Abstract
We evaluated the healing potential of human fetal aorta–derived CD133+ progenitor cells and their conditioned medium (CD133+ CCM) in a new model of ischemic diabetic ulcer. Streptozotocin-induced diabetic mice underwent bilateral limb ischemia and wounding. One wound was covered with collagen containing 2×104 CD133+ or CD133− cells or vehicle. The contralateral wound, covered with only collagen, served as control. Fetal CD133+ cells expressed high levels of wingless (Wnt) genes, which were downregulated following differentiation into CD133− cells along with upregulation of Wnt antagonists secreted frizzled-related protein (sFRP)-1, -3, and -4. CD133+ cells accelerated wound closure as compared with CD133− or vehicle and promoted angiogenesis through stimulation of endothelial cell proliferation, migration, and survival by paracrine effects. CD133+ cells secreted high levels of vascular endothelial growth factor (VEGF)-A and interleukin (IL)-8. Consistently, CD133+ CCM accelerated wound closure and reparative angiogenesis, with this action abrogated by coadministering the Wnt antagonist sFRP-1 or neutralizing antibodies against VEGF-A or IL-8. In vitro, these effects were recapitulated following exposure of high-glucose-primed human umbilical vein endothelial cells to CD133+ CCM, resulting in stimulation of migration, angiogenesis-like network formation and induction of Wnt expression. The promigratory and proangiogenic effect of CD133+ CCM was blunted by sFRP-1, as well as antibodies against VEGF-A or IL-8. CD133+ cells stimulate wound healing by paracrine mechanisms that activate Wnt signaling pathway in recipients. These preclinical findings open new perspectives for the cure of diabetic ulcers.
Keywords: ischemia, wound healing, diabetes, stem cells, angiogenesis
Chronic wounds represent a relevant clinical and socioeconomic burden, with diabetic foot ulcers alone causing costs of 300 million pounds per annum to the United Kingdom National Health System.1 Diabetic patients with foot ulcers associated with peripheral vascular disease manifest the worst outcome, with higher amputation and mortality rates than patients carrying nonischemic ulcers.2,3 Although the efficacy of a topical gel formulation of recombinant human platelet-derived growth factor-BB was recently demonstrated in patients with nonischemic neuropathic ulcers,4 most ischemic ulcers are refractory to conventional treatment and growth factor (GF) therapy.5 Therefore, new strategies for the cure of life-threatening ischemic ulcers are urgently awaited.
Preliminary evidence supports the potential of adult or fetal stem/progenitor cells for the healing of skin ulcers.6-8 However, because of the lack of an appropriate preclinical model, no information is available regarding the effectiveness of cell therapy on ischemic diabetic foot ulcers. The healing activity of stem cells is credited to their ability to transdifferentiate into the vascular and nonvascular components of injured tissue, as well as to secretion of GFs, which may activate endogenous modulators of angiogenesis in the recipient.9-11 Notably, fetal stem cells show significant advantages over their adult counterparts in terms of proliferative capacity, engraftment kinetics, and differentiation plasticity. Fetal stem cells abundantly express CD133, an antigenic marker associated with high clonogenic potential and asymmetrical division; both of these are typical “stemness” features.12 In a nondiabetic murine ischemic hindlimb model, we recently reported that transplantation of a low number of CD133+ human fetal aorta-derived vascular progenitor cells promotes reparative neovascularization and skeletal myocyte regeneration, thereby supporting limb salvage.13 We also showed that fetal CD133+ cells release large amounts of vascular endothelial growth factor (VEGF)-A.13 VEGF-A is a potent stimulator of the phosphatidylinositol 3-kinase–protein kinase B (Akt) pathway, which exerts proangiogenic and prosurvival effects through, among others, phosphorylation/activation of endothelial nitric oxide synthase and phosphorylation/inactivation of glycogen-synthase kinase 3β and forkhead box O (FOXO) transcription factors.14,15 Expanding our present knowledge of stem cell action, we analyzed the role of Wingless (Wnt) gene products, which previously have been implicated in stem cell self-renewal.16 Wnt proteins of the canonical pathway bind to Frizzled (Fz) receptors, which then form a complex with the coreceptor LRP (LDL receptor–related protein). Through several cytoplasmic relay components, the signal is transduced to β-catenin, which enters the nucleus to modulate the expression of target genes.17 The noncanonical pathway is independent of LRP/β-catenin and encompasses the Wnt/Ca2+ and Wnt/planar cell polarity pathways.18 Wnts play a key role in embryonic vasculogenesis, by modulating the expansion of primitive VEGF receptor 2–positive vascular progenitor cells,16,19 as well as in postnatal angiogenesis.20,21 In addition, Wnt/β-catenin signaling is implicated in physiological and pathological wound cicatrisation.22,23
Here, we used a newly developed mouse model of diabetic ischemic foot ulcer to study the therapeutic activity of fetal CD133+ cells and their conditioned medium. We furthermore elucidated mechanistic aspects of CD133+ cell action in the setting of diabetic wound healing.
