Abstract
Over the past two decades, extensive research has focused on arterial remodelling in both physiological and pathological ageing. The concept now describes the growth as well as the rearrangement of cellular components and extracellular matrix, resulting in either reduction or increase in vessel lumen. In diabetes, remodelling extends to capillaries, microvascular beds, and arteries of different calibre. This process is paralleled by accelerated atherosclerosis and accounts for an increased incidence of ischaemic complications. The incapacity of pre-existing and de novo formed collaterals to bypass atherosclerotic occlusions, combined with a decline in tissue capillary density, is responsible for the delayed recovery from ischaemia and ultimately leads to organ failure. The mechanisms of vascular remodelling are incompletely understood, but metabolic and mechanical factors seem to play an important role. Hyperglycaemia represents the main factor responsible for the fast progression of atherosclerosis as well as microangiopathy. However, intensive blood glucose control alone is insufficient to reduce the risk of macrovascular complications. Pharmacological control of oxidative stress and stimulation of nitric oxide release have proved to exert beneficial effects on vascular remodelling in experimental diabetic models. New approaches of regenerative medicine using vascular progenitor cells for the treatment of ischaemic disease have been shown to be safe and are now being tested for efficacy in preclinical and clinical trials.
Keywords: Diabetes, Remodelling, Angiogenesis, Endothelial progenitor cells, Advanced glycation end products
1. Introduction
With the number of people with diabetes mellitus (DM) rising exponentially, the disease represents one of the greatest medical and socioeconomic challenges worldwide. Despite adapted treatment strategies, vascular complications represent the leading cause of morbidity and mortality in diabetic patients.1 Therefore, nutritional and environmental interventions, together with new mechanistic therapies, are urgently needed to combat the new epidemics of ischaemic disease.
DM-associated vascular disease manifests with endothelial cell (EC) dysfunction, follows structural changes of large and small arteries with tissue hypoperfusion and hypoxia. These alterations recapitulate, in an accelerated version, the process of arterial remodelling with associated senescence that occurs with ageing.2 In particular, diabetic subjects frequently show signs of accelerated atherosclerosis, undergo acute coronary syndromes, myocardial infarction with silent myocardial ischaemia, peripheral artery disease, and stroke.3 Despite advances in interventional techniques, DM portends an adverse outcome following revascularization, and intimal hyperplastic remodelling still represents a common complication in diabetic patients.4 Furthermore, DM impairs endogenous reperfusion mechanisms, i.e. activation of pre-existing arterial collaterals and generation of neo-vessels by arteriogenesis and angiogenesis, thereby worsening the recovery from an ischaemic insult.5,6 This is aggravated by the concurrent development of microvascular complications. Limb muscle microangiopathy, together with peripheral neuropathy, is a key determinant in the pathogenesis of life-threatening foot ulcers, which affect 10% of diabetic patients. Proliferative retinopathy, a major cause of blindness, is present in more than 50% of patients with advanced type 1 DM. Nephropathy affects 35% of diabetic subjects and can evolve in chronic renal failure.3,7
Vessel integrity, once believed to be maintained exclusively by resident cells, is now recognized to be supported by bone marrow (BM)-derived endothelial progenitor cells (EPC).8 Furthermore, local and BM-derived progenitor cells seemingly participate in re-endothelialization and arterial remodelling after vascular injury.9 In both types of DM, vascular disease has been shown to be associated with a decline in EPC number and function. Common biochemical alterations affecting both mature EC and EPC might therefore concur in diabetic vascular complications.10,11
This review illustrates the heterogeneous mechanisms of cellular and extracellular vascular wall remodelling, focusing on possible therapeutic targets for the prevention and treatment of diabetic complications.
2. Vascular remodelling
Remodelling affects capillaries and arteries of different calibre in both physiological (e.g. ageing) and pathological (e.g. atherosclerosis, hypertension, and diabetes) conditions. The term remodelling was originally coined for a complex set of vascular changes induced by chronic hypertension, including altered phenotype and function of EC and vascular smooth muscle cells (VSMC), as well as the extracellular matrix (ECM) structure and composition, leading to altered vessel wall-to-lumen ratio.12,13 Principally, mechanical factors (wall shear stress, wall circumferential stress) and hypoxia determine hypertensive vascular remodelling.14 In DM, metabolic factors, e.g. hyperglycaemia and oxidative stress, are important for microvascular remodelling. Interestingly, control of hyperglycaemia alone, while improving microvascular function, exerts only modest benefit on macrovascular complications.15 This is in keeping with frequent association of DM with additive macrovascular remodelling risk factors, i.e. hypertension. In type 2 DM patients, concomitant hypertension causes enhanced inward remodelling of small arteries and attenuation of vessel dilation.16 In this respect, specifically DM-associated vascular remodelling comprises chemical and biological modifications of the ECM, altered function of EC and VSMC, and changes in circulating cells/EC interaction via adhesion molecules, cytokines, and proteases (Figure 1). At the macrovascular level, these alterations cause the characteristic intima and media (IM) thickening that, along with increased stiffness and decreased vasomotion, are predictive for a high risk for cardiovascular events.17
Figure 1.
Alterations within intima and media of diabetic vessels. Endothelial cell apoptosis, reduced generation of nitric oxide (NO), and loss of endothelial cell junctions allow infiltration of macrophages and extravasation of plasma proteins. Leukocyte adhesion is facilitated by endothelial expression of adhesion molecules, aiding monocyte/macrophage infiltration and ultimately foam cell generation. Increased protein entrapment in the extracellular matrix, together with increased matrix deposition, and reduced degradation result in higher matrix volume. Low NO levels promote vascular smooth muscle cell (VSMC) proliferation and impede relaxation. Infiltrating macrophages and VSMC further increase intima/media (IM) thickness. Secretion of thrombogenic factors accelerates platelet adhesion and thrombus formation.
2.1 Extracellular alterations
Increased IM thickness and vessel rigidity in type 2 DM patients result in an higher pulse wave velocity, decreased compliance, and luminal dilatation.17 We will briefly focus on mechanisms responsible for wall stiffening, namely calcium and matrix protein deposition, increased protein glycoxidation, together with altered matrix degradation by matrix metalloproteinases (MMPs) and impaired VSMC relaxation. Calcification and increased expression of mediators of osteogenic differentiation of VSMC and pericytes are also seen in atherosclerotic plaques from non-diabetic patients;18 altered insulin and glucose levels, however, accelerate this process.19,20 Increased deposition of matrix proteins within the diabetic vessel wall was described several decades ago. Hyperglycaemia and subsequently advanced glycation end products (AGEs) were shown to increase basement membrane components and fibronectin expression in cultured EC.21-23 The term AGE comprises a multitude of non-enzymatically glycosylated proteins and lipids with altered chemical and biological properties. Specifically, in plasma and locally at the site of vascular complications, increased glucose levels induce protein glycation. Early glycation products slowly degrade to form several different AGEs.24 AGEs have emerged as key substances in diabetic vascular remodelling, mediating extra-cellular modifications, such as impaired ECM flexibility and increased matrix area by cross-linking of matrix proteins, e.g. through entrapment of molecules, such as low-density lipoproteins (LDL),25,26 intracellular signalling, and cell–cell interaction (vide infra) (Figure 1). In addition, AGEs can reduce the activity of MMPs, a family of endopeptidases involved not only in matrix degradation, but also in cell migration, proliferation, and survival.27,28 MMP activity is controlled at several levels, (i) gene expression, (ii) proteolytic activation of secreted pro-MMPs, and (iii) inhibitors (TIMPs) or activators (e.g. EMMPRIN). In the diabetic vessel wall, AGEs interfere with this system through alterations in activator protein 1 (AP-1) and transforming growth factor beta (TGF-β) signalling,29,30 and specific expression of MMP subtypes.31-33 Expression of MMPs and their regulators furthermore differs among different vascular cells in physiologic and disease conditions as well as in culture.34-36 Altered cellular composition of the diabetic vessel wall (EC depletion, macrophage, and VSMC increased invasion/proliferation) aggravates the imbalance between different MMP (vide infra). In DM, differences in substrate specificity and altered expression/activity of individual MMPs might partly explain the increased plaque instability, the higher ECM volume and rigidity, and the reduced vascular healing capacity.
