Abstract
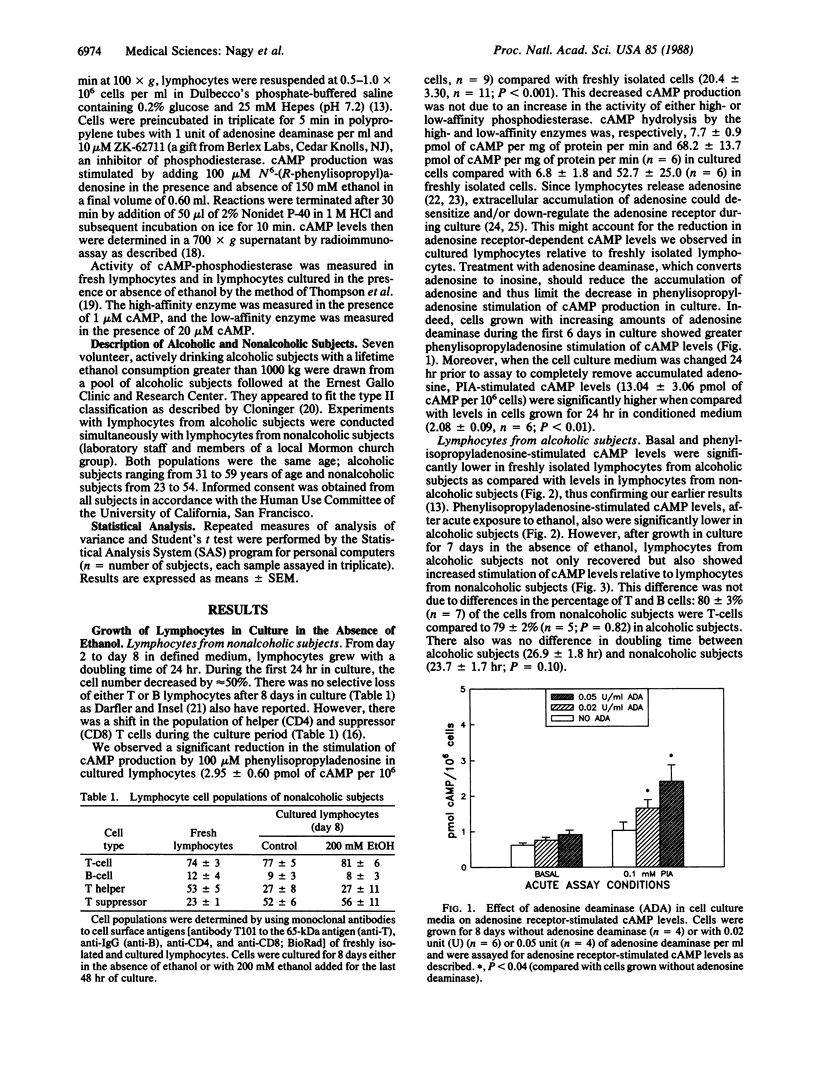
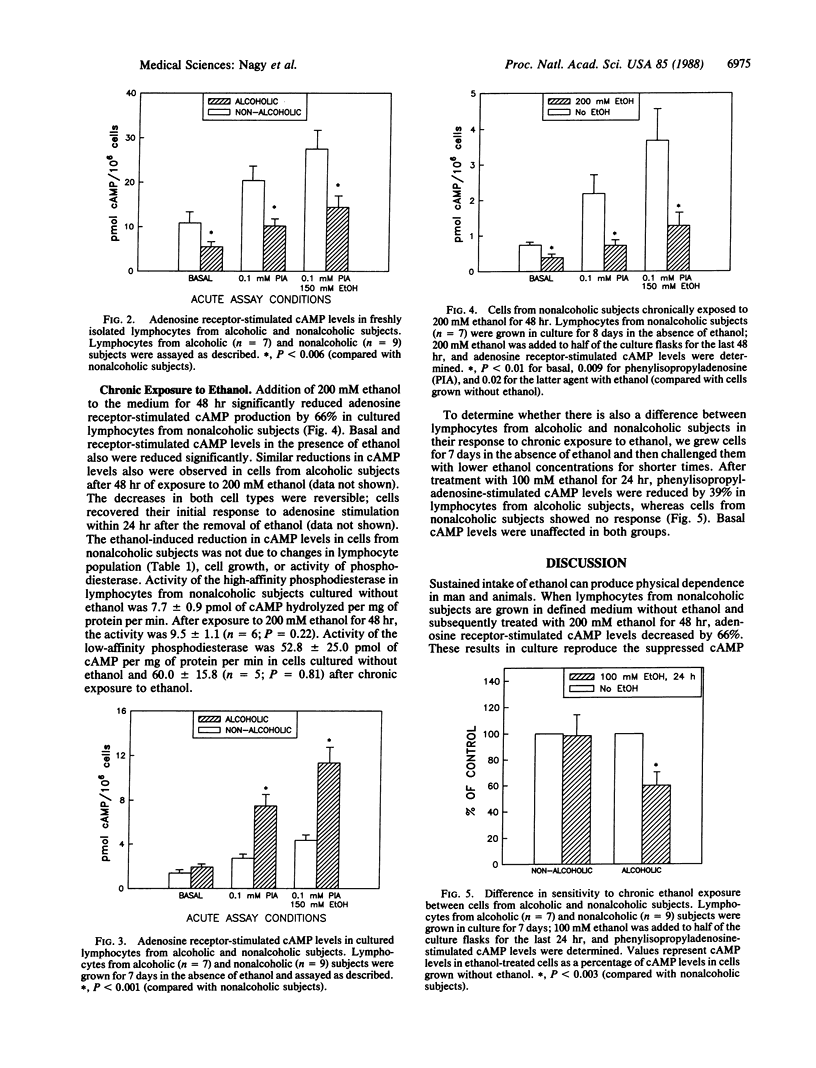
Previous work has shown that freshly isolated lymphocytes from alcoholic subjects show significantly reduced basal and adenosine receptor-stimulated cAMP levels. This decrease could be due to ethanol-induced cellular adaptation or to a genetic difference in the regulation of cAMP signal transduction. Therefore, we cultured human lymphocytes in defined medium without ethanol for 7-8 days and then examined differences in receptor-dependent cAMP accumulation between lymphocytes from alcoholic and nonalcoholic subjects. After four to six generations in culture without ethanol, lymphocytes from alcoholic subjects have significantly higher cAMP levels than do cells from nonalcoholic subjects. Thus, a difference in cAMP signal transduction is demonstrable in cells from alcoholic subjects grown without ethanol. We also found that cultured lymphocytes from both alcoholic and nonalcoholic subjects show a decrease in receptor-stimulated cAMP levels after exposure to 200 mM ethanol for 48 hr. To determine whether alcoholic subjects have increased sensitivity to ethanol, lymphocytes were exposed to only 100 mM ethanol for 24 hr. Under these conditions, receptor-dependent cAMP levels did not change in cells from nonalcoholic subjects. However, lymphocytes from alcoholic subjects showed a 39% decrease (P less than 0.003) in adenosine receptor-stimulated cAMP levels. Taken together, the results show that (i) chronic ethanol treatment in culture reproduces the suppression of cAMP levels found in circulating lymphocytes from alcoholic subjects and (ii) despite four to six cell divisions in culture without ethanol, lymphocytes from alcoholic subjects exhibit significantly increased adenosine receptor-dependent cAMP levels and increased sensitivity to chronic exposure to ethanol. These findings suggest that the suppression of cAMP levels observed in freshly isolated lymphocytes from alcoholic subjects results from both a direct effect of chronic exposure to ethanol and a genetic difference leading to altered cAMP signal transduction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cloninger C. R. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987 Apr 24;236(4800):410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Dar M. S., Mustafa S. J., Wooles W. R. Possible role of adenosine in the CNS effects of ethanol. Life Sci. 1983 Oct 3;33(14):1363–1374. doi: 10.1016/0024-3205(83)90819-6. [DOI] [PubMed] [Google Scholar]