Materials and Methods
Human Fetal Cells
Aortas from 11- to 12-week-old human fetuses (n=15) were obtained according to the ethical guidelines of the Network for European CNS Transplantation and Restoration (NECTAR) as described before.13 The experimental protocol was approved by the ethics committees of the National Neurological Institute “Carlo Besta” (Milan, Italy). CD133− cells were generated from CD133+ cells by serum-induced differentiation, as previously reported.13,24 Cell-conditioned medium (CCM) was obtained from cultures of 2×105 cells/mL after 48 hours of incubation.
Animal Procedures
All procedures complied with the standards stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Bethesda, Md, 1996) and were covered by ethical approvals from the Italian Ministry of Health and the United Kingdom Home Office. In 6- to 7-week-old male CD1 mice (Charles River Laboratories, Milan, Italy, and Morgate, UK) diabetes was induced by streptozotocin (Sigma), as described.25 Persistence of glycosuria of ≥10 g/L was checked over the duration of the experiments.
Four weeks after diabetes induction, bilateral hindlimb ischemia was induced by ligature of the proximal end of femoral arteries.13 At the same occasion, full-thickness wounds were created in the thigh dorsal skin of both legs using a sterile 5-mm-wide biopsy punch.26 The wounds were covered with type I collagen (Sigma) alone or collagen containing 2×104 CD133+ or CD133− cells. In separate experiments, wounds were covered with Extracel-HP hydrogel (Tebu-Bio, Le Perray en Yvelines, France),27 containing undiluted CD133− or CD133+ CCM with or without the Wnt inhibitor secreted frizzled-related protein (sFRP)-1, or CD133+ CCM was applied together with neutralizing antibodies against VEGF, interleukin (IL)-6, or IL-8. Contralateral wounds were covered with hydrogel containing nonconditioned culture medium (NCCM). After surgery, animals were maintained in individual cages with food and water ad libitum and in a temperature and humidity-controlled environment. Clinical outcome was established by determining the rate of wound closure.26
An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org.
Results
Characterization of CD133+ and CD133− Cells
Progenitor cells derived from human fetal aorta, expressed CD133, CD117 (c-Kit), and CXCR4 but only low levels of mature endothelial markers, such as CD31 and CD146, as previously reported.13 Exposure to serum-induced differentiation associated with the loss of CD133 and CD117 and the acquisition of endothelial antigens (Online Figure I, A and B, in the online data supplement, available at http://circres. ahajournals.org). Gene expression analysis verified downregulation of several stem cell–associated genes during serum-induced maturation (Online Table I).
Furthermore, differentiation of CD133+ into CD133− cells was associated with downregulation of Wnt4, Wnt5A, Wnt7A, Wnt7B, and Wnt10A and upregulation of sFRPs (Online Figure I, C). Inversely, culture of CD133+ cells in the presence of sFRP-1 caused a 25% reduction in CD133 expression (Online Figure I, D), without altering the abundance of CD31 (data not shown).
CD133+ Cells Accelerate Wound Closure in Diabetes
To assess the added effect of ischemia on diabetic wound healing, limb wounds were produced bilaterally together with unilateral femoral artery occlusion in streptozotocin-induced diabetic mice. Laser Doppler flowmetry confirmed an initial reduction of limb blood flow on the side of artery occlusion by 82%. Blood flow was still reduced to 52% as compared to the contralateral side on day 7. Wound closure was significantly delayed in ischemic as compared to nonischemic wounds (Online Figure II).
Next, to study the impact of cell or medium administration on wound healing, we produced wounds and limb ischemia bilaterally. The right side was covered with plain collagen gel alone and served as internal control, whereas gel on the left side wound contained either 2×104 CD133+ or CD133− cells or the vehicle. Transplantation of CD133+ cells accelerated the rate of wound closure in streptozotocin-induced diabetic mice, whereas no effect was observed in groups given CD133− cells or collagen as compared with the contralateral side (Figure 1A and Online Figure III). Two-way ANOVA detected a treatment effect among groups (P<0.05), with no interaction between treatment and time. In addition, Bonferroni post test analysis revealed an improved clinical outcome in the CD133+ treatment group as compared with collagen or CD133− cells. Neither cell therapy nor collagen accelerated hindlimb hemodynamic recovery (data not shown).
Figure 1.

Accelerated wound closure in the presence of CD133+ cells (A, middle) is associated with higher temporary wound capillarization at day 7 (B). Capillary density declines to levels of wounds treated with CD133− cells or collagen gel alone by day 14 (C) (n=12 mice per group). *P<0.05 vs collagen, +P<0.05 vs CD133− cells. Bar=20 μm.
