably rapid inactivation of the released sporozoites. All fresh oocyst inocula contained >95% intact oocysts. The number of days needed for 100% loss of intact oocysts coincided closely with the period of time needed for complete loss of infectivity, despite the fact that 60 to 80% of the original 1,000 oocysts were partially excysted when they were inoculated into the mice, indicating their lack of infectivity (Table 2). Apparently, repeated cycles of daily warming and cooling, like those that occur naturally in fecal deposits exposed to daily doses of solar radiation (Fig. 1), result in partial excystation of substantial numbers of oocysts (Table 2), and either eventual death or some other inactivating mechanism occurs for the sporozoites that are retained within the oocysts.
TABLE 2.
Percentages of intact, partially excysted, and empty (shell) oocysts induced by exposing C. parvum oocysts to 24-h temperature cycles
| Temp treatmenta | Length of exposure (days)b | % ofc: |
||
|---|---|---|---|---|
| Intact oocysts | Partial oocysts | Empty oocysts | ||
| 10°C cycle | 0d | 97.0 ± 1.0 | 2.3 ± 0.6 | 0.7 ± 0.6 |
| 1 | 93.0 ± 1.3 | 4.0 ± 0.6 | 3.2 ± 0.8 | |
| 5 | 88.2 ± 1.1 | 5.6 ± 0.3 | 6.2 ± 0.7 | |
| 15 | 83.9 ± 1.5 | 7.9 ± 0.9 | 8.2 ± 0.9 | |
| 30 | 79.3 ± 2.6 | 9.7 ± 1.1 | 11.0 ± 1.5 | |
| 60 | 73.5 ± 3.9 | 11.1 ± 2.4 | 15.4 ± 2.1 | |
| 90 | 61.6 ± 1.6 | 20.1 ± 2.1 | 18.3 ± 2.2 | |
| Heat-inactivated oocysts | 89.2 ± 0.8 | 5.7 ± 0.9 | 5.1 ± 0.5 | |
| 20°C cycle | 0d | 96.7 ± 0.5 | 2.3 ± 0.6 | 1.0 ± 0.9 |
| 1 | 86.6 ± 2.0 | 6.5 ± 2.0 | 6.8 ± 2.0 | |
| 5 | 64.6 ± 2.0 | 17.7 ± 1.2 | 17.7 ± 3.1 | |
| 10 | 43.8 ± 6.0 | 47.2 ± 6.4 | 9.0 ± 1.3 | |
| 28 | 28.5 ± 5.4 | 36.4 ± 4.8 | 35.0 ± 5.8 | |
| 56 | 10.2 ± 2.5 | 68.08 ± 4.9 | 21.8 ± 2.5 | |
| 70 | 0 | 80.7 ± 6.5 | 19.3 ± 6.5 | |
| Heat-inactivated oocysts | 90.4 ± 1.8 | 5.4 ± 1.2 | 4.2 ± 0.6 | |
| 30°C cycle | 0d | 95.5 ± 1.4 | 2.6 ± 0.5 | 1.9 ± 0.9 |
| 1 | 56.1 ± 3.6 | 31.0 ± 3.4 | 12.9 ± 4.0 | |
| 5 | 42.2 ± 3.7 | 47.1 ± 3.8 | 10.7 ± 1.3 | |
| 7 | 15.1 ± 2.4 | 65.3 ± 6.1 | 19.6 ± 4.2 | |
| 10 | 4.5 ± 1.0 | 60.3 ± 1.9 | 35.1 ± 1.1 | |
| 13 | 0.9 ± 0.9 | 48.2 ± 4.4 | 50.9 ± 5.3 | |
| 15 | 0 | 66.3 ± 5.0 | 33.7 ± 5.0 | |
| Heat-inactivated oocysts | 86.0 ± 1.8 | 6.1 ± 0.8 | 7.9 ± 1.6 | |
The 24-h thermal profiles mimicked the temperatures in bovine fecal pats shown in Fig. 1.
One day was equivalent to a single 24-h temperature cycle shown in Fig. 1.
The percentages were determined by dividing the number of intact, partially excysted, or empty oocysts by the number of all oocyst forms and then multiplying the result by 100. The data are arithmetic means ± standard deviations.
Fresh oocysts without any thermal treatment were used.
Acknowledgments
This work was conducted under the auspices of the Bernice Barbour Communicable Disease Laboratory with financial support from the Bernice Barbour Foundation, Hackensack, NJ, through a grant to the Center of Equine Health, University of California, Davis. Additional financial support was provided by the Sustainable Agriculture Research & Education Program, Division of Agriculture and Natural Resources, University of California, and by the California Beef Council.
