Abstract
Cryptosporidium is a genus of waterborne protozoan parasites that causes significant gastrointestinal disease in humans. These parasites can accumulate in environmental biofilms and be subsequently released to contaminate water supplies. Natural microbial assemblages were collected each season from an eastern Pennsylvania stream and used to grow biofilms in laboratory microcosms in which influx, efflux, and biofilm retention were determined from daily oocyst counts. For each seasonal biofilm, oocysts attached to the biofilm quickly during oocyst dosing. Upon termination of oocyst dosing, the percentage of oocysts retained within the biofilm decreased to a new steady state within 5 days. Seasonal differences in biofilm retention of oocysts were observed. The spring biofilm retained the greatest percentage of oocysts, followed (in decreasing order) by the winter, summer, and fall biofilms. There was no statistically significant correlation between the percentage of oocysts attached to the biofilm and (i) any measured stream water quality parameter (including temperature, pH, conductivity, and dissolved organic carbon concentration) or (ii) experimental temperature. Seasonal differences in oocyst retention persisted when biofilms were tested with stream water from a different season. These data suggest that seasonal variation in the microbial community and resulting biofilm architecture may be more important to oocyst transport in this stream site than water quality. The biofilm attachment and detachment dynamics of C. parvum oocysts have implications for public health, and the drinking water industry should recognize that the potential exists for pathogen-free water to become contaminated during the distribution process as a result of biofilm dynamics.
Cryptosporidium is a genus of waterborne protozoan parasites that cause a gastrointestinal disease in humans (cryptosporidiosis) that can be prolonged and life-threatening for people with compromised immune systems. Recent advances in medical treatment for cryptosporidiosis exist but are not entirely effective for immunocompromised patients (1). In addition, conventional water treatment does not effectively target Cryptosporidium oocysts because the oocysts' small size (4 to 8 μm) limits the ability of filters to remove them and, more importantly, oocysts are resistant to chlorine (25). Therefore, environmental control of Cryptosporidium is important to protect public health. To determine the risk of human exposure and infection, the fate and transport of Cryptosporidium oocysts in the environment, including biofilms, should be examined.
Within the past two decades, biofilms have been recognized as ubiquitous habitats found on most surfaces exposed to water (20, 24). Environmental biofilms can rapidly accumulate pathogens at densities much higher than water column densities, and the potential for gradual or sudden pathogen loss from the biofilm exists long after entrapment (8, 22). Biofilm sloughing events are commonplace, occurring when a biofilm detaches from the substrate to be resuspended as large particles in the water column, and may result in the release of pathogen reservoirs from the biofilm into the water column (8).
Biofilms have been identified as a possible contamination source for drinking water supplies, which may lead to infections for which the source cannot be identified (7, 9). An example of the impact of biofilm sloughing events on human health is seen in the cryptosporidiosis outbreak that occurred in Lancashire County, England, in March 2000 (10). After the outbreak, the oocyst source was identified as cattle feces from adjacent farmland that contaminated the drinking water after abnormally heavy rainfall. The water source was subsequently changed to two upland impounding reservoirs containing filtered surface water. However, oocysts persisted in the water distribution system for 19 days, with large peaks associated with major water main disturbance events, including the initial flushing of the system and a burst in the main supply pipe. This persistence of oocysts in the water supply was attributed to the release of oocysts trapped in biofilms on the interior surface of the distribution pipes and may have contributed to additional infections.
Several studies have examined pathogen transport dynamics in biofilms using glass or latex beads of various sizes as surrogates for pathogens (5, 8, 16, 17). A few studies examined the attachment of C. parvum oocysts to biofilms but did not use natural microbial assemblages to make the biofilms (3, 23) or quantify how many oocysts attached or sloughed (9, 22). Rogers and Keevil (22) showed that oocysts attached to a biofilm composed of a natural microbial assemblage collected from a reservoir at a concentration of 1,400 oocysts/cm2 after the addition of 108 oocysts in 10 ml of sterile water. Dai and Hozalski (3) and Searcy et al. (23) used pure culture biofilms to demonstrate oocyst attachment; however, only Searcy et al. (23) accounted for sloughing, although no oocyst release from the biofilm was seen during the course of their experiments. Helmi et al. (9) noted attachment and detachment of oocysts from a natural biofilm but did not include a quantitative analysis to account for all oocysts in the flow system over time. None of these studies examined pathogen attachment seasonally over the course of a year. Seasonal changes in temperature, precipitation, and water quality (including nutrient availability) may have significant impacts on the microbial composition and functional structure of a biofilm (14). These changes include structural changes in the biofilm thickness and morphology, as well as changes in the water composition and suspended matter. In addition, seasonal changes in stream flow dynamics may alter biofilm composition and morphology, as well as oocyst attachment and release patterns.