2.2 Intracellular alterations
2.2.1 Endothelial cells
Because of their incapacity to regulate glucose influx, EC represent a unique target for DM-induced damage. Protein glycation and excessive generation of reactive oxygen species (ROS) impair EC function and viability.37 A more ‘leaky’ endothelial layer results in increased extravasation of plasma proteins. Among the mechanisms responsible for enhanced permeability is reduced density of tight and adherens junctions in diabetic EC due to decreased expression and increased destruction by proteases like μ-calpain.38 In small vessels, also the loss of pericytes contributes to hyper-permeability (vide infra).
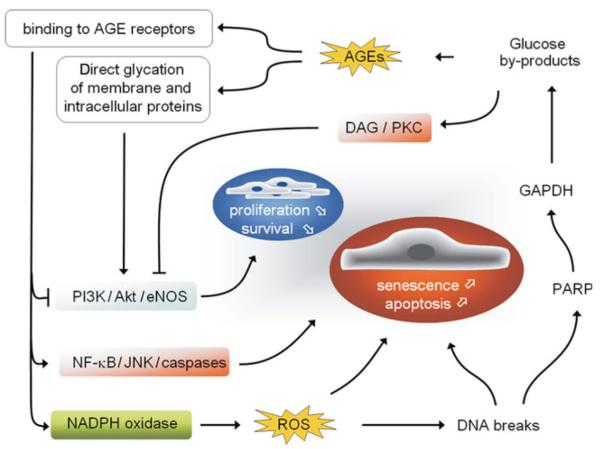
EC signalling is furthermore affected by glycation of ECM components, AGE receptor (e.g. RAGE) binding, and glycation/glycoxidation of intracellular proteins and transcription factors.37,39 RAGE signalling and high intracellular glucose levels increase both mitochondrial and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent ROS generation (Figure 2).40,41 While low ROS level is a physiological signalling mechanism for EC, e.g. promoting EC proliferation,42 excessive ROS is cytotoxic, and contributes to impaired angiogenesis and diabetic cardiovascular complications.43,44 In DM, ROS levels rise due to excessive superoxide production accompanied by inadequacy of scavenger mechanisms. Loss of the antioxidant enzyme glutathione peroxidase-1 aggravates atherosclerosis in diabetic mice, while administration of antioxidants rescues impaired endothelial function.45-47 High ROS levels induce DNA strand breaks. Poly (ADP ribose) polymerase (PARP) is then activated in the attempt of repairing the DNA damage. In chronic hyperglycaemia, however, this protective mechanism is detrimental. PARP inhibits glyceraldehyde phosphate dehydrogenase (GAPDH), with consequent accumulation of glucose by-products, which fuel AGE and diacylglycerol (DAG)/protein kinase C (PKC) pathways and thereby amplify ROS-induced endothelial damage (Figure 2).48 Both high glucose and ROS promote EC apoptosis through nuclear factor-kappaB (NFκB) and c-Jun NH2-terminal kinase (JNK) pathways by caspase activation (Figure 2).49,50
Figure 2.
Reactive oxygen species (ROS) induced damage in diabetic endothelial cells. Reactive oxygen species-induced DNA strand breaks induce upregulation of poly(ADP ribose) polymerase (PARP) that in turn inhibits glyceraldehyde phosphate dehydrogenase (GAPDH). The resulting glucose by-products provide substrates for both advanced glycation end product (AGE) formation and for the diacylglycerol (DAG)/protein kinase C (PKC) pathway. AGE receptors activate intracellular signalling cascades leading to apoptosis through inhibition of PI3K/Akt/eNOS signalling and activation of the NF-κB and c-Jun NH2-terminal kinase (JNK) pathways as well as NADPH oxidase-dependent ROS generation.
RAGE activation stimulates a variety of intracellular signalling pathways, including the mitogen-activated protein kinase (MAPK) pathway via apoptosis signal-regulating kinase 1 (ASK1).51 Interestingly, ASK1 activation causes transcriptional induction of plasminogen activator inhibitor-1 (PAI-1), a major inhibitor of fibrinolysis that may contribute to delayed resolution of thrombosis in diabetic patients.52 Caspase activation is normally under the inhibitory control of the phosphatidylinositol 3-kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) axis.53 PI3K signalling through Akt is a powerful pro-survival and pro-angiogenic mechanism, resulting among other effects, in the phosphorylation/activation of eNOS and nitric oxide (NO) generation.54 In atherosclerosis, and especially within the diabetic vessel wall, eNOS is dysfunctional.55 Besides reduced phosphorylation of eNOS at Ser1177, due to decreased Akt activity, and eNOS inactivation via the DAG/PKC pathway, eNOS dysfunction is ascribed to oxidation of the cofactor tetrahydrobiopterin (BH4), a mechanism referred to as ‘eNOS uncoupling’.56 Uncoupled eNOS produces oxygen radicals instead of NO, adding to the increased oxidative stress and to reduced NO levels.
2.2.2 Vascular smooth muscle cells
Situated within ECM with enhanced rigidity and beneath a layer of EC, which fail to provide appropriate levels of vasoactive substances,57 diabetic VSMC meet strong counteracting conditions. Those external factors are accompanied by VSMC failure to respond to vasoactive stimuli. Although VSMC are exposed to the same cues as EC, namely high glucose and oxidative stress, their response is the opposite with regard to proliferation and migration capacities, contributing to the disease-associated progressive atherosclerosis and restenosis.58,59
The signalling cascades involved are similar in VSMC and in EC, including ROS and AGE generation increase, mediated by RAGE, NADPH oxidase, PKC, and NFκB pathways combined with reduction in PI3K/Akt/eNOS signalling. Both, insulin and glucose enhance the proliferative ability of cultured VSMC and reduce apoptosis,60 but the underlying mechanisms are still not fully understood. In vitro, high glucose down-regulates PKC-β in VSMC, resulting in the activation of proliferation.61 Other PKC isoforms, however, show different behaviour: PKC-α up-regulation by hyperglycaemia is responsible for the increased expression of growth factors (GFs) and their receptors, such as TGF-β and TGF-β receptor-1.62 PKC signalling is also implicated in hyperglycaemia-mediated reduction of VSMC apoptosis.63
VSMC contractility and survival are also controlled by the renin–aldosterone–angiotensin system, with its effector angiotensin II (AngII). High glucose enhances AngII response, through up-regulation of the AngII AT1 receptor, and enhanced ROS production.64,65 Activation of extracellular signal-regulated kinases (ERK)1/2, JNK, and p38 MAPK by AngII is enhanced by high glucose, and in turn results in AngII up-regulation in VSMC.65,66 Other signs of vascular remodelling attributed to AngII comprise vessel wall calcification, enhanced permeability, and monocyte infiltration. Increased VSMC migration in DM is furthermore attributed to RAGE-mediated up-regulation of cytokines and GFs, e.g. TGF-β1, platelet-derived growth factor (PDGF), and tumour necrosis factor α.67 Digestion of the internal elastic lamina by MMPs furthermore facilitates VSMC migration/invasion of the intimal layer.
