Abstract
Background
Smaller hippocampal volume has been reported in some adult and pediatric studies of unipolar major depressive disorder. It is not clear whether the smaller hippocampal volume precedes or is a consequence of the illness. Early-life adversity is associated with both smaller hippocampal volume and increased vulnerability to depressive disorder. Hippocampal changes might mediate the relationship between early-life adversity and depressive illness in a subset of patients. However, there are no reports of longitudinal clinical studies that examined this issue.
Methods
Thirty adolescents with unipolar major depressive disorder, 22 adolescent volunteers with no personal history of a psychiatric illness including depression, but who were at high-risk for developing depression by virtue of parental depression (high-risk group), and 35 adolescent volunteers with no personal or family history of a psychiatric disorder (controls) underwent volumetric magnetic resonance imaging studies. Information also was gathered on early and recent adverse experiences with standard interviews. The participants were followed for up to 5 years to assess the onset and clinical course of depression.
Results
Depressed and high-risk groups had significantly smaller left and right hippocampal volumes than controls. Higher levels of early-life adversity were associated with smaller hippocampal volumes. Smaller hippocampal volume partially mediated the effect of early-life adversity on depression during longitudinal follow-up.
Conclusions
Smaller hippocampal volume in adolescents at high-risk for depression suggests that it might be a vulnerability marker for the illness. Early-life adversity might interact with genetic vulnerability to induce hippocampal changes, potentially increasing the risk for depressive disorder.
Keywords: abuse, adolescent, depression, high-risk, hippocampus, stress
INTRODUCTION
Depression is a world-wide problem particularly affecting teens and young adults (1). Commonly starting in the teens, there is a high risk of recurrence or persistence into adulthood along with ongoing psychosocial difficulties, including disruption in interpersonal relationships, early pregnancy, low educational attainment, poor occupational functioning and unemployment, as well as elevated risk for suicidal behavior (2–4). Efforts to identify youth at risk for depression may have significant personal and public health impact (4).
There is compelling evidence that early adverse experiences, such as parental loss and maltreatment, are associated with a higher risk for developing depression in later life (5–10). Based on the extensive literature in animals and limited studies in humans, it is believed that stress endured early in life, during a time of high neuronal plasticity, results in a persistent sensitization of the limbic-hypothalamic-pituitary-adrenal system to even mild stress in later life, contributing to the development and maintenance of depression (5, 11–14).
Preclinical research indicates that the hippocampus is highly susceptible to stress, possibly because of the protracted ontogeny, persistent post-natal neurogenesis and relatively high levels of glucocorticoid receptors (15–18). Smaller hippocampal volume has been reported in youngsters and adults exposed to early-life adversity (19), and also in individuals with depression (20–21). However, the nature of associations among early-life adversity, hippocampal volume deficit and depressive disorder are not well-understood. One possibility is that the relationship between early-life adversity and depression may be indirect, and their association might be mediated by hippocampal size. Alternatively, there might be an interaction between early-life adversity and hippocampal size in increasing the vulnerability to depression; early-life adversity might have a worse effect on depression particularly in individuals with a smaller hippocampal volume. Another possibility is that early-life adversity and depression interact to induce changes in the hippocampus, with smaller hippocampal size occurring particularly in depressed patients who experienced early-life adversity (20–22). Under this scenario, smaller hippocampal size is a consequence of depressive disorder (as opposed to being a vulnerability marker for the illness), with greater reduction in size as the illness progresses. The literature is not clear on this issue. Some studies in adults reported that hippocampal volume was smaller with greater duration of illness and/or number of depressive episodes (20–21, 23–26). However, smaller hippocampal volume was observed in depressed adolescents, many of whom had relatively short duration of illness compared with adults (20–21, 27). Investigations in at-risk individuals will be helpful in determining whether hippocampal size deficit occurs before the clinical manifestation of depression. To the best of our knowledge, there are no reports on hippocampal volume in a high-risk population although one study found relatively normal hippocampal shape in non-affected co-twins discordant for depression (28).
Using a longitudinal design, the current study was undertaken to examine the relationships among early-life adversity, morphological changes in the hippocampus and vulnerability to depressive disorder in three groups of adolescents: volunteers at low-risk for depression (control group), healthy volunteers at high-risk for depression (high-risk group), and volunteers with major depressive disorder (depressed group). We hypothesized that: (1) high-risk and depressed adolescents will have higher levels of early-life adversity and lower hippocampal volumes than controls; (2) regardless of the group status, higher levels of early-life adversity will be associated with lower hippocampal volumes after accounting for chronic stress during adolescence; and (3) lower hippocampal volume will mediate and/or moderate the relationship between early-life adversity and depressive episodes during prospective follow-up.