This study provides novel information about C. parvum oocyst attachment to biofilms grown in the laboratory from natural microbial assemblages collected seasonally (i.e., in January, April, July, and October) from Monocacy Creek in Bethlehem, PA. Previous work (26) showed that (i) a significant fraction of C. parvum oocysts adhered to the surface of experimental biofilms during a 3-day oocyst dosing period, (ii) a portion of the adhered oocysts immediately released from the biofilm, and (iii) a portion of the oocysts remained attached to the biofilm for a period of days after termination of oocyst dosing. Here, we test the hypotheses that (i) oocyst retention by biofilms varies seasonally and (ii) seasonal changes in water quality influence oocyst retention.
MATERIALS AND METHODS
Preparation of biofilm inoculum.
Using a plastic brush, biofilms were gently scraped from rocks in Monocacy Creek (Bethlehem, PA) into 500 ml of creek water in July 2008 (summer biofilm), October 2008 (fall biofilm), January 2009 (winter biofilm), and April 2009 (spring biofilm). The 500-ml biofilm suspension was vacuum filtered through a 6-μm-pore-size cellulose filter paper that removed algae (which were not evenly distributed throughout the natural biofilm and would not survive laboratory experiments performed in the absence of sunlight), large particles that would clog the flow system tubing, and grazers that could disturb the biofilm structure and consume oocysts. The filtrate was centrifuged (1,754 × g for 15 min), and the resulting biofilm cell pellet was resuspended in 1 ml of filtered creek water. Cell concentration was quantified by DAPI staining (21). Cells were split into aliquots (5 × 106 cells each) and stored in cryovials of filtered creek water containing 30% glycerol. Cells were stored at −80°C until used to inoculate flow chambers.
Water quality parameters.
Approximately 20 liters of creek water were collected at the same time as the biofilms, filter sterilized (0.22-μm-pore-size filter) within 24 h of collection, and stored at 4°C until used for an experiment. Water quality analysis was performed with 500 ml of the collected water, and the remaining 19.5 liters was kept to use as medium in the flow chamber systems. The creek water temperature at the time of biofilm collection was measured in the field by using a mercury thermometer. After filtration, the pH (Orion Ross Series electrode; Thermo Scientific, Waltham, MA) and conductivity (Orion model 105; Thermo Scientific) were measured in the laboratory within 24 h of collection. Dissolved organic carbon (DOC) concentration was measured by using a Shimadzu (Columbia, MD) total organic carbon analyzer (TOV-V CPH).
Oocysts.
Mouse-shed C. parvum oocysts (Iowa isolate) were obtained from Waterborne, Inc. (New Orleans, LA), and used within 3 weeks of shedding.
Experimental setup.
Single-channel convertible flow chambers (24 by 8 by 4 mm [length by width by height]) with glass coverslips (Stovall Life Science, Inc., Greensboro, NC) were inoculated with 5 × 106 Monocacy Creek biofilm cells for 24 h before the flow was started. Filter-sterilized Monocacy Creek water (collected at the same time as each biofilm culture, except for the water exchange experiment described below) was used as the flow medium for all experiments. A 12-channel peristaltic pump (IPC pump; Ismatec, Glattbrugg, Switzerland) was used to maintain a constant flow of 0.2 mm/s (0.96 ml/s) for all experiments (2).
All four seasonal biofilms were grown on the laboratory benchtop at room temperature (20 to 25°C). An additional experiment, to assess the impact of experimental temperature on biofilm growth and oocyst retention, was performed with the winter culture biofilm grown (i) on the benchtop at the experimental temperature (20 to 25°C) (identical to the other experiments) and (ii) in an incubator at the ambient temperature of Monocacy Creek when the biofilm culture was harvested (5°C). To test the impact of seasonal differences in water chemistry on biofilm growth and oocyst retention, a water exchange experiment was performed by using Monocacy Creek biofilms collected in the fall with Monocacy Creek water collected in the spring as the flow medium. The opposite experiment, using Monocacy Creek biofilm collected in the spring with Monocacy Creek water collected in the fall, was also performed. These data sets were compared to the experiments using the fall and spring biofilms grown with water collected at the same time as the biofilm.
For mass balance analysis, C. parvum oocysts (104/day) were added to 500 ml of constantly stirred influent water each day for 3 days. The 3-day oocyst dosing period was followed by 5 days of filtered creek water flow without oocyst addition. Influent water was replaced each day, and remaining influent and effluent waters at the end of each 24-hour flow period were processed as described below to recover and quantify oocysts. The total number of oocysts that passed through the flow chamber system was calculated by subtracting the number of oocysts remaining in the influent water after 24 h from the total added to the influent reservoir each day (104). The number of oocysts in the effluent was determined by direct counts using a hemacytometer. The percentage of oocysts in the biofilm after each 24 h period was calculated from the difference between the number of oocysts that passed through the flow chamber system and the number of oocysts in the effluent after each 24-h period; the number calculated at the end of the 8 days matched (within 7%) the number determined by direct count of oocysts recovered from the biofilm.