Capillarization was increased at days 3 and 7 in wounds transplanted with CD133+ cells as compared with CD133− cell– or collagen-treated ulcers (Online Figure IV and Figure 1B) but returned to levels comparable to CD133− cell or collagen treatment at day 14 (Figure 1C). Furthermore, in ulcers collected at 3 days from CD133+ cell transplantation, a higher number of endothelial cells (ECs) stained positive for the proliferation marker MCM-2, and a lower number showed apoptosis-associated TUNEL positivity, as compared with CD133− cell– or collagen-treated wounds (Figure 2A and 2B). An increase in EC proliferation was still evident in CD133+ cell-transplanted ulcers collected a 7 days (0.81±0.04 versus 0.37±0.02 MCM-2 positive nuclei per vessel in CD133− cell–treated ulcers, P<0.05; Online Figure V, A and B). Immunohistochemistry studies also demonstrated an extremely thin endothelial lining of the forming blood vessels in the diabetic wounds, which is fragmental and exhibits gaps of all sizes with bleeding into the surrounding tissue, as indicated by the existence of numerous erythrocytes in the perivascular tissue. CD133+ cell treatment improves this condition without completely omitting the gaps and the extreme stretching of many vessel walls.
Figure 2.

Endothelial cell proliferation is stimulated in diabetic wounds by CD133+ cells (A), whereas apoptosis is reduced (B). CD133− cells did not influence endothelial apoptosis or proliferation (n=5 per group). *P<0.05 vs collagen, +P<0.05 vs CD133− cells. Bar=10 μm.
CD133+ Cells Mediate Wound Healing by Paracrine Mechanisms
Human cells derived from transplanted CD133+ or CD133− cells were rarely recognized in the wound granulation tissue harvested 3 days after transplantation (5.4±1.7 and 4.3±2.4 cells/mm2, respectively, P=NS). Fluorescence-activated cell-sorting (FACS) analysis, as well as quantitative PCR of excised wounds, confirmed that only low numbers of cells remained in the wounds after 3 days, with no difference between cell groups (CD133+ donor cells/wound: 34±9 [FACS], 43±7 [quantitative PCR]; CD133− donor cells per wound: 36±11 [FACS], 18±6 [quantitative PCR]; P=NS). Immunohistochemistry indicated a drastic reduction in the number of human nuclear antigen-positive (hNA+) cells in diabetic wounds collected at later stages, namely at 7 days posttransplantation (0.3±0.1 cells/mm2, P<0.05 versus day 3), with no human cells being detectable at 14 days (Online Figure V, C and D). Furthermore, the rare hNA+ cells were located at 2- to 5-cell-diameter distance from microvessels, indicating that these residual elements were not integrated in the wound vasculature (Online Figure V, C). This led us to suspect a paracrine mechanism underlying the supportive effect on wound healing and capillarization described above.
We have previously shown that CD133+ cells produce large amounts of VEGF-A.13 By cytokine bead array, we verified this and identified additional proangiogenic factors, secreted at higher levels by CD133+ as compared to CD133− cells. Highest levels were detected for IL-6 and IL-8 among interleukins, VEGF-A and granulocyte-colony stimulating factor (G-CSF) among GF, and monocyte chemoattractant protein-1 (CCL2) among chemokines (Figure 3). In accordance with those findings, administration of CD133+ CCM instead of cells supported wound closure, whereas CD133− CCM was ineffective (Figure 4A). The healing action of CD133+ CCM was associated with increased wound vascularization at 7 days as compared with wounds given CD133− CCM or NCCM (Figure 4B and 4D). However, no difference in capillary density was detected among groups at 14 days (Figure 4C).
Figure 3.

Cytometric bead array indicated higher secretion of interleukins (A), GFs (B), and chemokines (C) by CD133+ cells as compared to CD133− cells (n=4 per group). **P<0.01, ***P<0.005 vs CD133− cells.
Figure 4.

Administration of CD133+ CCM facilitated wound closure in diabetic animals over a time course of 14 days, whereas CD133− CCM was ineffective (A). Wound closure was accompanied by temporarily higher capillarization of wounds treated with CD133+ CCM on day 7 (B and D). Capillary density decreased to levels only slightly above wounds treated with collagen alone by day 14 (C). CD133− CCM did not accelerate wound closure or stimulate capillarization at any time point (A through C) (n=6 per group). **P<0.01 vs collagen, ++P<0.01 vs CD133− cells. Bar=10 μm.
CD133+ CCM Promotes Endothelial Cell Migration and Survival In Vitro
To gain insight into the mechanisms underlying CD133+ CCM-induced capillarization, we first performed a Matrigel-based in vitro angiogenesis assay. Corresponding to in vivo capillary density data, CD133+ CCM potentiated human umbilical vein endothelial cell (HUVEC) network formation as compared to NCCM or CD133− CCM (Figure 5A).
Figure 5.