2.2.3 Pericytes/podocytes
In microvessels, EC and basal lamina are surrounded by a discontinuous layer of pericytes in direct contact with EC through gaps in the basal lamina. A similar position is maintained by the podocytes in renal glomeruli. These cell types through both direct cell–cell contact and paracrine signalling, regulate EC survival, proliferation, and migration, and stabilize nascent neovessels during angiogenesis. Pericytes share characteristics with VSMC, e.g. contractility in response to vasoactive stimuli, and in vitro studies suggest that they can give rise to VSMC. However, it is still unclear whether pericytes are VSMC precursors, or both independently derived from a common progenitor, with pericytes maintaining higher plasticity.
Under physiologic conditions oxygen induces pericyte contraction. High levels of oxidative stress, however, evoked by high glucose and AGEs, induce pericyte and podocyte apoptosis through mechanisms similar to those in EC. For example, forkhead box transcription factors, known mediators of apoptosis, are activated upon p38 activation and Akt dephosphorylation in the presence of glycated collagen.68 These effects are amplified by loss of insulin-mediated pro-survival signalling.69 Moreover, high glucose up-regulates phagocyte-type NAD(P)H oxidase in pericytes increasing ROS production.70 Increased MMP-2 activity and reduced TIMP3 expression in DM concur in promoting pericyte apoptosis via detachment from the matrix.71-73 Similar to pericytes, podocytes are lost in renal glomeruli as a consequence of ROS-induced apoptosis.74 Furthermore, modulation of ion channel activity, e.g. P2X7 purinoceptors, might contribute to accelerated pericyte/podocyte death, as recently postulated.75-77 Pericyte impairment, as in the diabetic retina, leads to uncontrolled growth of immature and ‘leaky’ vessels, easily broken causing haemorrhagic damage and vision loss.
2.3 Cell–cell interactions
Diabetes-associated EC dysfunction facilitates vascular inflammation via GF and cytokine secretion, and adhesion molecules expression.78 Increased leukocyte affinity to EC has been linked to the pathogenesis of diabetic microangiopathy and atherogenesis.79,80 Furthermore, facilitated leukocyte trans-endothelial migration (TEM) due to increased endothelial permeability and cytokine/GF generation contributes to IM thickening and plaque instability, symptomatic for diabetic atherosclerosis.81,82 High insulin and glucose levels increase adhesion molecule expression on leukocytes and EC, assisting initial rolling and later firm adhesion preliminary to TEM.83,84 As discussed before, both AGEs and AngII activate transcription factors (NF-κB and AP-1) in a ROS-dependent manner, inducing adhesion molecule gene expression.85 Consistently, RAGE signalling inhibition, NF-κB inactivation, and ROS scavenging reduce monocyte adhesion to the endothelium and retard the development of vasculopathies in diabetic patients.86-88 Excessive thrombus formation in diabetic patients is attributed at least in part to AngII-mediated up-regulation of PAI-1 in response to RAGE activation. Furthermore, glycoxidation of diabetic platelet cell membrane proteins is associated with accelerated aggregation and decreased sensitivity towards aspirin.89,90 Finally, AGEs in the trans-endothelial space induce the production of chemoattractants for monocytes.86 This process is auto-amplifying, since activated monocytes that infiltrate the vessel wall produce ROS and inflammatory cytokines, which in turn stimulate ROS generation in VSMC. The above-described mechanisms of DM-induced atherogenesis follow the pattern observed during arterial ageing,91,92 indicating common mechanisms for arterial remodelling, e.g. eNOS uncoupling and systemic insulin resistance.93,94
3. Impairment of angiogenesis and vasculogenesis
Clinical and experimental evidence indicates that altered remodelling of arterial collaterals as well as de novo vascularization play a key role in impaired recovery from ischaemia in DM. We and others used a model of severe hind limb ischaemia to investigate the cellular and molecular mechanisms of disturbed angiogenesis in diabetic animal models.6,95
3.1 Angiogenesis inducers and inhibitors
Both forms of diabetes feature an insufficient surge of endothelial GFs at sites of ischaemia, namely members of the vascular endothelial GF (VEGF) and insulin-like GF families, hindering reparative neovascularization.6,96 Impaired VEGF signalling translates into reduced monocyte chemotaxis to sites of ischaemia, where those cells are putatively implicated in the formation of new arterial collaterals.97 Studies from Tanii et al.98 contradict, however, the primary involvement of VEGF-mediated mechanisms.
Microangiopathy and peripheral neuropathy often develop concomitantly and aggravate each other. The neurotrophin nerve growth factor (NGF) is produced by EC, which also express NGF receptors.99 In DM, impaired NGF signalling together with overexpression of the neurotrophin-related death receptor p75 increases EC apoptosis and impairs wound healing.100,101 Other neuropeptides from sensory neurons are implicated in angiogenesis and their deficit could participate in the delayed repair of diabetic ulcers.102
Disequilibrium of angiogenesis promoters and inhibitors can lead to exuberant but dysfunctional neovascularization, as seen in the diabetic retina, as well as vascular destabilization, as observed in skeletal and cardiac muscle, thus supporting a high degree of heterogeneity of diabetic vascular pathology.103,104
3.2 Vasculogenesis: dysfunction of diabetic endothelial progenitor cells
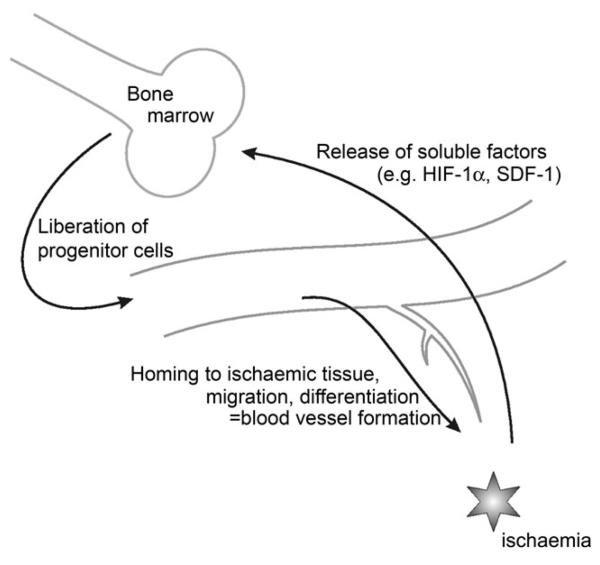
Neovascularization accomplished with contribution of stem cells (SC) and BM-derived EPC, termed post-natal vasculogenesis, is a multi-step process cooperating with regeneration facilitated by resident vascular cells (Figure 3). Circulating EPC from type 2 DM patients are numerically and functionally altered and correlate inversely with levels of haemoglobin A1C and cardiovascular risk factors.10 The finding of an association between EPC dysfunction and cardiovascular complications is important but not sufficient to draw pathogenetic conclusions. Furthermore, the specific location and mechanisms of EPC damage and reduction remain unknown. The initial stage of vasculogenesis is represented by BM–SC activation and transmigration to the central BM ‘vascular niche’.105,106 BM–SC express high levels of antioxidant enzymes.107 Not surprisingly, therefore, oxidative stress does not play a major role in high glucose-induced EPC dysfunction.108 A recent report from Li et al.109 indicates that circulating EPC number in diabetic mice is significantly reduced with arterial injury; however, the number of EPC in BM in diabetic mice was greater. This important observation underlines the possibility that DM-induced EPC damage may occur after liberation from the BM, with exposure to high glucose. Those findings also lead us to speculate that the EPC increase in BM of diabetic animals may represent a compensatory mechanism for increased mortality of those cells in the circulation.