Oocyst recovery from flow system.
After each 24-h flow period, remaining influent and effluent waters were processed by membrane filtration and immunomagnetic separation (IMS) to recover and purify oocysts. Membrane filtration was performed as described previously (15) with a slight modification: only the 3-μm-pore-size filter was used (no macropore prefilter was used). IMS was performed on the biological material retained on the membrane filter using the Virusys IMS kit (Virusys Co., Sykesville, MD) according to the manufacturer's recommendations; oocysts were dissociated from the magnetic beads by using 0.05 M HCl, and the oocyst suspension was neutralized by using 0.5 M NaOH.
At the end of each experiment, the flow was stopped, and the biofilm thickness was measured at four locations across the length of each flow chamber using bright-field DICT settings on a scanning confocal microscope (Zeiss LSM 510 META laser scanning microscope). The biofilm was scraped from the flow chambers using plastic sterile loops, resuspended in sterile creek water, and processed by membrane filtration and IMS. The IMS products of all samples were counted on a hemacytometer. Hemacytometer counts were corrected for membrane filtration and IMS processing losses. An average IMS recovery of 65% ± 4.2% (determined by four trials using 104 oocysts in deionized water) was used. Membrane filtration recoveries were found to be consistent within each day but varied between days. As a result, a membrane filtration recovery control was performed each day using 104 oocysts in 1 liter of deionized water to obtain a daily correction factor for membrane filtration. The summer biofilm data presented here were also presented by Wolyniak et al. (26).
Statistical analysis.
Independent t tests were performed to determine whether there was a significant difference between (i) the percentage of oocysts attached to the biofilm at day 3 versus day 8 within any given season, (ii) the percentage of oocysts attached to the biofilm at day 3 between seasons, (iii) the percentage of oocysts attached to the biofilm at day 8 between seasons, (iv) the percentage of oocysts attached to the biofilm at day 3 and day 8 using fall biofilm cultures and fall or spring water, (v) the percentage of oocysts attached to the biofilm at day 3 and day 8 using spring biofilm cultures and fall or spring water, and (vi) the average biofilm thickness between seasons. Spearman correlation coefficients were used to determine whether there were any correlations between (i) the percentage of oocysts attached to the biofilm at day 3 or day 8 and any of the water quality parameters tested (i.e., temperature, pH, specific conductivity, and DOC concentration) and (ii) the standard deviation of biofilm thickness measurements (a measure of surface roughness) and the percentage of oocysts attached to the biofilm at day 3 or day 8. All statistical analyses were performed with the Analyze-It add-in (Analyze-It Software, Ltd., Leeds, England) for Microsoft Excel.
RESULTS
Mass balance analysis accounted for oocysts added to the flow system with errors of <7% (Table 1) . In a control flow chamber with no biofilm growth (i.e., a clean glass surface), 1% or less of the oocysts were lost within the system, indicating that very few oocysts attached to any abiotic surface within the flow chamber or tubing.
TABLE 1.
Oocyst retention and biofilm thicknessa
| Season | % Oocysts ± SE |
Avg biofilm thickness (μm) | ||
|---|---|---|---|---|
| In biofilm at end of dosing (day 3) | In biofilm at end of expt (day 8) | Accounted for in flow system | ||
| Summer 2008 | 64 ± 3.2 | 28 ± 0.05 | 107 ± 0.93 | 42 ± 3.5 |
| Fall 2008 | 46 ± 4.5 | 24 ± 0.67 | 104 ± 0 | 28 ± 5.1 |
| Winter 2009 | 72 ± 0.89 | 47 ± 3.2 | 97.5 ± 0.51 | 34 ± 10 |
| Spring 2009 | 78 ± 1.4 | 65 ± 1.7 | 94.5 ± 0.53 | 25 ± 11 |
| Winter 2009 (20°C) | 77 ± 0.62 | 54 ± 1.4 | 94.5 ± 1.6 | 30 ± 12 |
| Winter 2009 (5°C) | 76 ± 0.31 | 53 ± 2.7 | 103.5 ± 0.48 | 33 ± 11 |
The table presents a summary of the percentage of oocysts attached to each biofilm at the end of the dosing period (day 3) and end of the experiment (day 8), the total percent of oocysts accounted for in the flow system, and the average biofilm thickness for each set of seasonal biofilms. All errors are percent standard error (n = 2, except biofilm thickness, where n = 8).