- Darfler F. J., Insel P. A. Serum-free culture of resting, PHA-stimulated, and transformed lymphoid cells, including hybridomas. Exp Cell Res. 1982 Apr;138(2):287–295. doi: 10.1016/0014-4827(82)90177-x. [DOI] [PubMed] [Google Scholar]
- Diamond I., Wrubel B., Estrin W., Gordon A. Basal and adenosine receptor-stimulated levels of cAMP are reduced in lymphocytes from alcoholic patients. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1413–1416. doi: 10.1073/pnas.84.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis G., Goldberg D. M. A reduced nicotinamide adenine dinucleotide--linked kinetic assay for adenosine deaminase activity. J Lab Clin Med. 1970 Sep;76(3):507–517. [PubMed] [Google Scholar]
- Fredholm B. B., Sandberg G., Ernström U. Cyclic AMP in freshly prepared thymocyte suspensions, Evidence for stimulation by endogenous adenosine. Biochem Pharmacol. 1978;27(23):2675–2682. doi: 10.1016/0006-2952(78)90041-2. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Collier K., Diamond I. Ethanol regulation of adenosine receptor-stimulated cAMP levels in a clonal neural cell line: an in vitro model of cellular tolerance to ethanol. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2105–2108. doi: 10.1073/pnas.83.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. L., Tabakoff B. Ethanol does not modify opiate-mediated inhibition of striatal adenylate cyclase. J Neurochem. 1986 Mar;46(3):812–816. doi: 10.1111/j.1471-4159.1986.tb13044.x. [DOI] [PubMed] [Google Scholar]
- Hynie S., Lanefelt F., Fredholm B. B. Effects of ethanol on human lymphocyte levels of cyclic AMP. In vitro: Potentiation of the response to isoproterenol, prostaglandin E2 or adenosine stimulation. Acta Pharmacol Toxicol (Copenh) 1980 Jul;47(1):58–65. doi: 10.1111/j.1600-0773.1980.tb02026.x. [DOI] [PubMed] [Google Scholar]
- Kenimer J. G., Nirenberg M. Desensitization of adenylate cyclase to prostaglandin E1 or 2-chloroadenosine. Mol Pharmacol. 1981 Nov;20(3):585–591. [PubMed] [Google Scholar]
- Mochly-Rosen D., Chang F. H., Cheever L., Kim M., Diamond I., Gordon A. S. Chronic ethanol causes heterologous desensitization of receptors by reducing alpha s messenger RNA. Nature. 1988 Jun 30;333(6176):848–850. doi: 10.1038/333848a0. [DOI] [PubMed] [Google Scholar]