Figure 3.
Ischaemia-induced vasculogenesis. In response to ischaemia, secreted soluble factors, like hypoxia-inducible factor-1α (HIF-1α) and stromal cell-derived factor-1, induce proliferation, differentiation, and liberation of vascular progenitor cells from the BM. In the tissue, EPC adhere to the vessel wall and migrate along gradients of chemotactic factors (e.g. VEGF, SDF-1).
We previously showed that moderate increase in glucose levels impairs cell cycling and migration and increases apoptosis of cultured human EPC.36 Consistently, human diabetic CD34+ progenitor cells show altered migratory ability towards stromal cell-derived factor-1 (SDF-1).110 Furthermore, diabetic EPC show impaired integrative capacity in neovasculature of ischaemic organs and reduced re-endothelialization ability after arterial injury.109 Recent evidence shows that eNOS uncoupling is central in EPC mobilization and function in humans as well as in a DM animal model.111,112 Elevation of asymmetric dimethylarginine, an endogenous NOS inhibitor, in DM, could contribute to this phenomenon.113 Glycoxidated proteins are suspected for the reduced availability and dysfunction of EPC in DM, as in vitro studies showed that activation of the Akt/p53/p21 pathway in healthy EPC cultured in the presence of oxidized small and dense LDL (ox-dmLDL), results in a senescent-like growth arrest.114,115 Apart from endogenous liabilities, diabetic EPC release unidentified factors that accelerate microvascular EC ageing.10,11 The mobilization of SC from BM to sites of injury is considered instrumental to the repair and stabilization of vascular damage.18 Initial evidence suggests however that DM could convert this mechanism into an adverse process, with recruitment of pro-inflammatory progenitors prevailing on those endowed of regenerative potential. In addition to SC from distant sources, local adventitial progenitor cells may contribute to vascular remodelling. Following vascular injury, those progenitor cells migrate into the intima and differentiate into smooth muscle cells116 but are also capable of participating in the formation of peri-adventitial vascular sprouts, thus establishing the basement for arterial collateralization.117 Thus, adventitial progenitor cells might play a Dr Jekyll–Mr Hyde role in the development of arteriosclerosis, angioplasty-induced restenosis, vein graft atherosclerosis, and reparative vascular growth.
4. Regenerative therapies
New mechanistic insights in the pathogenesis of endothelial dysfunction were rapidly translated into new therapeutic opportunities. Among emerging strategies, ROS and AGE scavengers, PKCβ inhibitors,118-121,122 and potentiation of eNOS activity with BH4, statins, and thiazolidinediones (glitazones) reportedly alleviate endothelial dysfunction in diabetic animals.123,124 Clinical trials demonstrated the ability of statins and glitazones to reduce the incidence of cardiovascular events such as myocardial infarction and stroke in diabetic patients (see Hamilton et al.125 for review). In this final section of this review, we will concentrate on new approaches of regenerative vascular medicine, namely therapeutic angiogenesis and SC therapy. Based on the hypothesis that VEGF signalling is decreased in the diabetic heart, Yoon et al.126 injected a plasmid DNA encoding VEGF165 in the myocardium of diabetic rats, thereby restoring microvascular homeostasis and preventing heart failure development. In addition, VEGF gene therapy proved to stimulate angiogenesis in ischaemic limbs of diabetic mice.6 It was argued that the short duration and leaky neovascularization induced by this GF may be worrisome in diabetes. Furthermore, different VEGF isoforms and splice variants have been shown to induce different signalling mechanisms, suggesting better contemplation before clinical usage of this protein. There has been, in general, a disappointing outcome regarding angiogenesis in clinical trials,127 with only recombinant human PDGF-BB being clinically approved for the treatment of diabetic neuropathic ulcers, yet none for ischaemic ulcers.128 This claims for the introduction of pleiotropic angiogenic agents able to address the multi-factorial determinants of diabetic endotheliopathy. Our group previously showed that gene therapy with human kallikrein prevents endothelial dysfunction and microangiopathy in limb muscles of mice with type 1 DM.95,129,130 Several mechanisms of kallikrein action in this setting are conceivable, including improvement of ECM flexibility by its protease function and increased kinin release. With superimposed limb ischaemia, kallikrein promotes arterial collateralization through generation of kinins and NO, but independently of VEGF.131 The kinin pathway has furthermore been shown to mediate the pro-angiogenic effects of angiotensin-converting enzyme inhibition in DM.104
Schatteman and colleagues132 first analysed the role of EPC in DM-related microangiopathies. CD34+ circulating cells from type 1 DM patients produced fewer EC per ml of blood and exogenous non-diabetic CD34+ cells accelerated blood flow recovery in a diabetic model of limb ischaemia. Moreover, heterologous transplantation of non-diabetic BM-derived progenitor cells promotes vasculogenesis and wound healing in type 2 DM mice, whereas homologous, diabetic progenitor cells favour cicatrization but inhibit vasculogenesis.133 Thus autologous SC therapy for diabetic vascular regeneration may have limitations both intrinsic in progenitor cells and imposed by the diabetic environment. Furthermore, differences may exist with regard to EPC function and curative properties in type 1 and 2 DM. Finally, a crucial point determining the therapeutic benefit of EPC is represented by their ability to secrete pro-angiogenic factors, which may be diminished or substituted by inflammatory cytokines in diabetic EPC.
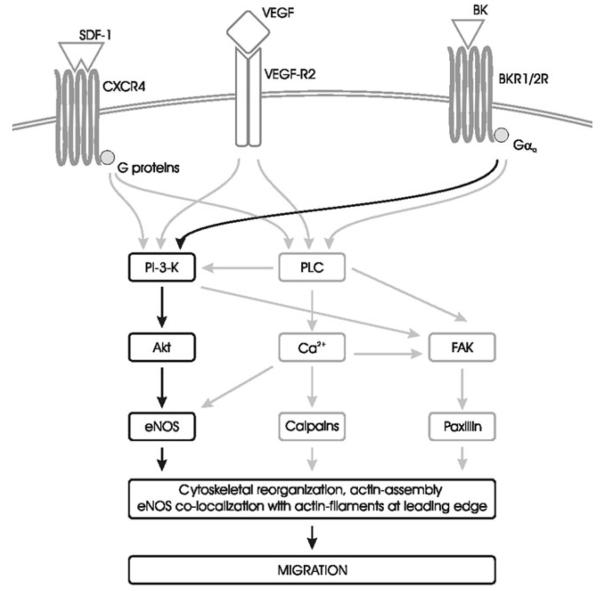
Future directions include attempting to rescue those functional defects and improving EPC recruitment/engraftment.134 One candidate is NO, which is a common mediator of intracellular pathways in EC and EPC (Figure 4). Recent studies indicate statin and, to an even greater extent, NO-donating statins potently stimulate reparative angiogenesis and arteriogenesis in type 1-DM.135 Of note, statins improve the migratory and survival capacity of EPC via the PI3K/Akt/eNOS axis and NO-donating statins further amplify these effects.135
Figure 4.