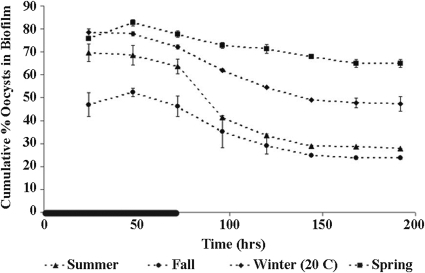
Oocyst attachment to each seasonal biofilm quickly reached a steady state while oocysts were supplied to the system via the inflow and decreased to a new steady state once the oocyst supply was removed from the inflow (Fig. 1 and 2). The decrease in oocyst attachment after the dosing period terminated was significant for all seasonal biofilms: there was a significant difference in the number of oocysts attached to each biofilm at day 3 (i.e., the end of the oocyst dosing period) versus day 8 (i.e., 5 days after oocyst dosing was terminated) for all biofilms (winter, P < 0.01; spring, P = 0.01; summer, P < 0.01; fall, P < 0.01; winter, 20°C, P < 0.01; winter, 5°C, P < 0.01).
FIG. 1.
Seasonal changes in oocyst retention by biofilms using a standard laboratory water temperature (20 to 25°C). The cumulative percentage of oocysts associated with the biofilm over time for summer, fall, winter, and spring biofilm cultures was determined. Error bars show the percent standard error (n = 2) and are smaller than the symbols where not visible. The black line on the time axis indicates the period of oocyst dosing (0 to 72 h).
FIG. 2.
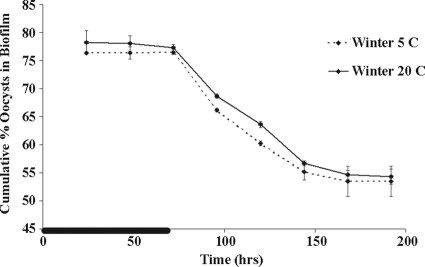
Testing the effect of experimental water temperature on oocyst retention by biofilm. The cumulative percentage of oocysts associated with the biofilm over time with winter culture biofilm grown at 5°C (ambient biofilm collection temperature) and at 20 to 25°C (normal laboratory experimental temperature) was determined. Error bars indicate the percent standard error (n = 2) and are smaller than the symbols where not visible. The black line on the time axis indicates the period of oocyst dosing (0 to 72 h).
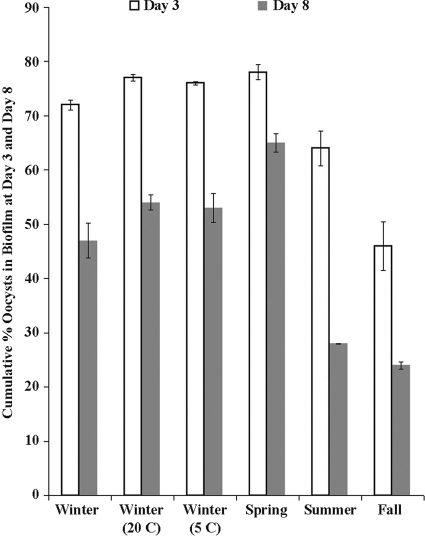
The steady-state fractions of attached oocysts varied seasonally, with spring biofilms holding the most oocysts, followed by (in decreasing order) winter, summer, and fall biofilms (Fig. 1 and 3). The number of oocysts attached to the biofilm at day 3 was significantly different (P < 0.05) among all of the seasonal biofilms, with the exception of the summer and winter biofilms (P = 0.12). The summer biofilms were significantly thicker than the fall (P < 0.0001), winter (P = 0.05), and spring (P < 0.0001) biofilms (Table 1).
FIG. 3.
Cumulative percentage of oocysts associated with the biofilm at the end of oocyst dosing (day 3) and the end of the experiment (day 8). The error bars indicate the percent standard error (n = 2).
The experimental temperature used in the microcosm experiments (20 to 25°C except for the one winter temperature experiment that was also run at the ambient temperature of Monocacy Creek when the winter biofilm culture was harvested, 5°C) did not affect oocyst attachment on the one occasion that it was tested (March 2009) (Fig. 2). The percentage of oocysts attached to the winter biofilm at 20°C on day 3 (average, 77.3%, n = 2) was not statistically different (P = 0.25) from the percentage of oocysts attached to the winter biofilm at 5°C on day 3 (average, 76.4%, n = 2). In addition, the percentage of oocysts attached to the winter biofilm at 20°C at the end of the experiment (day 8) (average, 54.3%, n = 2) was not statistically different (P = 0.65) than the percentage of oocysts attached to the winter biofilm at 5°C at the end of the experiment (day 8) (average, 53.5%, n = 2).