Treatment of HUVECs with CD133+ CCM enhanced network formation (A), gap closure (B), and chemotaxis (C), whereas CD133− CCM was less potent. CD133+ CCM blunted HG-induced apoptosis (D). Data are means±SEM. **P<0.01, ***P<0.005 vs nonconditioned medium (NCCM), $P<0.05 vs CD133− cells, ##P<0.01 vs normal glucose.
Network formation, even in a simplified in vitro assay, relies on the interplay of several distinct cellular processes, which we addressed separately. First, we focused on directed migration of ECs, which is crucial for organized capillary growth. HUVECs migrated toward CD133+ CCM in gap closure (“scratch”), as well as in “transwell” chemotaxis assays (Figure 5B and 5C). Next, we analyzed apoptosis, which is typically activated in ECs from diabetic patients or after exposure to hyperglycemic (HG) culture conditions. Consistently, we found higher activities of caspases 3 and 7 in HG-cultured ECs, which was prevented by the addition of CD133+ CCM, but not CD133− CCM, to the HG medium (Figure 5D).
One critical nexus in the network controlling migration, proliferation, and apoptosis is governed by the protein kinase Akt. In the presence of CD133+ CCM, we detected higher phosphorylation states of Akt at Ser473, which has been described to be crucial for its activity (Online Figure VI, A).14 Accordingly, transfection of HUVECs with dominant-negative Akt reduced CD133+ CCM-mediated survival in HG conditions by 50% (P<0.05 versus green fluorescent protein–transfected cells, data not shown). Akt itself exerts its effects in part via the phosphorylation of endothelial nitric oxide synthase (associated with activation and increased generation of the endothelial survival factor nitric oxide [NO]) and via the forkhead transcription factor FOXO1 (associated with inactivation and blockade of its proapoptotic function). In the presence of CD133+ CCM, endothelial nitric oxide synthase and FOXO1 showed higher phosphorylation levels as compared to NCCM, a result that agrees with the above described overall prosurvival action of CD133+ CCM (Online Figure VI, B and C). We could not detect significant difference among treatment groups with regard to glycogen-synthase kinase 3β phosphorylation (Online Figure VI, D).
Paracrine Mechanisms Implicated in CD133+ CCM-Regenerative Action
As described above, we detected high levels of VEGF-A, IL-6, and IL-8 in the CCM of CD133+ cells and therefore suspected their involvement in the therapeutic and angiogenic action of CD133+ CCM. In vivo, neutralizing antibodies against VEGF-A or IL-8 inhibited the healing effect of CD133+ CCM, thus confirming the critical role of both factors in wound closure and capillarization, whereas capturing IL-6 did not affect CD133+ CCM-induced wound closure or capillarization (Online Figure VII, A and B). In in vitro scratch assays, neutralizing antibodies against VEGF-A, IL-6, or IL-8 attenuated the acceleration of HUVEC gap closure by CD133+ CCM (Online Figure VII, C). Neutralizing VEGF-A, IL-6, or IL-8, however, failed to suspend the protective action of CD133+ CCM on HG-induced apoptosis (data not shown).
CD133+ Cells Activate Wnt Signaling In Vivo and In Vitro
Recent evidence suggests a link between the VEGF-A and Wnt pathways, with Wnt potentiating the susceptibility of ECs to VEGF-A signals.28 The findings that CD133+ cells express Wnt genes and release VEGF-A prompted us to investigate whether CD133+ cells may paracrinally activate the Wnt signaling pathway in the recipient’s wounds.
To this aim, we screened for Wnt genes regulated by the treatment with CD133+ cells in diabetic wounds. RNA was extracted from tissue collected at day 3 postwounding and subjected to quantitative RT-PCR. Wnt4, Wnt5A, Wnt5B, Wnt7A, and Wnt7B genes were present in all wound samples (data not shown). Interestingly, the expression of only Wnt7A was increased in CD133+ cell–treated wounds as compared with either CD133− cell– or collagen-treated wounds (Online Figure VIII).
Next, we evaluated the involvement of Wnt in promotion of angiogenesis by human progenitor cells. HUVECs cultured under HG conditions expressed Wnt2B, Wnt3, Wnt4, Wnt5A, Wnt9A, and, to a lesser extent, Wnt8B and Wnt16 (data not shown). Following exposure to CD133+ CCM, we observed an upregulation of Wnt3, Wnt5A, and Wnt9A as compared with HUVECs exposed to CD133− CCM or control NCCM (Online Figure IX). Importantly, addition of with anti–VEGF-A antibodies to CD133+ CCM prevented the induction of Wnt3, Wnt5A, and Wnt9A by CD133+ CCM (data not shown), thus implying a role of VEGF-A in the modulation of Wnt expression.