Intracellular signalling pathways for migration. Migration can be activated by heterogeneous chemokine gradients through a redundant array of receptors. Two major classes of receptors are implicated: G protein-coupled receptors and tyrosine kinase receptors. PI3K and phospholipase C (PLC) activation of the Akt/eNOS pathway increases intracellular calcium and induce focal adhesion assembly, thus mediating cytoskeletal reorganization. BK, bradykinin; BKR1/2R, bradykinin receptor 1 or 2; FAK, focal adhesion kinase.
5. Summary
EC and EPC dysfunction pairs with a complex set of cellular and structural modifications within the vessel wall leading to diabetic vascular complications. We believe that EPC will have a central role in new targeted therapies for diabetes angiopathies, but more data establishing their nature and function are to be achieved.
Funding
British Heart Foundation (PG/06/035/20641, PG/06/096/21325); Juvenile Diabetes Foundation Research (1/2004/124); European Federation for the Study of Diabetes; Ministero della Pubblica Istruzione Università e Ricerca (MIUR) (RBIN04N8YA to P.M.); Ministero della Salute (PSCARDIO ex art.56/12/2005 to P.M.); Wellcome trust (WT083018 to N.K.); British Heart Foundation Basic Science Lectureship to C.E.
Footnotes
Conflict of interest: none declared.
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi N. The burden of diabetes and its complications: trends and implications for intervention. Diabetes Res Clin Pract. 2007;76:S3–S12. doi: 10.1016/j.diabres.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Mathew V, Gersh BJ, Williams BA, Laskey WK, Willerson JT, Tilbury RT, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation. 2004;109:476–480. doi: 10.1161/01.CIR.0000109693.64957.20. [DOI] [PubMed] [Google Scholar]
- 5.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 6.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Diabetic retinopathy. Diabetes Care. 2003;26:S99–S102. doi: 10.2337/diacare.26.2007.s99. [DOI] [PubMed] [Google Scholar]
- 8.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 9.Inoue T, Sata M, Hikichi Y, Sohma R, Fukuda D, Uchida T, et al. Mobilization of CD34-positive bone marrow-derived cells after coronary stent implantation: impact on restenosis. Circulation. 2007;115:553–561. doi: 10.1161/CIRCULATIONAHA.106.621714. [DOI] [PubMed] [Google Scholar]
- 10.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 11.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 12.Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension. 1993;21:391–397. doi: 10.1161/01.hyp.21.4.391. [DOI] [PubMed] [Google Scholar]
- 13.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, et al. Vascular remodeling. Hypertension. 1996;28:505–506. [PubMed] [Google Scholar]
- 14.Pries AR, Reglin B, Secomb TW. Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension. 2005;46:725–731. doi: 10.1161/01.HYP.0000184428.16429.be. [DOI] [PubMed] [Google Scholar]
- 15.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schofield I, Malik R, Izzard A, Austin C, Heagerty A. Vascular structural and functional changes in type 2 diabetes mellitus: evidence for the roles of abnormal myogenic responsiveness and dyslipidemia. Circulation. 2002;106:3037–3043. doi: 10.1161/01.cir.0000041432.80615.a5. [DOI] [PubMed] [Google Scholar]
- 17.Henry RM, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Kamp O, et al. Carotid arterial remodeling: a maladaptive phenomenon in type 2 diabetes but not in impaired glucose metabolism: the Hoorn study. Stroke. 2004;35:671–676. doi: 10.1161/01.STR.0000115752.58601.0B. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson FL, Liu Y, Rucka AK, Jeziorska M, Hoyland JA, Heagerty AM, et al. Contribution of VCAF-positive cells to neovascularization and calcification in atherosclerotic plaque development. J Pathol. 2007;211:362–369. doi: 10.1002/path.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen NX, Duan D, O'Neill KD, Moe SM. High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol Dial Transplant. 2006;21:3435–3442. doi: 10.1093/ndt/gfl429. [DOI] [PubMed] [Google Scholar]
- 20.Olesen P, Nguyen K, Wogensen L, Ledet T, Rasmussen LM. Calcification of human vascular smooth muscle cells: associations with osteoprotegerin expression and acceleration by high-dose insulin. Am J Physiol Heart Circ Physiol. 2007;292:H1058–H1064. doi: 10.1152/ajpheart.00047.2006. [DOI] [PubMed] [Google Scholar]
- 21.Cagliero E, Maiello M, Boeri D, Roy S, Lorenzi M. Increased expression of basement membrane components in human endothelial cells cultured in high glucose. J Clin Invest. 1988;82:735–738. doi: 10.1172/JCI113655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanzaki T, Shiina R, Saito Y, Zardi L, Morisaki N. Transforming growth factor-beta receptor and fibronectin expressions in aortic smooth muscle cells in diabetic rats. Diabetologia. 1997;40:383–391. doi: 10.1007/s001250050691. [DOI] [PubMed] [Google Scholar]
- 23.Anderson SS, Wu K, Nagase H, Stettler-Stevenson WG, Kim Y, Tsilibary EC. Effect of matrix glycation on expression of type IV collagen, MMP-2, MMP-9 and TIMP-1 by human mesangial cells. Cell Adhes Commun. 1996;4:89–101. doi: 10.3109/15419069609010765. [DOI] [PubMed] [Google Scholar]
- 24.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 25.Hammes HP, Weiss A, Hess S, Araki N, Horiuchi S, Brownlee M, et al. Modification of vitronectin by advanced glycation alters functional properties in vitro and in the diabetic retina. Lab Invest. 1996;75:325–338. [PubMed] [Google Scholar]
- 26.Howard EW, Benton R, Ahern-Moore J, Tomasek JJ. Cellular contraction of collagen lattices is inhibited by nonenzymatic glycation. Exp Cell Res. 1996;228:132–137. doi: 10.1006/excr.1996.0308. [DOI] [PubMed] [Google Scholar]
- 27.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 28.Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006;69:614–624. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Jormsjo S, Ye S, Moritz J, Walter DH, Dimmeler S, Zeiher AM, et al. Allele-specific regulation of matrix metalloproteinase-12 gene activity is associated with coronary artery luminal dimensions in diabetic patients with manifest coronary artery disease. Circ Res. 2000;86:998–1003. doi: 10.1161/01.res.86.9.998. [DOI] [PubMed] [Google Scholar]
- 30.Jesmin S, Sakuma I, Hattori Y, Kitabatake A. Role of angiotensin II in altered expression of molecules responsible for coronary matrix remodeling in insulin-resistant diabetic rats. Arterioscler Thromb Vasc Biol. 2003;23:2021–2026. doi: 10.1161/01.ATV.0000094235.78783.D1. [DOI] [PubMed] [Google Scholar]
- 31.Portik-Dobos V, Anstadt MP, Hutchinson J, Bannan M, Ergul A. Evidence for a matrix metalloproteinase induction/activation system in arterial vasculature and decreased synthesis and activity in diabetes. Diabetes. 2002;51:3063–3068. doi: 10.2337/diabetes.51.10.3063. [DOI] [PubMed] [Google Scholar]
- 32.Gao L, Kang L, Chen Q, Chen C, Xu B, Jiang S. Advanced glycation end products inhibit production and activity of matrix metalloproteinase-2 in human umbilical vein endothelial cells. J Int Med Res. 2007;35:709–715. doi: 10.1177/147323000703500517. [DOI] [PubMed] [Google Scholar]