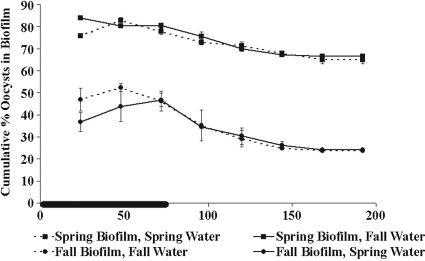
In the experiment to test for a water chemistry effect (reciprocal exposure of spring and fall biofilms to fall and spring stream water), the large seasonal difference in oocyst retention persisted regardless of the water used in the test (Fig. 4). The percentage of oocysts attached to the fall biofilm grown with spring water was not statistically different from the percentage of oocysts attached to the fall biofilm grown with fall water at either day 3 (P = 0.88) or day 8 (P = 0.27). Similarly, there was no statistical difference between the percentages of oocysts attached to the spring biofilm grown with fall water and the spring biofilm grown with spring water at either day 3 (P = 0.19) or day 8 (P = 0. 31).
FIG. 4.
Effect of changing flow media on oocyst retention by biofilm. The cumulative percentage of oocysts associated with the biofilm over time with fall biofilm grown with fall water, fall biofilm grown with spring water, spring biofilm grown with fall water, and spring biofilm grown with spring water was determined. The error bars show the percent standard error (n = 2) and are smaller than the symbols where not visible. The black line on the time axis indicates the period of oocyst dosing (0 to 72 h).
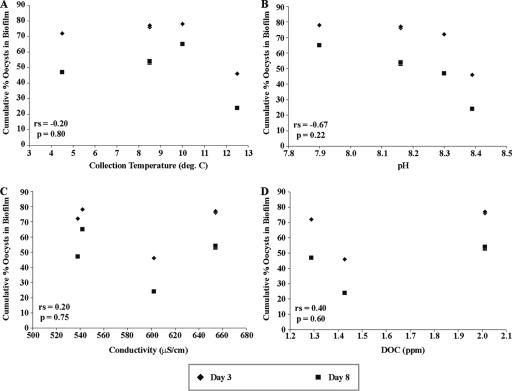
A trend of decreasing oocyst attachment with increasing water pH was observed (Spearman correlation coefficient [rs] = −0.67); however, this trend was not statistically significant (P = 0.22) (Fig. 5). No other correlations were observed between oocyst attachment and collection temperature, specific conductivity, or DOC concentration of the water (Fig. 5).
FIG. 5.
Correlation of several water quality measures and oocyst retention by biofilms. The percentages of oocysts attached to biofilms at day 3 and day 8 versus water temperature at the time of biofilm collection (A), pH (B), conductivity (C), and DOC concentration (D) were determined. Spearman correlation coefficients and P values for each parameter are indicated on each panel.
A trend of increasing oocyst attachment with increasing standard deviation of biofilm thickness (an indication of surface roughness) was observed (rs = 0.60) but was not statistically significant (P = 0.21).
DISCUSSION
The methods used for these experiments were effective in tracking oocysts throughout the flow system during the course of the experiment and allowed a full mass balance analysis to be performed without concern about oocyst attachment to the abiotic surfaces in the system.
As seen previously (26), oocyst attachment to the biofilms quickly reached a steady state, and a portion of the oocysts remained attached to the biofilm after the oocyst supply was removed. Flood and Ashbolt (8) also saw biofilm deposition of 100-nm latex surrogate beads reach a steady state within 24 h and remain stable over 7 months, with the concentration of beads in the biofilm at the end of the 7-month experiment 2 orders of magnitude higher than the greatest water concentration. Helmi et al. (9) similarly reported that most attachment occurred within the first hour of biofilm exposure to C. parvum oocysts. Similar to our findings, Helmi et al. (9) also reported a declining release of oocysts from the biofilm between days 2 and 34 (after a single day of oocyst dosing on day 1), with ca. 30% of the oocysts remaining in the biofilm on day 34. These data suggest that biofilms are potentially significant reservoirs of oocysts that can be released back into the water column over a period of a few days and possibly longer, even after a water supply has been classified as safe. The seasonal differences seen in the present study support our first hypothesis (Fig. 1 and 3) and compound the unknown public health risks associated with oocysts in biofilms.