We are grateful to the dedicated livestock and natural resource farm advisors of the University of California Cooperative Extension for collecting temperature profiles for bovine fecal pats throughout California. We are also grateful to Ted Jones at the Stanford Center for DNA Sequence and Technology, Stanford University, for programming the temperature profiles.
Footnotes
Published ahead of print on 18 December 2009.
REFERENCES
- 1.Agresti, A. 2002. Building and applying logistic regression models, p. 211-266. In A. Agresti (ed.), Categorical data analysis, 2nd ed. John Wiley & Sons, New York, NY.
- 2.Anderson, B. C. 1986. Effect of drying on the infectivity of cryptosporidia-laden calf feces for 3- to 7-day-old mice. Am. J. Vet. Res. 47:2272-2273. [PubMed] [Google Scholar]
- 3.Arrowood, M. J., and C. R. Sterling. 1987. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J. Parasitol. 73:314-319. [PubMed] [Google Scholar]
- 4.Atwill, E. R., M. D. Pereira, L. H. Alonso, C. Elmi, W. B. Epperson, R. Smith, W. Riggs, L. V. Carpenter, D. A. Dargatz, and B. Hoar. 2006. Environmental load of Cryptosporidium parvum oocysts from cattle manure in feedlots from the central and western United States. J. Environ. Qual. 35:200-206. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, S. A., and J. Schijven. 2002. Release of Cryptosporidium and Giardia from dairy calf manure: impact of solution salinity. Environ. Sci. Technol. 36:3916-3923. [DOI] [PubMed] [Google Scholar]
- 6.Craun, G. F., R. L. Calderon, and M. F. Craun. 2005. Outbreaks associated with recreational water in the United States. Int. J. Environ. Health Res. 15:243-262. [DOI] [PubMed] [Google Scholar]
- 7.Davies, C. M., N. Altavilla, M. Krogh, C. M. Ferguson, D. A. Deere, and N. J. Ashbolt. 2005. Environmental inactivation of Cryptosporidium oocysts in catchment soils. J. Appl. Microbiol. 98:308-317. [DOI] [PubMed] [Google Scholar]
- 8.Dziuban, E. J., J. L. Liang, G. F. Craun, V. Hill, P. A. Yu, J. Painter, M. R. Moore, R. L. Calderon, S. L. Roy, and M. J. Beach. 2006. Surveillance for waterborne disease and outbreaks associated with recreational water—United States, 2003-2004. MMWR Surveill. Summ. 55:1-30. [PubMed] [Google Scholar]
- 9.Erickson, M. C., and Y. R. Ortega. 2006. Inactivation of protozoan parasites in food, water, and environmental systems. J. Food Prot. 69:2786-2808. [DOI] [PubMed] [Google Scholar]
- 10.Fayer, R., and T. Nerad. 1996. Effects of low temperatures on viability of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 62:1431-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fayer, R., J. M. Trout, and M. C. Jenkins. 1998. Infectivity of Cryptosporidium parvum oocysts stored in water at environmental temperatures. J. Parasitol. 84:1165-1169. [PubMed] [Google Scholar]
- 12.Ferguson, C. M., C. M. Davies, C. Kaucner, M. Krogh, J. Rodehutskors, D. A. Deere, and J. Ashbolt. 2007. Field scale quantification of microbial transport from bovine faeces under simulated rainfall events. J. Water Health 5:83-95. [DOI] [PubMed] [Google Scholar]
- 13.Finch, G. R., C. W. Daniels, E. K. Black, F. W. Schaefer III, and M. Belosevic. 1993. Dose response of Cryptosporidium parvum in outbred neonatal CD-1 mice. Appl. Environ. Microbiol. 59:3661-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujino, T., T. Matsui, F. Kobayashi, K. Haruki, Y. Yoshino, J. Kajima, and M. Tsuji. 2002. The effect of heating against Cryptosporidium oocysts. J. Vet. Med. Sci. 64:199-200. [DOI] [PubMed] [Google Scholar]
- 15.Hardin, J., and J. Hilbe. 2001. Generalized linear models and extensions, p. 101-114. Stata Press, College Station, TX.
