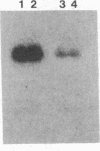
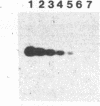
Abstract
Amino acid sequence similarity of the reverse transcriptases encoded by retroviruses and hepadnaviruses was first reported by Toh, H., Hayashida, H. & Miyata, T. (1983) Nature (London) 305, 827-829. The regions of similarity extend over a small number of amino acids and require the introduction of gaps through the open reading frame. By using an octapeptide region as the sole criterion for "taxonomic" classification, we have grouped the oncoviruses into two distinct categories and the lentiviruses and hepadnaviruses into two additional groupings. This classification suggests that murine and feline leukemia viruses may be more closely related to the viruses that are associated with leukemia in primates and cattle than had been appreciated. We have exploited a portion of this region because of the minimal translational codon degeneracy of the conserved residues. Unique oligonucleotides from this region have been designed and used in the primer-directed in vitro DNA amplification of the hepadnaviruses as a model system. In addition, mixtures of oligonucleotides with various sequences but of the same length were demonstrated to be efficient primers. The amplification procedure enabled dramatic increases in sensitivity and coincident detection of mammalian and avian genomes. This approach will be a valuable tool to detect and characterize members of viral groups. In addition, since short stretches of similarity have been frequently identified in related but distinct genes, such an approach could prove a valuable asset to molecular studies in general.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Kingston I. B. Isolation of a genomic clone for bovine pancreatic trypsin inhibitor by using a unique-sequence synthetic DNA probe. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6838–6842. doi: 10.1073/pnas.80.22.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Tucker A. D., Philipson L. Primary structural relationships may reflect similar DNA replication strategies. Virology. 1986 Mar;149(2):208–216. doi: 10.1016/0042-6822(86)90122-4. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Blumberg B. S., Gerstley B. J., Hungerford D. A., London W. T., Sutnick A. I. A serum antigen (Australia antigen) in Down's syndrome, leukemia, and hepatitis. Ann Intern Med. 1967 May;66(5):924–931. doi: 10.7326/0003-4819-66-5-924. [DOI] [PubMed] [Google Scholar]
- Burns J. C., Geha R. S., Schneeberger E. E., Newburger J. W., Rosen F. S., Glezen L. S., Huang A. S., Natale J., Leung D. Y. Polymerase activity in lymphocyte culture supernatants from patients with Kawasaki disease. 1986 Oct 30-Nov 5Nature. 323(6091):814–816. doi: 10.1038/323814a0. [DOI] [PubMed] [Google Scholar]
- Chardin P., Tavitian A. The ral gene: a new ras related gene isolated by the use of a synthetic probe. EMBO J. 1986 Sep;5(9):2203–2208. doi: 10.1002/j.1460-2075.1986.tb04485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I. M., Callahan R., Tronick S. R., Schlom J., Aaronson S. A. Major pol gene progenitors in the evolution of oncoviruses. Science. 1984 Jan 27;223(4634):364–370. doi: 10.1126/science.6197754. [DOI] [PubMed] [Google Scholar]
- Deen K. C., Sweet R. W. Murine mammary tumor virus pol-related sequences in human DNA: characterization and sequence comparison with the complete murine mammary tumor virus pol gene. J Virol. 1986 Feb;57(2):422–432. doi: 10.1128/jvi.57.2.422-432.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue P. R., Hoover E. A., Beltz G. A., Riedel N., Hirsch V. M., Overbaugh J., Mullins J. I. Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. J Virol. 1988 Mar;62(3):722–731. doi: 10.1128/jvi.62.3.722-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitelson M. A., Millman I., Halbherr T., Simmons H., Blumberg B. S. A newly identified hepatitis B type virus in tree squirrels. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2233–2237. doi: 10.1073/pnas.83.7.