To verify the importance of Wnt signaling in vivo, we next applied the Wnt antagonist sFRP together with CD133+ CCM onto diabetic wounds. Importantly, sFRP-1 abolished the facilitation of wound closure and reparative angiogenesis by CD133+ CCM (Figure 6). Consistent with in vivo data, the supportive action of CD133+ CCM on in vitro network formation by HUVECs was negated in the presence of sFRP-1 (Online Figure X). However, if sFRP-1 was added to NCCM, HUVEC network formation was facilitated, in agreement with results published before.21 Similarly, CD133+ CCM-induced HUVEC migration was blunted by sFRP-1 (Online Figure XI). No effect of sFRP-1 on HUVEC survival in the presence of CD133+ CCM under HG conditions was detected (data not shown). To elucidate further distinct Wnt signaling mechanisms mediated by CD133+ CCM, we studied network formation, as well as gap closure and survival in HUVECs, in the presence or absence of Dkk-1. Dkk-1 inhibits canonical Wnt signaling by binding to LRP, which is thereupon removed from the membrane via kremen proteins.29 Dkk-1 tended to reduce HUVEC network formation facilitated by CD133+ CCM (Online Figure X), whereas HUVEC gap closure mediated by CD133+ CCM was not affected by addition of Dkk-1 (Online Figure XI).
Figure 6.
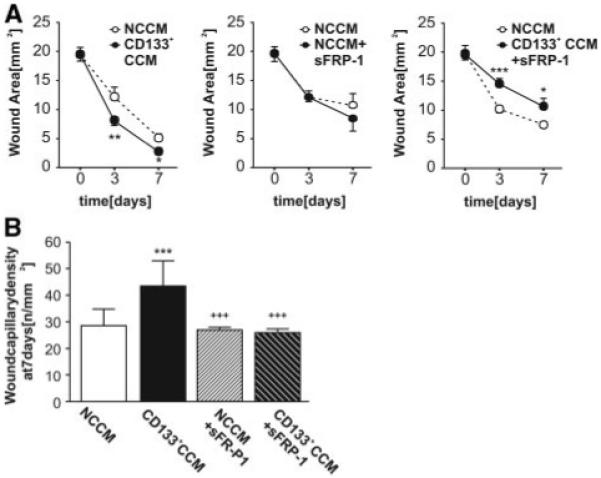
Wound closure, facilitated by unmodified CD133+ CCM, is retarded as compared to vehicle, when Wnt antagonist sFRP-1 is added (A). Treatment of wounds with sFRP-1 in the absence of CD133+ CCM did not modulate wound closure (A). Likewise, capillarization of wounds was supported by CD133+ CCM only in the absence of sFRP-1 (B) (n=7 per group). *P<0.05, **P<0.01, ***P<0.005 vs NCCM, +++P<0.005 vs CD133+ CCM.
In CD133+ CCM-stimulated ECs, we performed a luciferase-based reporter assay to detect β-catenin–induced gene expression via binding to Tcf/Lef elements. Surprisingly, we could not find transcriptional activation in response to either CD133+ or CD133− CCM (Online Figure XI), thus suggesting the involvement of β-catenin-independent mechanisms in CD133+ CCM-induced effects.
Discussion
Great enthusiasm has been generated by recent preclinical stem cell therapy trials on wounds created by punching the interscapular region of diabetic mice.6-8,30 In diabetic patients, lesions of this kind are caused by accidental nonadverted traumas and easily heal with rest and conventional treatment. Therefore, extrapolation of those promising results to the precarious situation of ischemic foot ulcers is premature. To recreate a situation analogous to the ischemic diabetic foot, we developed a new model, which consists of excisional full-thickness wounds in ischemic limbs of streptozotocin-induced diabetic mice. We verified that the association of diabetes and ischemia leads to a severe impairment in wound cicatrisation. In this model, transplantation of angiocompetent cells, derived from differentiation of peripheral blood mononuclear cells of healthy donors, were unable to facilitate wound closure (P. Madeddu, L. Barcelos, unpublished observation, 2008). We evaluated whether fetal progenitor cells, which previously proved to be therapeutically effective in a limb ischemia model,13 succeed where adult cells failed. Besides CD133, those fetal progenitors expressed stemness markers not shared by their CD133− progeny, such as TERT, Syk, and uPAR, which have previously been described to be involved in stem cell maintenance, angiogenesis, and vasculogenesis.31-35
Although fetal CD133+ cells express Wnt, under differentiation, they strongly downregulate Wnt4 and Wnt7A and upregulate of sFRP-1, sFRP-3, and sFRP-4. Interestingly, sFRP-1 was able to reduce CD133 expression in fetal progenitor cells without inducing the acquisition of mature endothelial markers. Given the complexity of signaling events governing differentiation, we would not expect the identical mimicking of CD133− cell phenotype by only the presence of this one Wnt antagonist. The reduction in CD133 expression, however, verifies maintenance of CD133+ identity in our model to strongly rely on Wnt signaling, as has been shown for other stem cells before,36 and suggests that Wnt antagonism may trigger initial phases of differentiation by overriding the negative control exerted by Wnt.37 Maintenance of stemness was relevant to the outcome of our preclinical trial: topical application of CD133+ cells onto ischemic diabetic limb ulcers accelerated healing, whereas CD133− cells were ineffective.