The quick steady-state deposition of particles has been found to be independent of cell density in the biofilm or biofilm thickness, but dependent on the particle surface properties (i.e., surface charge and hydrophobic properties) and the physical properties of the biofilm (i.e., surface roughness and pore size) (12, 23). Accordingly, biofilm thickness did not appear to play a role in how many oocysts attached to the biofilms in the present study, as also shown by others (5, 12, 16, 17). In the present study, summer biofilms were the only biofilms that were significantly different in thickness, but their thicker biomass did not yield an increase in the number of oocysts attached to the biofilm (Table 1). Although not significant, a trend of increasing oocyst attachment with increasing surface roughness (measured as the standard deviation of biofilm thickness measurements) was observed, as seen by others using 1-μm microbeads (5) and C. parvum oocysts (23). In addition, the experimental conditions (specifically, experimental temperature) did not appear to impact oocyst attachment to the biofilms either (Fig. 2).
Particle surface properties may have caused some of the differences in oocyst attachment between biofilms. C. parvum oocysts are neutral around pH 2 to 3 and become increasingly negatively charged as the pH increases (11). In addition, biofilm surfaces are generally negatively charged as a result of the presence of anionic carboxyl, sulfate, and phosphoryl groups in the biofilm matrix (6). A trend of decreasing oocyst attachment with increasing pH was observed in the present study, which is consistent with the electrostatic repulsion that would be expected as oocysts become more negatively charged at higher pH levels. However, this relationship is not significant, suggesting that there may be other forces influencing the interaction. Alternatively, others have shown that dissolved cations can bind to oocyst surface proteins and neutralize these proteins that extend from the oocyst surface, thus reducing steric repulsion (11). Further analysis of the waters used here and similar experiments using a wider range of water chemistries are needed to explore this influence of water chemistry.
Oocyst release from the biofilm was observed throughout our experiments, in contrast to Searcy et al. (23), who observed no oocyst release after 24 h of oocyst-free water flow through the biofilm system or with a 40-fold increase in flow velocity. These differences may be a result of the type of biofilm (Searcy et al. used a pure culture Pseudomonas aeruginosa biofilm) or the chemistry of the flow media (Searcy et al. used laboratory growth media to promote biofilm growth, whereas filtered creek water was used here). In addition, the difference in biofilm thickness between the pure culture biofilm used by Searcy et al. (2.81 to 7.68 μm thick) and the environmental biofilm used in the present study (25 to 42 μm thick) may have led to the additional sloughing seen here. Previous studies have shown increased sloughing with increasing biofilm thickness as a result of increased shear stress (4, 13). Similar to our findings, Helmi et al. (9) also observed a continuous, yet declining oocyst release from biofilms over 32 days.
In our study, experimental biofilms were seen to be in a steady state of growth and sloughing after the 24-h inoculation period (i.e., the biofilm thickness was consistent). Biofilm sloughing into the effluent was visible and necessitated the use of IMS to purify oocysts for counting. Because oocysts attached to and remained at the biofilm surface (26), which is the area most greatly affected by sloughing, oocysts were likely released from the biofilm as a result of sloughing. It is not possible in this system to distinguish between oocysts that passed through the system without attaching to the biofilm and those that attached and subsequently sloughed. The oocysts that remained in the biofilm in the days after the oocyst dosing period likely attached to more stable or sheltered portions of the biofilm that did not slough.
Two possible factors controlling the percentage of oocyst attachment to the biofilm are the water quality and the characteristics of the biofilm itself. Searcy et al. (23) noted that, along with biofilm architecture, surface-chemical interactions and background water chemistry affect pathogen deposition in biofilms, as reflected by the significant difference in the fraction of C. parvum oocysts that attached to the biofilm using two different growth medias (LB broth and Jensen medium). Experiments performed by Searcy et al. (23) showed that changing the growth medium impacts the surface charge of oocysts and therefore affects their attachment to a pure culture biofilm. This change in flow medium chemistry could be analogous to water chemistry changes with season; however, we did not see any significant changes across seasons in the bulk water chemistry in the present study (Fig. 5), and none of the water quality parameters tested (biofilm collection temperature, pH, conductivity, and DOC concentration) showed any significant correlation with the percentage of oocysts that attached to the biofilm (Fig. 5). In addition, biofilms grown with water from a different season showed no significant difference in oocyst attachment from biofilms grown with water collected at the same time as the biofilm culture (Fig. 4). Because the large seasonal differences in oocyst retention between fall and spring biofilms persisted when tested with stream water from the other season, it is likely that oocyst retention is influenced by seasonal changes in the microbial community. Our hypothesis that there is a direct effect of water quality on oocyst retention is not supported by these data.