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H., Leunissen J., Goldbach R., Lomonossoff G., Zimmern D. Homologous sequences in non-structural proteins from cowpea mosaic virus and picornaviruses. EMBO J. 1984 Apr;3(4):855–861. doi: 10.1002/j.1460-2075.1984.tb01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Chen T. N., Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982 Jan;41(1):51–65. doi: 10.1128/jvi.41.1.51-65.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., DeFreitas E. C., Harper M. E., Sandberg-Wollheim M., Sheremata W. A., Robert-Guroff M., Saxinger C. W., Feinberg M. B., Wong-Staal F., Gallo R. C. Multiple sclerosis and human T-cell lymphotropic retroviruses. Nature. 1985 Nov 14;318(6042):154–160. doi: 10.1038/318154a0. [DOI] [PubMed] [Google Scholar]
- Kröger B., Horak I. Isolation of novel human retrovirus-related sequences by hybridization to synthetic oligonucleotides complementary to the tRNA(Pro) primer-binding site. J Virol. 1987 Jul;61(7):2071–2075. doi: 10.1128/jvi.61.7.2071-2075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Darby G. Related functional domains in virus DNA polymerases. EMBO J. 1987 Jan;6(1):169–175. doi: 10.1002/j.1460-2075.1987.tb04735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Purifoy D. J., Powell K. L., Darby G. Site-specific mutagenesis of AIDS virus reverse transcriptase. 1987 Jun 25-Jul 1Nature. 327(6124):716–717. doi: 10.1038/327716a0. [DOI] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion P. L., Oshiro L. S., Regnery D. C., Scullard G. H., Robinson W. S. A virus in Beechey ground squirrels that is related to hepatitis B virus of humans. Proc Natl Acad Sci U S A. 1980 May;77(5):2941–2945. doi: 10.1073/pnas.77.5.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Seal G., Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980 Dec;36(3):829–836. doi: 10.1128/jvi.36.3.829-836.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Kimber K. L., Summers J. W., Mason W. S. Mapping of the cohesive overlap of duck hepatitis B virus DNA and of the site of initiation of reverse transcription. J Virol. 1984 Jul;51(1):181–191. doi: 10.1128/jvi.51.1.181-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Ogston C. W., Jonak G. J., Rogler C. E., Astrin S. M., Summers J. Cloning and structural analysis of integrated woodchuck hepatitis virus sequences from hepatocellular carcinomas of woodchucks. Cell. 1982 Jun;29(2):385–394. doi: 10.1016/0092-8674(82)90155-6. [DOI] [PubMed] [Google Scholar]
- Ono M., Toh H., Miyata T., Awaya T. Nucleotide sequence of the Syrian hamster intracisternal A-particle gene: close evolutionary relationship of type A particle gene to types B and D oncovirus genes. J Virol. 1985 Aug;55(2):387–394. doi: 10.1128/jvi.55.2.387-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Yasunaga T., Miyata T., Ushikubo H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol. 1986 Nov;60(2):589–598. doi: 10.1128/jvi.60.2.589-598.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power M. D., Marx P. A., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986 Mar 28;231(4745):1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- Prince A. M. An antigen detected in the blood during the incubation period of serum hepatitis. Proc Natl Acad Sci U S A. 1968 Jul;60(3):814–821. doi: 10.1073/pnas.60.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Steele P. E., O'Neill R. R., Rabson A. B., Martin M. A. Nucleotide sequence of a full-length human endogenous retroviral segment. J Virol. 1985 Jun;54(3):764–772. doi: 10.1128/jvi.54.3.764-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Nucleotide sequence of an infectious molecularly cloned genome of ground squirrel hepatitis virus. J Virol. 1984 Aug;51(2):367–375. doi: 10.1128/jvi.51.2.367-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., O'Connell A., Millman I. Genome of hepatitis B virus: restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4597–4601. doi: 10.1073/pnas.72.11.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiollais P., Charnay P., Vyas G. N. Biology of hepatitis B virus. Science. 1981 Jul 24;213(4506):406–411. doi: 10.1126/science.6264599. [DOI] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]