- Newby A. C., Holmquist C. A. Adenosine production inside rat polymorphonuclear leucocytes. Biochem J. 1981 Nov 15;200(2):399–403. doi: 10.1042/bj2000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. E., Levitzki A. Desensitization of normal rat kidney cells to adenosine. Biochem Pharmacol. 1983 Jan 1;32(1):137–140. doi: 10.1016/0006-2952(83)90665-2. [DOI] [PubMed] [Google Scholar]
- Proctor W. R., Dunwiddie T. V. Behavioral sensitivity to purinergic drugs parallels ethanol sensitivity in selectively bred mice. Science. 1984 May 4;224(4648):519–521. doi: 10.1126/science.6324348. [DOI] [PubMed] [Google Scholar]
- Rabin R. A., Molinoff P. B. Activation of adenylate cyclase by ethanol in mouse striatal tissue. J Pharmacol Exp Ther. 1981 Jan;216(1):129–134. [PubMed] [Google Scholar]
- Richelson E., Stenstrom S., Forray C., Enloe L., Pfenning M. Effects of chronic exposure to ethanol on the prostaglandin E1 receptor-mediated response and binding in a murine neuroblastoma clone (N1E-115). J Pharmacol Exp Ther. 1986 Dec;239(3):687–692. [PubMed] [Google Scholar]
- Saito T., Lee J. M., Hoffman P. L., Tabakoff B. Effects of chronic ethanol treatment on the beta-adrenergic receptor-coupled adenylate cyclase system of mouse cerebral cortex. J Neurochem. 1987 Jun;48(6):1817–1822. doi: 10.1111/j.1471-4159.1987.tb05741.x. [DOI] [PubMed] [Google Scholar]
- Saito T., Lee J. M., Tabakoff B. Ethanol's effects on cortical adenylate cyclase activity. J Neurochem. 1985 Apr;44(4):1037–1044. doi: 10.1111/j.1471-4159.1985.tb08722.x. [DOI] [PubMed] [Google Scholar]
- Stenstrom S., Enloe L., Pfenning M., Richelson E. Acute effects of ethanol and other short-chain alcohols on the guanylate cyclase system of murine neuroblastoma cells (clone N1E-115). J Pharmacol Exp Ther. 1986 Feb;236(2):458–463. [PubMed] [Google Scholar]
- Stenstrom S., Richelson E. Acute effect of ethanol on prostaglandin E1-mediated cyclic AMP formation by a murine neuroblastoma clone. J Pharmacol Exp Ther. 1982 May;221(2):334–341. [PubMed] [Google Scholar]
- Tabakoff B., Hoffman P. L., Lee J. M., Saito T., Willard B., De Leon-Jones F. Differences in platelet enzyme activity between alcoholics and nonalcoholics. N Engl J Med. 1988 Jan 21;318(3):134–139. doi: 10.1056/NEJM198801213180302. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Terasaki W. L., Epstein P. M., Strada S. J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv Cyclic Nucleotide Res. 1979;10:69–92. [PubMed] [Google Scholar]
- Valverius P., Hoffman P. L., Tabakoff B. Effect of ethanol on mouse cerebral cortical beta-adrenergic receptors. Mol Pharmacol. 1987 Aug;32(1):217–222. [PubMed] [Google Scholar]