Impaired wound healing in diabetic patients results from multifactorial deficits, including inefficient reparative angiogenesis,26,38 as well as aberrant control of cell survival39; thus it may be clinically relevant that transplantation of fetal CD133+ cells restored reparative angiogenesis in murine diabetic ulcers through stimulation of EC survival, proliferation, and migration.
The therapeutic efficiency of cell therapy depends on the adequate recruitment of applied cells to the target tissue and their ability to produce substances capable of supporting the healing process.10 Using in vivo imaging methods, the extent of homing was shown to be rather low in most experimental and clinical studies (reviewed elsewhere40). Consistently, by 3 different assays, we showed a low rate of incorporation of CD133+ cells in diabetic wounds, which might result from the unfavorable environment at site of application. Our data do not exclude that vascular progenitor cells might directly support reparative angiogenesis under different circumstances, as supported by our previous observation that intramuscularly injected human fetal CD133+ cells were able to incorporate in limb muscle neovasculature.13 In view of the short persistence after application onto diabetic wounds, it is plausible that CD133+ cells exert favorable effects on healing through a burst release of remedial factors and/or stimulation of the sustained paracrine reaction of the tissue of the recipient, as reported previously for other progenitor cells.9 We specified a complex combination of cytokines and GFs produced by CD133+ cells. Similarities could be found with substances released by endothelial progenitor cells41; however, much higher concentrations were detected in the fetal CD133+ CCM, thus accounting for the greater healing potential of these cells. We found that CD133+ CCM elicited promigratory and prosurvival effects on human ECs. Neutralizing antibodies against VEGF-A, IL-6, and IL-8 reduced HUVEC migration but failed to suspend the protective action of CCM on high glucose–induced apoptosis (data not shown). The Akt-FOXO pathway seems to be implicated in the latter phenomenon because transfection of HUVECs with dominant-negative Akt reduced CD133+ CCM-mediated survival in HG conditions. Other factors that were found increased in CD133+ CCM, such as angiogenin, reportedly activate Akt-dependent survival pathway in ECs.42 Thus, the combination rather than a single released agent seems to be responsible for the overall effects of the CD133+ CCM on HUVECs. In vivo, cicatrisation promoted by CD133+ CCM seems to be mainly ascribed to VEGF-A and IL-8, as denoted by the use of neutralizing antibodies.
Wnts are potent regulators of stem cell fate and skin maintenance and regeneration,22 as well as angiogenesis.20,21 This study provides pioneering evidence linking stem cell paracrine action to activation of Wnt signaling in the host. CD133+ cells induced endogenous expression of distinct Wnt genes in vivo and in vitro. Therefore, it appears that besides expressing both canonical and noncanonical components of the Wnt family, CD133+ cells also selectively upregulate some of the Wnt genes that are constitutively expressed by ECs. The importance of these expressional changes is supported by our finding that stimulation of wound cicatrisation and neovascularization by CD133+ CCM is inhibited by the Wnt antagonist sFRP-1. These results were mirrored by in vitro functional assays, in which the same antagonist abrogated the stimulatory effect of CD133+ CCM on HUVEC migration and network formation.
At least 2 classes of Wnt antagonists have been reported.29 The first class includes sFRP-1, which acts as scavenger of Wnt. The second class encompasses members of the Dkk family. Dkk-1 binds Kremen and the coreceptor LPR5/6, which is essential for canonical signaling, but does not prevent Wnt from associating with Fz. Dkk-1 was not efficient as sFRP-1 in inhibiting EC migration and network formation stimulated by CD133+ CCM. By using a reporter assay, we could not demonstrate any change in β-catenin–induced transcription activity in HUVECs stimulated by CD133+ CCM. Altogether, these results indicate that the mechanisms implicated in CD133+ CCM-induced stimulation of capillary-like network formation involve the interaction of Wnt with Fz on ECs and support the emerging concept that noncanonical Wnt pathways play a role in angiogenesis.43
Our results indicate the participation of VEGF-A in the stimulatory effect of CD133+ CCM on Wnt expression in human ECs. Reciprocally, the Wnt signaling pathway was found to strongly upregulate VEGF-A and IL-8, thereby supporting angiogenesis.44,45 However, recent microarray analyses revealed the complexity of Wnt targetome, the primary level including proteolytic enzymes (eg, matrix metalloproteinase-7), transcription regulators (eg, c-Myc), and pathway regulators (eg, VEGF-A), the secondary level being either effectors (eg, the c-Myc target gene p21) or target pathways (eg, VEGF receptor tyrosine kinase pathway) and the tertiary level encompassing targets of the target pathways (eg, the VEGF target gene DSCR1) (reviewed elsewhere46). Exploring these mechanisms was beyond the focus of the present study.