Because no clear correlation between oocyst attachment to biofilms and any measured water quality parameter was found, and changing the flow water produced no difference in oocyst attachment, the observed seasonal trend of oocyst attachment (Fig. 1) may be a result of the biofilm architecture, biofilm community composition, and the resulting extrapolymeric substance (EPS) and protein production. Moss et al. (14) reported distinct biofilm compositions that were consistent throughout the summer and winter. Seasonal changes in composition may affect the biofilm architecture and/or EPS production and, in turn, affect oocyst attachment. In fact, we observed a trend linking a higher standard deviation of biofilm thickness measurements (an indication of surface roughness) to a higher percentage of oocysts attached to the biofilm, which is further evidence that a change in biofilm architecture may influence oocyst attachment. Although the water quality data presented here show no correlation with oocyst attachment, larger changes in these water quality parameters as well as others, including nutrient and ion concentrations, may influence oocyst attachment patterns. The water quality parameters measured here showed a small range over the course of the year. Future testing using biofilms from streams with larger water quality ranges may reveal a role of water chemistry in oocyst attachment.
Biofilm composition has been reported to be affected by seasonal changes, including temperature, nutrients, and sunlight (14, 27). For example, the source and molecular weight of DOC can vary seasonally and affect its biolability and, subsequently, the microbes that can use that carbon source and the overall composition, structure, and functioning of the biofilm community (18, 19, 27). Further study of oocyst attachment to biofilms from sites with different sources and concentrations of DOC is in progress and will help to determine whether DOC plays a role in how oocysts attach to the biofilm.
The results shown here for the Monocacy Creek winter biofilm are significantly different from those presented for one prior experiment with a winter biofilm culture from the same creek (26). In the previous experiment, the biofilm held 40% of the oocysts on day 3 and 4.8% of the oocysts on day 5 (which was the end of the experiment), compared to the 72% on day 3 and 47% on day 8 observed in the current study. No water quality data was collected in January 2007. However, these differences may be explained by the differences in weather patterns during the 2 years in which biofilms were collected, including air temperature, which would impact stream water temperature and the microbial communities present. The biofilm used in the previous experiments (26) was collected in January 2007, when the monthly average and range for air temperature (average, 1.8°C; monthly range, −9.4 to 17°C) were higher than January 2009 (average, −2.5°C; monthly range, −11 to 5.1°C). Although experimental temperature did not affect oocyst attachment in laboratory experiments, the higher air temperatures in January 2007 could have resulted in a different biofilm community composition as a result of the higher temperature itself or as a result of secondary effects including the availability and type of DOC (due to changes in organic material decomposition rates and algal production). Future analysis of the biofilm community compositions may aid in explaining the observed seasonal and intra-annual differences in oocyst attachment. Although we cannot rule out the influence of changes in water quality on oocyst retention by biofilms in other streams, we suggest that for Monocacy Creek, seasonal and intra-annual variations in the microbial community composition, perhaps mediated by average weather conditions, have the greatest influence on oocyst retention. The data do not suggest that any biofilm from a stream in a given season will necessarily behave the same each year. The biofilms present at any given time will reflect the stream and weather conditions at that time, which may average out to show a seasonal response over long periods, but will vary year to year.
The work presented here has important implications for the drinking water industry because oocysts that remain in biofilms long-term may pose a public health threat. Water utilities routinely monitor microbial concentrations in raw and finished waters, but rarely, if ever, monitor biofilms because of a lack of standardized sampling and laboratory protocols and the challenge of accessing biofilms without disrupting the water distribution system. Therefore, increased knowledge of pathogen dynamics in biofilms is important to understand the public health threat posed by biofilms in raw water supplies and water distribution systems. Continued work will aid in determining a mechanism for oocyst attachment to biofilms by further examining seasonal trends of oocyst attachment across streams with different water chemistries.
Acknowledgments
This research was funded by National Science Foundation CAREER award 0545687 to K.L.J.
Footnotes
Published ahead of print on 18 December 2009.