- 33.Death AK, Fisher EJ, McGrath KC, Yue DK. High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetes. Atherosclerosis. 2003;168:263–269. doi: 10.1016/s0021-9150(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 34.Chung AW, Hsiang YN, Matzke LA, McManus BM, van Breemen C, Okon EB. Reduced expression of vascular endothelial growth factor paralleled with the increased angiostatin expression resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in human type 2 diabetic arterial vasculature. Circ Res. 2006;99:140–148. doi: 10.1161/01.RES.0000232352.90786.fa. [DOI] [PubMed] [Google Scholar]
- 35.Ho FM, Liu SH, Lin WW, Liau CS. Opposite effects of high glucose on MMP-2 and TIMP-2 in human endothelial cells. J Cell Biochem. 2007;101:442–450. doi: 10.1002/jcb.21192. [DOI] [PubMed] [Google Scholar]
- 36.Krankel N, Adams V, Linke A, Gielen S, Erbs S, Lenk K, et al. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol. 2005;25:698–703. doi: 10.1161/01.ATV.0000156401.04325.8f. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Brodsky SV, Goligorsky DM, Hampel DJ, Li H, Gross SS, et al. Glycated collagen I induces premature senescence-like phenotypic changes in endothelial cells. Circ Res. 2002;90:1290–1298. doi: 10.1161/01.res.0000022161.42655.98. [DOI] [PubMed] [Google Scholar]
- 38.Scalia R, Gong Y, Berzins B, Zhao LJ, Sharma K. Hyperglycemia is a major determinant of albumin permeability in diabetic microcirculation: the role of mu-calpain. Diabetes. 2007;56:1842–1849. doi: 10.2337/db06-1198. [DOI] [PubMed] [Google Scholar]
- 39.Giardino I, Edelstein D, Brownlee M. Nonenzymatic glycosylation in vitro and in bovine endothelial cells alters basic fibroblast growth factor activity. A model for intracellular glycosylation in diabetes. J Clin Invest. 1994;94:110–117. doi: 10.1172/JCI117296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 41.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 42.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, et al. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 43.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 44.Ebrahimian T, He Y, Schiffrin EL, Touyz RM. Differential regulation of thioredoxin and NAD(P)H oxidase by angiotensin II in male and female mice. J Hypertens. 2007;25:1263–1271. doi: 10.1097/HJH.0b013e3280acac60. [DOI] [PubMed] [Google Scholar]
- 45.Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallas-Bonke V, et al. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115:2178–2187. doi: 10.1161/CIRCULATIONAHA.106.664250. [DOI] [PubMed] [Google Scholar]
- 46.Yi X, Maeda N. a-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006;55:2238–2244. doi: 10.2337/db06-0251. [DOI] [PubMed] [Google Scholar]
- 47.Mackness B, Quarck R, Verreth W, Mackness M, Holvoet P. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1545–1550. doi: 10.1161/01.ATV.0000222924.62641.aa. [DOI] [PubMed] [Google Scholar]
- 48.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 49.Ho FM, Liu SH, Liau CS, Huang PJ, Lin-Shiau SY. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH(2)-terminal kinase and caspase-3. Circulation. 2000;101:2618–2624. doi: 10.1161/01.cir.101.22.2618. [DOI] [PubMed] [Google Scholar]
- 50.Ho FM, Lin WW, Chen BC, Chao CM, Yang CR, Lin LY, et al. High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-Jun NH2-terminal kinase pathway and prevented by PI3K/Akt/eNOS pathway. Cell Signal. 2006;18:391–399. doi: 10.1016/j.cellsig.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 52.Yokoi T, Fukuo K, Yasuda O, Hotta M, Miyazaki J, Takemura Y, et al. Apoptosis signal-regulating kinase 1 mediates cellular senescence induced by high glucose in endothelial cells. Diabetes. 2006;55:1660–1665. doi: 10.2337/db05-1607. [DOI] [PubMed] [Google Scholar]
- 53.Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, et al. Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005;24:3954–3963. doi: 10.1038/sj.onc.1208548. [DOI] [PubMed] [Google Scholar]
- 54.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sessa WC. Regulation of endothelial derived nitric oxide in health and disease. Mem Inst Oswaldo Cruz. 2005;100:15–18. doi: 10.1590/s0074-02762005000900004. [DOI] [PubMed] [Google Scholar]
- 56.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 57.Keynan S, Khamaisi M, Dahan R, Barnes K, Jackson CD, Turner AJ, et al. Increased expression of endothelin-converting enzyme-1c isoform in response to high glucose levels in endothelial cells. J Vasc Res. 2004;41:131–140. doi: 10.1159/000077132. [DOI] [PubMed] [Google Scholar]
- 58.Kunjathoor VV, Wilson DL, LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest. 1996;97:1767–1773. doi: 10.1172/JCI118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faries PL, Rohan DI, Takahara H, Wyers MC, Contreras MA, Quist WC, et al. Human vascular smooth muscle cells of diabetic origin exhibit increased proliferation, adhesion, and migration. J Vasc Surg. 2001;33:601–607. doi: 10.1067/mva.2001.111806. [DOI] [PubMed] [Google Scholar]
- 60.Avena R, Mitchell ME, Neville RF, Sidawy AN. The additive effects of glucose and insulin on the proliferation of infragenicular vascular smooth muscle cells. J Vasc Surg. 1998;28:1033–1038. doi: 10.1016/s0741-5214(98)70029-1. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto M, Acevedo-Duncan M, Chalfant CE, Patel NA, Watson JE, Cooper DR. Acute glucose-induced downregulation of PKC-betaII accelerates cultured VSMC proliferation. Am J Physiol Cell Physiol. 2000;279:C587–C595. doi: 10.1152/ajpcell.2000.279.3.C587. [DOI] [PubMed] [Google Scholar]
- 62.Lindschau C, Quass P, Menne J, Guler F, Fiebeler A, Leitges M, et al. Glucose-induced TGF-beta1 and TGF-beta receptor-1 expression in vascular smooth muscle cells is mediated by protein kinase C-alpha. Hypertension. 2003;42:335–341. doi: 10.1161/01.HYP.0000087839.72582.DD. [DOI] [PubMed] [Google Scholar]
- 63.Hall JL, Matter CM, Wang X, Gibbons GH. Hyperglycemia inhibits vascular smooth muscle cell apoptosis through a protein kinase C-dependent pathway. Circ Res. 2000;87:574–580. doi: 10.1161/01.res.87.7.574. [DOI] [PubMed] [Google Scholar]
- 64.Sodhi CP, Kanwar YS, Sahai A. Hypoxia and high glucose upregulate AT1 receptor expression and potentiate ANG II-induced proliferation in VSM cells. Am J Physiol Heart Circ Physiol. 2003;284:H846–H852. doi: 10.1152/ajpheart.00625.2002. [DOI] [PubMed] [Google Scholar]
- 65.Lavrentyev EN, Estes AM, Malik KU. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ Res. 2007;101:455–464. doi: 10.1161/CIRCRESAHA.107.151852. [DOI] [PubMed] [Google Scholar]
- 66.Natarajan R, Scott S, Bai W, Yerneni KK, Nadler J. Angiotensin II signaling in vascular smooth muscle cells under high glucose conditions. Hypertension. 1999;33:378–384. doi: 10.1161/01.hyp.33.1.378. [DOI] [PubMed] [Google Scholar]
- 67.Natarajan R, Bai W, Lanting L, Gonzales N, Nadler J. Effects of high glucose on vascular endothelial growth factor expression in vascular smooth muscle cells. Am J Physiol. 1997;273:H2224–H2231. doi: 10.1152/ajpheart.1997.273.5.H2224. [DOI] [PubMed] [Google Scholar]