In conclusion, this is the first study to demonstrate the efficacy and associated healing mechanisms of local therapy with CD133+ progenitor cells in a preclinical model of diabetic ischemic foot ulcer. The fetus-derived cells would be difficult to obtain for therapeutic applications. However, the finding that CD133+ CCM is also effective in stimulating wound cicatrisation, together with the discovery that the healing effect is associated with activation of the Wnt signaling pathway in the host, may have important implications for the cure of the ischemic complications of diabetes. Fetal CD133+ cells might be used in the future as a “factory” of therapeutic substances. Alternatively, synthetic replica of the CCM could be produced to obviate ethical concerns surrounding the direct use of fetal stem cells.
Supplementary Material
Acknowledgments
We thank Silvia Cristini (Besta Institute, Milan, Italy) for technical assistance. The Bristol Heart Institute is an associate member of the European Vascular Genomic Network of Excellence.
Sources of Funding
The study was supported by Resolve, a Program Grant of the European Commission under FP7 HEALTH-F4-2008-202047 and project grants of the Juvenile Diabetes Research Foundation and the British Heart Foundation (PG 06/035/20641 and PG/06/096/21325). P.C., G.M., and M.S. are British Heart Foundation PhD students. C.E. holds a British Heart Foundation Basic Science Lectureship.
Footnotes
Disclosures
None.
References
- 1.Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times. 2008;104:44–45. [PubMed] [Google Scholar]
- 2.Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26:491–494. doi: 10.2337/diacare.26.2.491. [DOI] [PubMed] [Google Scholar]
- 3.Campbell WB, Ponette D, Sugiono M. Long-term results following operation for diabetic foot problems: arterial disease confers a poor prognosis. Eur J Vasc Endovasc Surg. 2000;19:174–177. doi: 10.1053/ejvs.1999.1006. [DOI] [PubMed] [Google Scholar]
- 4.Wieman TJ, Becaplermin Gel Studies Group Clinical efficacy of becaplermin (rhPDGF-BB) gel. Am J Surg. 1998;176:74S–79S. doi: 10.1016/s0002-9610(98)00185-8. [DOI] [PubMed] [Google Scholar]
- 5.Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003;90:133–146. doi: 10.1002/bjs.4019. [DOI] [PubMed] [Google Scholar]
- 6.Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40:368–377. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- 7.Suh W, Kim KL, Kim JM, Shin IS, Lee YS, Lee JY, Jang HS, Lee JS, Byun J, Choi JH, Jeon ES, Kim DK. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells. 2005;23:1571–1578. doi: 10.1634/stemcells.2004-0340. [DOI] [PubMed] [Google Scholar]
- 8.Badillo AT, Redden RA, Zhang L, Doolin EJ, Liechty KW. Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell Tissue Res. 2007;329:301–311. doi: 10.1007/s00441-007-0417-3. [DOI] [PubMed] [Google Scholar]
- 9.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1:4. doi: 10.1186/1755-1536-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner W, Ansorge A, Wirkner U, Eckstein V, Schwager C, Blake J, Miesala K, Selig J, Saffrich R, Ansorge W, Ho AD. Molecular evidence for stem cell function of the slow-dividing fraction among human hematopoietic progenitor cells by genome-wide analysis. Blood. 2004;104:675–686. doi: 10.1182/blood-2003-10-3423. [DOI] [PubMed] [Google Scholar]
- 13.Invernici G, Emanueli C, Madeddu P, Cristini S, Gadau S, Benetti A, Ciusani E, Stassi G, Siragusa M, Nicosia R, Peschle C, Fascio U, Colombo A, Rizzuti T, Parati E, Alessandri G. Human fetal aorta contains vascular progenitor cells capable of inducing vasculogenesis, angiogenesis, and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol. 2007;170:1879–1892. doi: 10.2353/ajpath.2007.060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 15.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 17.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659–671. doi: 10.1016/j.cellsig.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Charles PC, Wu Y, Ren R, Pi X, Moser M, Barshishat-Kupper M, Rubin JS, Perou C, Bautch V, Patterson C. Gene expression profile signatures indicate a role for Wnt signaling in endothelial commitment from embryonic stem cells. Circ Res. 2006;98:1331–1339. doi: 10.1161/01.RES.0000220650.26555.1d. [DOI] [PubMed] [Google Scholar]
- 20.Ezan J, Leroux L, Barandon L, Dufourcq P, Jaspard B, Moreau C, Allières C, Daret D, Couffinhal T, Duplàa C. FrzA/sFRP-1, a secreted antagonist of the Wnt-Frizzled pathway, controls vascular cell proliferation in vitro and in vivo. Cardiovasc Res. 2004;63:731–738. doi: 10.1016/j.cardiores.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Dufourcq P, Couffinhal T, Ezan J, Barandon L, Moreau C, Daret D, Duplàa C. FrzA, a secreted frizzled related protein, induced angiogenic response. Circulation. 2002;106:3097–3103. doi: 10.1161/01.cir.0000039342.85015.5c. [DOI] [PubMed] [Google Scholar]