- 16.Hou, L., X. Li, L. Dunbar, R. Moeller, B. Palermo, and E. R. Atwill. 2004. Neonatal-mouse infectivity of intact Cryptosporidium parvum oocysts isolated after optimized in vitro excystation. Appl. Environ. Microbiol. 70:642-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter, P. R., and R. C. Thompson. 2005. The zoonotic transmission of Giardia and Cryptosporidium. Int. J. Parasitol. 35:1181-1190. [DOI] [PubMed] [Google Scholar]
- 18.Kato, S., M. B. Jenkins, E. Fogarty, and D. D. Bowman. 2002. Effects of freeze-thaw events on the viability of Cryptosporidium parvum oocysts in soil. J. Parasitol. 88:718-722. [DOI] [PubMed] [Google Scholar]
- 19.King, B. J., D. Hoefel, D. P. Daminato, S. Fanok, and P. T. Monis. 2008. Solar UV reduces Cryptosporidium parvum oocyst infectivity in environmental waters. J. Appl. Microbiol. 104:1311-1323. [DOI] [PubMed] [Google Scholar]
- 20.King, B. J., A. R. Keegan, P. T. Monis, and C. P. Saint. 2005. Environmental temperature controls Cryptosporidium oocyst metabolic rate and associated retention of infectivity. Appl. Environ. Microbiol. 71:3848-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King, B. J., and P. T. Monis. 2007. Critical processes affecting Cryptosporidium oocyst survival in the environment. Parasitology 134:309-323. [DOI] [PubMed] [Google Scholar]
- 22.Lewis, D. J., M. J. Singer, R. A. Dahlgren, and K. W. Tate. 2000. Hydrology in a California oak woodland watershed: a 17-year study. J. Hydrol. 230:106-117. [Google Scholar]
- 23.Li, X., E. R. Atwill, L. A. Dunbar, T. Jones, J. Hook, and K. W. Tate. 2005. Seasonal temperature fluctuations induce rapid inactivation of Cryptosporidium parvum. Environ. Sci. Technol. 39:4484-4489. [DOI] [PubMed] [Google Scholar]
- 24.McGuigan, K. G., F. Méndez-Hermida, J. A. Castro-Hermida, E. Ares-Mazás, S. C. Kehoe, M. Boyle, C. Sichel, P. Fernández-Ibáρez, B. P. Meyer, S. Ramalingham, and E. A. Meyer. 2006. Batch solar disinfection inactivates oocysts of Cryptosporidium parvum and cysts of Giardia muris in drinking water. J. Appl. Microbiol. 101:453-463. [DOI] [PubMed] [Google Scholar]
- 25.Medema, G. J., M. Bahar, and F. M. Schets. 1997. Survival of Cryptosporidium parvum, Escherichia coli, faecal enterococci and Clostridium perfringens in river water: influence of temperature and autochthonous microorganisms. Water Sci. Technol. 35:249-252. [Google Scholar]
- 26.Nichols, R. A., C. A. Paton, and H. V. Smith. 2004. Survival of Cryptosporidium parvum oocysts after prolonged exposure to still natural mineral waters. J. Food Prot. 67:517-523. [DOI] [PubMed] [Google Scholar]
- 27.Olson, M. E., J. Goh, M. Philips, N. Guselle, and T. A. McAllister. 1999. Giardia cyst and Cryptosporidium oocyst survival in water, soil, and cattle feces. J. Environ. Qual. 28:1991-1996. [Google Scholar]
- 28.Pokorny, N. J., S. C. Weir, R. A. Carreno, J. T. Trevors, and H. Lee. 2002. Influence of temperature on Cryptosporidium parvum oocyst infectivity in river water samples as detected by tissue culture assay. J. Parasitol. 88:641-643. [DOI] [PubMed] [Google Scholar]
- 29.Reinoso, R., and E. Bécares. 2008. Environmental inactivation of Cryptosporidium parvum oocysts in waste stabilization ponds. Microb. Ecol. 56:585-592. [DOI] [PubMed] [Google Scholar]
- 30.Robertson, L. J., A. T. Campbell, and H. V. Smith. 1992. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl. Environ. Microbiol. 58:3494-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson, L. J., and B. K. Gjerde. 2004. Effects of the Norwegian winter environment on Giardia cysts and Cryptosporidium oocysts. Microb. Ecol. 47:359-365. [DOI] [PubMed] [Google Scholar]
- 32.Schijven, J. F., S. A. Bradford, and S. Yang. 2004. Release of Cryptosporidium and Giardia from dairy cattle manure: physical factors. J. Environ. Qual. 33:1499-1508. [DOI] [PubMed] [Google Scholar]
- 33.Xiao, L., L. Escalante, C. Yang, I. Sulaiman, A. A. Escalante, R. J. Montali, R. Fayer, and A. A. Lal. 1999. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl. Environ. Microbiol. 65:1578-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]