REFERENCES
- 1.Abuvakar, I., S. H. Aliyu, C. Arumugam, N. K. Usman, and P. R. Hunter. 2007. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br. J. Clin. Pharmacol. 63:387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen, B. B., C. Sternberg, J. B. Anderson, R. J. Palmer, A. T. Nielsen, M. Givskov, and S. Molin. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20-42. [DOI] [PubMed] [Google Scholar]
- 3.Dai, X., and R. M. Hozalski. 2002. Effect of NOM and biofilm on the removal of Cryptosporidium parvum oocysts in rapid filters. Water Res. 36:3523-3532. [DOI] [PubMed] [Google Scholar]
- 4.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drury, W. J., W. G. Characklis, and P. S. Stewart. 1993. Interactions of 1-μm latex particles with Pseudomonas aeruginosa biofilms. Water Res. 27:1119-1126. [DOI] [PubMed] [Google Scholar]
- 6.Flemming, H. C. 1995. Sorption sites in biofilms. Water Sci. Technol. 32:27-33. [Google Scholar]
- 7.Flemming, H. C., S. L. Percival, and J. T. Walker. 2002. Contamination potential of biofilms in water distribution systems. Water Sci. Technol. 2:271-280. [Google Scholar]
- 8.Flood, J. A., and N. J. Ashbolt. 2000. Virus-sized particles can be entrapped and concentrated one hundred fold within wetland biofilms. Adv. Environ. Res. 3:403-411. [Google Scholar]
- 9.Helmi, K., S. Skraber, C. Gantzer, R. Willame, L. Hoffman, and H. Cauchie. 2008. Interactions of Cryptosporidium parvum, Giardia lamblia, vaccinal poliovirus type I, and bacteriophages φX174 and MS2 with a drinking water biofilm and a wastewater biofilm. Appl. Environ. Microbiol. 74:2079-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howe, A. D., S. Forster, S. Morton, R. Marshall, K. S. Osborn, P. Wright, and P. R. Hunter. 2002. Cryptosporidium oocysts in a water supply associated with a cryptosporidiosis outbreak. Emerg. Infect. Dis. 8:619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuznar, Z. A., and M. Elimelech. 2004. Adhesion kinetics of viable Cryptosporidium parvum oocysts to quartz surfaces. Environ. Sci. Technol. 38:6839-6845. [DOI] [PubMed] [Google Scholar]
- 12.Långmark, J., M. V. Storey, N. J. Ashbolt, and T. A. Stenström. 2005. Accumulation and fate of microorganisms and microspheres in biofilms formed in a pilot-scale water distribution system. Appl. Environ. Microbiol. 71:706-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgenroth, E., and P. A. Wilderer. 2000. Influence of detachment mechanisms on competition in biofilms. Water Res. 34:417-426. [Google Scholar]
- 14.Moss, J. A., A. Nocker, J. E. Lepo, and R. A. Snyder. 2006. Stability and change in estuarine biofilm bacterial community diversity. Appl. Environ. Microbiol. 72:5679-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oda, T., M. Sakagame, H. Ito, S. K. Yano, H. Rai, M. Kawabata, and S. Uga. 2000. Size selective continuous flow filtration method for detection of Cryptosporidium and Giardia. Water Res. 34:4477-4481. [Google Scholar]
- 16.Okabe, S., H. Kuroda, and Y. Watanabe. 1998. Significance of biofilm structure on transport of inert particulates into biofilms. Water Sci. Technol. 38:163-170. [Google Scholar]
- 17.Okabe, S., T. Yasuda, and Y. Watanabe. 1997. Uptake and release of inert fluorescence particles by mixed population biofilms. Biotechnol. Bioeng. 53:459-469. [DOI] [PubMed] [Google Scholar]
- 18.Olapade, O. A., and L. G. Leff. 2005. Seasonal response of stream biofilm communities to dissolved organic matter and nutrient enrichments. Appl. Environ. Microbiol. 71:2278-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olapade, O. A., and L. G. Leff. 2006. Influence of dissolved organic matter and inorganic nutrients on the biofilm bacterial community on artificial substrates in a northeastern Ohio, USA, stream. Can. J. Microbiol. 6:540-549. [DOI] [PubMed] [Google Scholar]
- 20.Percival, S. L., J. T. Walker, and P. R. Hunter. 2000. Microbiological aspects of biofilms and drinking water. CRC Press, Inc., New York, NY.
- 21.Porter, K. G., and Y. S. Keig. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 22.Rogers, J., and C. W. Keevil. 1995. Survival of Cryptosporidium parvum oocysts in biofilm and planktonic samples in a model system, p. 209-213. In W. B. Betts, D. Casemore, C. Fricker, H. Smith, and J. Watkins (ed.), Protozoan parasites and water. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 23.Searcy, K. E., A. I. Packman, E. R. Atwill, and T. Harter. 2006. Capture and retention of Cryptosporidium parvum oocysts by Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 72:6242-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szewzyk, U., R. Szewzyk, W. Manz, and K. H. Schleifer. 2000. Microbiological safety of drinking water. Annu. Rev. Microbiol. 54:81-127. [DOI] [PubMed] [Google Scholar]
- 25.Weir, S. C., N. J. Pokorny, R. A. Carreno, J. T. Trevors, and H. Lee. 2002. Efficacy of common laboratory disinfectants on the infectivity of Cryptosporidium parvum oocysts in cell culture. Appl. Environ. Microbiol. 68:2576-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolyniak, E. A., B. R. Hargreaves, and K. L. Jellison. 2009. Retention and release of Cryptosporidium parvum oocysts by experimental biofilms composed of a natural stream microbial community. Appl. Environ. Microbiol. 75:4624-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ylla, I., C. Borrego, A. M. Romani, and S. Sabater. 2009. Availability of glucose and light modulates the structure and function of a microbial biofilm. FEMS Microbiol. Ecol. 69:27-42. [DOI] [PubMed] [Google Scholar]