METHODS AND MATERIALS
These data are part of a larger ongoing study on the development and course of depression in adolescents, as well as the relationship between depression and substance use disorders. The studies were performed at two sites, Harbor-UCLA Medical Center and UT Southwestern Medical Center. Due to space limitations, the methods are described briefly and additional information is provided in Supplement 1.
Participants
With approvals from the local Institutional Review Boards, 30 adolescents with depression, 22 adolescents at high-risk for depression and 35 controls were recruited. All participants were between 12–20 years, and Tanner Stage III, IV or V of pubertal development (29–30). The depressed adolescents met criteria for major depressive disorder (31), with a minimum duration of four weeks. Adolescents with a current or prior history of mania, hypomania, substance use disorder symptoms, schizophrenia, schizoaffective disorder or autism were excluded from the study. Subjects also were excluded if there was a family history of bipolar disorder. The participants were free from psychotropic agents for at least eight weeks. Adolescents at high-risk for depression had no personal history of a psychiatric disorder, including depression, but at least one biological parent had a history of unipolar major depressive disorder. Controls were free from any type of psychopathology in their lifetime. Controls were not included in the study if any first-degree relative had history of a major psychiatric disorder. All participants were medically healthy and free from alcohol or illicit drug use.
Diagnostic Evaluation
The diagnosis of major depression and other psychiatric disorders was based on a semi-structured instrument, the Schedule for Affective Disorders and Schizophrenia for School-Age Children - the Present and Lifetime Version (K-SADS-PL; 32), designed to ascertain present and lifetime history of psychiatric illness according to DSM-IV criteria. It was administered separately to the adolescent and parent, and both were re-interviewed to resolve discrepancies. Summary scores were tabulated based on the information obtained from both informants. The Hamilton Depression Rating Scale (HDRS; 33) and the Children’s Global Assessment Scale (CGAS; 34), a global psychosocial functioning measure, also were completed. The adolescents completed the Beck Depression Inventory (BDI; 35) for self-assessment of depression severity.
The Family History-Research Diagnostic Criteria interview method (FH-RDC) was used to evaluate psychiatric disorders in family members (36). A parent was interviewed regarding life-time psychiatric disorders in all first-degree relatives of the adolescent subject (including the self, spouse and all offspring). Information on socioeconomic status (SES) was assessed with the Hollingshead Scale (37).
Early-Life Adversity
Information on early-life adversity was obtained with a semi-structured interview that had good inter-rater reliability (38). The adolescent and parent were interviewed separately. Information was obtained on seven subtypes of childhood adversity that persisted for 6 months or longer (separation/loss, life-threatening illness/injury, physical neglect, emotional abuse/assault, physical abuse/assault, witnessing violence, and sexual abuse/assault) that occurred prior to age 11 years. The adverse impact (1 = no adversity, and 5 = most severe form of adversity) was based on summaries of events, circumstances and their contexts. Information from both informants was combined for the summary ratings. Early-life adversity score was tabulated from the sum of ratings from the seven adversity domains.
Chronic Stress during Adolescence
In order to control for ongoing stress during adolescence, a semi-structured interview (39) elicited information about chronic stress in nine content areas (family, friends, romantic relationships, social life, school, work, finances, health of the subject, and health of the family members). The adolescent was interviewed by a trained clinician on the quality of relationships and performance in each domain within the past 6 months, and ratings were given for the magnitude of stress in each domain based on objective and behaviorally-specific criteria (1 = not at all stressful, and 5 = extremely stressful). Good inter-rater reliabilities have been established for the chronic stress ratings (40).
Magnetic Resonance Imaging (MRI)
Structural images of the brain were obtained using a General Electric (GE) 1.5 Tesla scanner with a transmit/receive quadrature RF head coil. To avoid large motions, the head was immobilized with tightly-fitting foam padding and a strap was fastened across the forehead. High-resolution T1 images of the entire brain were acquired in the sagittal plane (3D SPGR pulse sequence: TR = 19 ms; TE = 4.6 ms; flip angle = 30°; matrix = 192 × 256; FOV = 230 mm; slice thickness = 1.2 mm).
The raw data were converted to analyze image format using Automated Functional Neuro-Imaging software (AFNI; 41). Tissue segmentation and volumetric analysis were done using Analysis of Images, Networks and Systems Software (BRAINS2; 42). Image data were re-sampled to align with anterior commissure-posterior commissure and then anatomical boundaries of the left hippocampus, right hippocampus and total brain volume (TBV) were traced manually using established methods, differentiating the amygdala and hippocampus (43–44). All planes were used to trace the hippocampus with most tracing done in the coronal plane because it had the best resolution. Sagittal and axial planes were used as references (45–46).