- 22.Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 23.Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, Merchant A, Galiano RD, Tomic-Canic M. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167:59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alessandri G, Pagano S, Bez A, Benetti A, Pozzi S, Iannolo G, Baronio M, Invernici G, Caruso A, Muneretto C, Bisleri G, Parati E. Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages. Lancet. 2004;364:1872–1883. doi: 10.1016/S0140-6736(04)17443-6. [DOI] [PubMed] [Google Scholar]
- 25.Emanueli C, Graiani G, Salis MB, Gadau S, Desortes E, Madeddu P. Prophylactic gene therapy with human tissue kallikrein ameliorates limb ischemia recovery in type 1 diabetic mice. Diabetes. 2004;53:1096–1103. doi: 10.2337/diabetes.53.4.1096. [DOI] [PubMed] [Google Scholar]
- 26.Graiani G, Emanueli C, Desortes E, Van Linthout S, Pinna A, Figueroa CD, Manni L, Madeddu P. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of type 1 diabetic mice. Diabetologia. 2004;47:1047–1054. doi: 10.1007/s00125-004-1414-7. [DOI] [PubMed] [Google Scholar]
- 27.Riley CM, Fuegy PW, Firpo MA, Shu XZ, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis using dual growth factor-loaded crosslinked glycosaminoglycan hydrogels. Biomaterials. 2006;27:5935–5943. doi: 10.1016/j.biomaterials.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein D, Demory A, Peyre F, Kroll J, Augustin HG, Helfrich W, Kzhyshkowska J, Schledzewski K, Arnold B, Goerdt S. Wnt2 acts as a cell type-specific, autocrine growth factor in rat hepatic sinusoidal endothelial cells cross-stimulating the VEGF pathway. Hepatology. 2008;47:1018–1031. doi: 10.1002/hep.22084. [DOI] [PubMed] [Google Scholar]
- 29.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Wang J, Scott PG, Tredget EE. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007;15(suppl 1):S18–S26. doi: 10.1111/j.1524-475X.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- 31.Natesan S. Telomerase extends a helping hand to progenitor cells. Trends Biotechnol. 2005;23:1–3. doi: 10.1016/j.tibtech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Yanagi S, Kurosaki T, Yamamura H. The structure and function of nonreceptor tyrosine kinase p72syk expressed in hematopoietic cells. Cell Signal. 1995;7:185–193. doi: 10.1016/0898-6568(94)00088-s. [DOI] [PubMed] [Google Scholar]
- 33.Furuhata S, Ando K, Oki M, Aoki K, Ohnishi S, Aoyagi K, Sasaki H, Sakamoto H, Yoshida T, Ohnami S. Gene expression profiles of endo-thelial progenitor cells by oligonucleotide microarray analysis. Mol Cell Biochem. 2007;298:125–138. doi: 10.1007/s11010-006-9359-4. [DOI] [PubMed] [Google Scholar]
- 34.Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost. 2001;86:324–333. [PubMed] [Google Scholar]
- 35.Lacroix R, Sabatier F, Mialhe A, Basire A, Pannell R, Borghi H, Robert S, Lamy E, Plawinski L, Camoin-Jau L, Gurewich V, Angles-Cano E, Dignat-George F. Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood. 2007;110:2432–2439. doi: 10.1182/blood-2007-02-069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 37.Jacobsen SE. Defining ‘stemness’: Notch and Wnt join forces? Nat Immunol. 2005;6:234–236. doi: 10.1038/ni0305-234. [DOI] [PubMed] [Google Scholar]
- 38.Romano Di Peppe S, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, Capogrossi MC. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 2002;9:1271–1277. doi: 10.1038/sj.gt.3301798. [DOI] [PubMed] [Google Scholar]
- 39.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45:514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Kim HM, Kang DK, Kim HY, Kang SS, Chang SI. Angiogenin-induced protein kinase B/Akt activation is necessary for angiogenesis but is independent of nuclear translocation of angiogenin in HUVE cells. Biochem Biophys Res Commun. 2007;352:509–513. doi: 10.1016/j.bbrc.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 43.Masckauchán TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, Khoo A, Tycko B, Brown AM, Kitajewski J. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell. 2006;17:5163–5172. doi: 10.1091/mbc.E06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–6054. [PubMed] [Google Scholar]
- 46.Vlad A, Rohrs S, Klein-Hitpass L, Muller O. The first five years of the Wnt targetome. Cell Signal. 2008;20:795–802. doi: 10.1016/j.cellsig.2007.10.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