- 68.Chuang PY, Yu Q, Fang W, Uribarri J, He JC. Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int. 2007;72:965–976. doi: 10.1038/sj.ki.5002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kobayashi T, Puro DG. Loss of insulin-mediated vasoprotection: early effect of diabetes on pericyte-containing microvessels of the retina. Invest Ophthalmol Vis Sci. 2007;48:2350–2355. doi: 10.1167/iovs.06-1357. [DOI] [PubMed] [Google Scholar]
- 70.Manea A, Raicu M, Simionescu M. Expression of functionally phagocyte-type NAD(P)H oxidase in pericytes: effect of angiotensin II and high glucose. Biol Cell. 2005;97:723–734. doi: 10.1042/BC20040107. [DOI] [PubMed] [Google Scholar]
- 71.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 72.Yang R, Liu H, Williams I, Chaqour B. Matrix metalloproteinase-2 expression and apoptogenic activity in retinal pericytes: implications in diabetic retinopathy. Ann N Y Acad Sci. 2007;1103:196–201. doi: 10.1196/annals.1394.000. [DOI] [PubMed] [Google Scholar]
- 73.Barth JL, Yu Y, Song W, Lu K, Dashti A, Huang Y, et al. Oxidised, glycated LDL selectively influences tissue inhibitor of metalloproteinase-3 gene expression and protein production in human retinal capillary pericytes. Diabetologia. 2007;50:2200–2208. doi: 10.1007/s00125-007-0768-z. [DOI] [PubMed] [Google Scholar]
- 74.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 75.Vonend O, Turner CM, Chan CM, Loesch A, Dell'Anna GC, Srai KS, et al. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int. 2004;66:157–166. doi: 10.1111/j.1523-1755.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- 76.Sugiyama T, Kawamura H, Yamanishi S, Kobayashi M, Katsumura K, Puro DG. Regulation of P2X7-induced pore formation and cell death in pericyte-containing retinal microvessels. Am J Physiol Cell Physiol. 2005;288:C568–C576. doi: 10.1152/ajpcell.00380.2004. [DOI] [PubMed] [Google Scholar]
- 77.Puro DG. Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments. MicroCirculation. 2007;14:1–10. doi: 10.1080/10739680601072099. [DOI] [PubMed] [Google Scholar]
- 78.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 2007;112:375–384. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- 79.Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, et al. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest. 1998;101:1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen NG, Abbasi F, Lamendola C, McLaughlin T, Cooke JP, Tsao PS, et al. Mononuclear cell adherence to cultured endothelium is enhanced by hypertension and insulin resistance in healthy nondiabetic volunteers. Circulation. 1999;100:940–943. doi: 10.1161/01.cir.100.9.940. [DOI] [PubMed] [Google Scholar]
- 81.Vlassara H. Advanced nonenzymatic tissue glycosylation: cell-mediated interactions implicated in the complications associated with diabetes and aging. Blood Purif. 1990;8:223–232. doi: 10.1159/000169970. [DOI] [PubMed] [Google Scholar]
- 82.Okouchi M, Okayama N, Imai S, Omi H, Shimizu M, Fukutomi T, et al. High insulin enhances neutrophil transendothelial migration through increasing surface expression of platelet endothelial cell adhesion molecule-1 via activation of mitogen activated protein kinase. Diabetologia. 2002;45:1449–1456. doi: 10.1007/s00125-002-0902-x. [DOI] [PubMed] [Google Scholar]
- 83.Esposito C, Fasoli G, Plati AR, Bellotti N, Conte MM, Cornacchia F, et al. Long-term exposure to high glucose up-regulates VCAM-induced endothelial cell adhesiveness to PBMC. Kidney Int. 2001;59:1842–1849. doi: 10.1046/j.1523-1755.2001.0590051842.x. [DOI] [PubMed] [Google Scholar]
- 84.Okouchi M, Okayama N, Shimizu M, Omi H, Fukutomi T, Itoh M. High insulin exacerbates neutrophil-endothelial cell adhesion through endothelial surface expression of intercellular adhesion molecule-1 via activation of protein kinase C and mitogen-activated protein kinase. Diabetologia. 2002;45:556–559. doi: 10.1007/s00125-001-0773-6. [DOI] [PubMed] [Google Scholar]
- 85.Wendt T, Harja E, Bucciarelli L, Qu W, Lu Y, Rong LL, et al. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis. 2006;185:70–77. doi: 10.1016/j.atherosclerosis.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 86.Renier G, Mamputu JC, Serri O. Benefits of gliclazide in the atherosclerotic process: decrease in monocyte adhesion to endothelial cells. Metabolism. 2003;52:13–18. doi: 10.1016/s0026-0495(03)00212-9. [DOI] [PubMed] [Google Scholar]
- 87.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 88.Figarola JL, Shanmugam N, Natarajan R, Rahbar S. Anti-inflammatory effects of the advanced glycation end product inhibitor LR-90 in human monocytes. Diabetes. 2007;56:647–655. doi: 10.2337/db06-0936. [DOI] [PubMed] [Google Scholar]
- 89.Suzuki M, Akimoto K, Hattori Y. Glucose upregulates plasminogen activator inhibitor-1 gene expression in vascular smooth muscle cells. Life Sci. 2002;72:59–66. doi: 10.1016/s0024-3205(02)02182-3. [DOI] [PubMed] [Google Scholar]
- 90.Watala C, Pluta J, Golanski J, Rozalski M, Czyz M, Trojanowski Z, et al. Increased protein glycation in diabetes mellitus is associated with decreased aspirin-mediated protein acetylation and reduced sensitivity of blood platelets to aspirin. J Mol Med. 2005;83:148–158. doi: 10.1007/s00109-004-0600-x. [DOI] [PubMed] [Google Scholar]
- 91.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 92.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 93.Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res. 2006;4:53–65. doi: 10.3121/cmr.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest. 2006;116:1813–1822. doi: 10.1172/JCI29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Emanueli C, Graiani G, Salis MB, Gadau S, Desortes E, Madeddu P. Prophylactic gene therapy with human tissue kallikrein ameliorates limb ischemia recovery in type 1 diabetic mice. Diabetes. 2004;53:1096–1103. doi: 10.2337/diabetes.53.4.1096. [DOI] [PubMed] [Google Scholar]
- 96.Brown DL, Kane CD, Chernausek SD, Greenhalgh DG. Differential expression and localization of insulin-like growth factors I and II in cutaneous wounds of diabetic and nondiabetic mice. Am J Pathol. 1997;151:715–724. [PMC free article] [PubMed] [Google Scholar]
- 97.Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: A potential predictor for the individual capacity to develop collaterals. Circulation. 2000;102:185–190. doi: 10.1161/01.cir.102.2.185. [DOI] [PubMed] [Google Scholar]
- 98.Tanii M, Yonemitsu Y, Fujii T, Shikada Y, Kohno R, Onimaru M, et al. Diabetic microangiopathy in ischemic limb is a disease of disturbance of the platelet-derived growth factor-BB/protein kinase C axis but not of impaired expression of angiogenic factors. Circ Res. 2006;98:55–62. doi: 10.1161/01.RES.0000197842.38758.45. [DOI] [PubMed] [Google Scholar]
- 99.Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- 100.Graiani G, Emanueli C, Desortes E, Van Linthout S, Pinna A, Figueroa CD, et al. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice. Diabetologia. 2004;47:1047–1054. doi: 10.1007/s00125-004-1414-7. [DOI] [PubMed] [Google Scholar]
- 101.Salis MB, Graiani G, Desortes E, Caldwell RB, Madeddu P, Emanueli C. Nerve growth factor supplementation reverses the impairment, induced by Type 1 diabetes, of hindlimb post-ischaemic recovery in mice. Diabetologia. 2004;47:1055–1063. doi: 10.1007/s00125-004-1424-5. [DOI] [PubMed] [Google Scholar]
- 102.Wiedermann CJ, Auer B, Sitte B, Reinisch N, Schratzberger P, Kahler CM. Induction of endothelial cell differentiation into capillary-like structures by substance P. Eur J Pharmacol. 1996;298:335–338. doi: 10.1016/0014-2999(95)00818-7. [DOI] [PubMed] [Google Scholar]
- 103.Weihrauch D, Lohr NL, Mraovic B, Ludwig LM, Chilian WM, Pagel PS, et al. Chronic hyperglycemia attenuates coronary collateral development and impairs proliferative properties of myocardial interstitial fluid by production of angiostatin. Circulation. 2004;109:2343–2348. doi: 10.1161/01.CIR.0000129225.67353.1F. [DOI] [PubMed] [Google Scholar]
- 104.Ebrahimian TG, Tamarat R, Clergue M, Duriez M, Levy BI, Silvestre JS. Dual effect of angiotensin-converting enzyme inhibition on angiogenesis in type 1 diabetic mice. Arterioscler Thromb Vasc Biol. 2005;25:65–70. doi: 10.1161/01.ATV.0000149377.90852.d8. [DOI] [PubMed] [Google Scholar]
- 105.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 106.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 107.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, et al. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 108.Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR, Huang PH, et al. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 109.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 110.Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, et al. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes. 2006;55:102–109. [PubMed] [Google Scholar]
- 111.Bauersachs J, Thum T. Endothelial progenitor cell dysfunction: mechanisms and therapeutic approaches. Eur J Clin Invest. 2007;37:603–606. doi: 10.1111/j.1365-2362.2007.01833.x. [DOI] [PubMed] [Google Scholar]
- 112.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 113.Thum T, Tsikas D, Stein S, Schultheiss M, Eigenthaler M, Anker SD, et al. Suppression of endothelial progenitor cells in human coronary artery disease by the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. J Am Coll Cardiol. 2005;46:1693–1701. doi: 10.1016/j.jacc.2005.04.066. [DOI] [PubMed] [Google Scholar]
- 114.Rosso A, Balsamo A, Gambino R, Dentelli P, Falcioni R, Cassader M, et al. p53 Mediates the accelerated onset of senescence of endothelial progenitor cells in diabetes. J Biol Chem. 2006;281:4339–4347. doi: 10.1074/jbc.M509293200. [DOI] [PubMed] [Google Scholar]
- 115.Scheubel RJ, Kahrstedt S, Weber H, Holtz J, Friedrich I, Borgermann J, et al. Depression of progenitor cell function by advanced glycation end-products (AGEs): potential relevance for impaired angiogenesis in advanced age and diabetes. Exp Gerontol. 2006;41:540–548. doi: 10.1016/j.exger.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 116.Torsney E, Hu Y, Xu Q. Adventitial progenitor cells contribute to arteriosclerosis. Trends Cardiovasc Med. 2005;15:64–68. doi: 10.1016/j.tcm.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 117.Invernici G, Emanueli C, Madeddu P, Cristini S, Gadau S, Benetti A, et al. Human fetal aorta contains vascular progenitor cells capable of inducing vasculogenesis, angiogenesis, and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol. 2007;170:1879–1892. doi: 10.2353/ajpath.2007.060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 119.Shoji T, Koyama H, Morioka T, Tanaka S, Kizu A, Motoyama K, et al. Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes. Diabetes. 2006;55:2245–2255. doi: 10.2337/db05-1375. [DOI] [PubMed] [Google Scholar]
- 120.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 121.Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 122.Devangelio E, Santilli F, Formoso G, Ferroni P, Bucciarelli L, Michetti N, et al. Soluble RAGE in type 2 diabetes: association with oxidative stress. Free Radic Biol Med. 2007;43:511–518. doi: 10.1016/j.freeradbiomed.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 123.Cai S, Khoo J, Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res. 2005;65:823–831. doi: 10.1016/j.cardiores.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 124.Tawfik HE, El-Remessy AB, Matragoon S, Ma G, Caldwell RB, Caldwell RW. Simvastatin improves diabetes-induced coronary endothelial dysfunction. J Pharmacol Exp Ther. 2006;319:386–395. doi: 10.1124/jpet.106.106823. [DOI] [PubMed] [Google Scholar]
- 125.Hamilton SJ, Chew GT, Watts GF. Therapeutic regulation of endothelial dysfunction in type 2 diabetes mellitus. Diab Vasc Dis Res. 2007;4:89–102. doi: 10.3132/dvdr.2007.026. [DOI] [PubMed] [Google Scholar]
- 126.Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, et al. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation. 2005;111:2073–2085. doi: 10.1161/01.CIR.0000162472.52990.36. [DOI] [PubMed] [Google Scholar]
- 127.Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003;90:133–146. doi: 10.1002/bjs.4019. [DOI] [PubMed] [Google Scholar]
- 128.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21:822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 129.Emanueli C, Salis MB, Pinna A, Stacca T, Milia AF, Spano A, et al. Prevention of diabetes-induced microangiopathy by human tissue kallikrein gene transfer. Circulation. 2002;106:993–999. doi: 10.1161/01.cir.0000027104.33206.c8. [DOI] [PubMed] [Google Scholar]
- 130.Emanueli C, Caporali A, Krankel N, Cristofaro B, Van Linthout S, Madeddu P. Type-2 diabetic Lepr(db/db) mice show a defective micro-vascular phenotype under basal conditions and an impaired response to angiogenesis gene therapy in the setting of limb ischemia. Front Biosci. 2007;12:2003–2012. doi: 10.2741/2205. [DOI] [PubMed] [Google Scholar]
- 131.Emanueli C, Salis MB, Van Linthout S, Meloni M, Desortes E, Silvestre JS, et al. Akt/protein kinase B and endothelial nitric oxide synthase mediate muscular neovascularization induced by tissue kallikrein gene transfer. Circulation. 2004;110:1638–1644. doi: 10.1161/01.CIR.0000142051.36244.83. [DOI] [PubMed] [Google Scholar]
- 132.Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000;106:571–578. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stepanovic V, Awad O, Jiao C, Dunnwald M, Schatteman GC. Leprdb diabetic mouse bone marrow cells inhibit skin wound vascularization but promote wound healing. Circ Res. 2003;92:1247–1253. doi: 10.1161/01.RES.0000074906.98021.55. [DOI] [PubMed] [Google Scholar]
- 134.Tamarat R, Silvestre JS, Le Ricousse-Roussanne S, Barateau V, Lecomte-Raclet L, Clergue M, et al. Impairment in ischemia-induced neovascularization in diabetes: bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am J Pathol. 2004;164:457–466. doi: 10.1016/S0002-9440(10)63136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Emanueli C, Monopoli A, Kraenkel N, Meloni M, Gadau S, Campesi I, et al. Nitropravastatin stimulates reparative neovascularisation and improves recovery from limb ischaemia in type-1 diabetic mice. Br J Pharmacol. 2007;150:873–882. doi: 10.1038/sj.bjp.0707142. [DOI] [PMC free article] [PubMed] [Google Scholar]