TBV was measured using a standard protocol which includes the combined volume of the cerebral hemispheres, cerebellum and the brainstem (47). Both hippocampal volumes were adjusted for TBV using the formula left/right hippocampal volume/TBV*100. Anatomical measurements were conducted by trained, reliable raters. Intra-class correlations (n=15) were as follows: left hippocampus: 0.91; right hippocampus: 0.92; and TBV: 0.98. All raters were blind to the diagnostic status and other clinical information.
Follow-up Evaluation
Participants were followed longitudinally after the baseline assessment at 6-month intervals for up to 5 years. In addition to obtaining information from the K-SADS-PL during each follow-up evaluation, the Longitudinal Interval Follow-up Evaluation (LIFE) was used to document the onset and clinical course of depression. The LIFE is a semi-structured instrument used for charting the clinical course of depression and other psychiatric disorders during longitudinal follow-up (48). Development of a depressive episode was defined as a rating of ≥5 on the Psychiatric Status Rating (PSR) component of the LIFE for a minimum of 4-week duration. Remission from a depressive episode was defined as a rating of ≤2 for ≥12 weeks on the PSR. Recurrence was defined as a PSR rating of ≥5 for four or more weeks with a minimum duration of 12 weeks between episodes. The conventional criterion for remission/recovery is 8 weeks, and the minimum duration for onset/recurrent depressive episode is 2 weeks (31, 49). In the current study, more stringent duration criteria were used to ensure the stability of symptoms because depressive symptoms in youngsters tend to be more variable, with greater heterogeneity in clinical response and course, than adults (4, 50). Information from the diagnostic assessments was rated by an independent clinician “blind” to the baseline clinical and MRI data.
Statistical Analysis
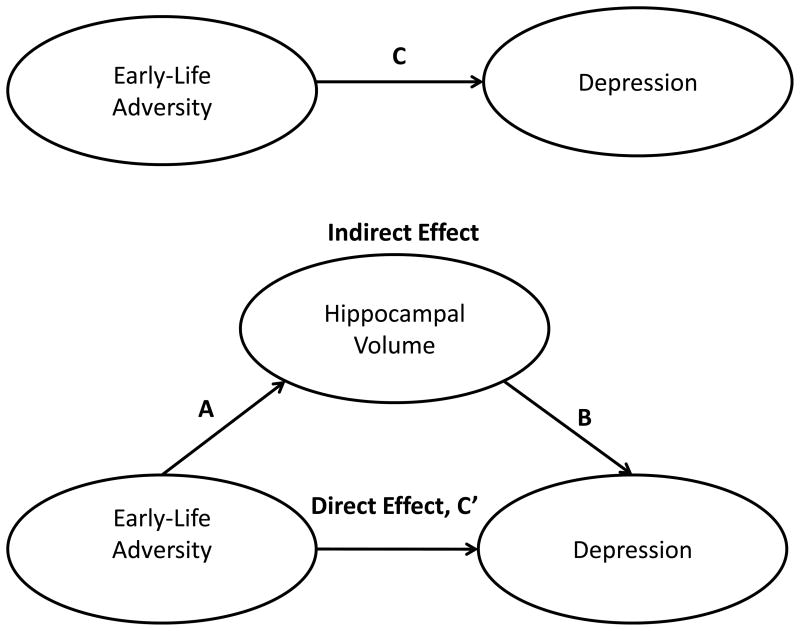
Descriptive analyses employed chi-square, analysis of variance (ANOVA) and correlations as warranted by variable characteristics. Multivariate analysis of variance (MANOVA) was conducted to compare hippocampal volumes by group; following an overall significant MANOVA, univariate ANOVAs compared individual means. Kaplan-Meier survival analysis was used to compute the probability of depressive episodes during follow-up of varying lengths. The mediation analyses were performed using three regression (Cox and Linear) equations as described by MacKinnon et al. (51; see Figure 1). These equations established the total effects of early-life adversity on depression (path C) and hippocampal volume (path A), the effect of hippocampal volume on depression after controlling for early-life adversity (path B), and the direct effect of early-life adversity on depression controlling for hippocampal volume (path C′). An estimate of the indirect or mediated effect (AB) was calculated as the product of A and B (52). The program, PRODCLIN, was used to calculate the 95% confidence interval (CI) of the indirect effect (53). Alpha was set at .05. The interaction effect of early-life adversity and hippocampal size on depression was tested using Cox regression after computing the product of early-life adversity and hippocampal size.
Figure 1.
Statistical mediational model to depict the associations among early-life adversity, hippocampal volume and depression in adolescents.
RESULTS
Demographic and Clinical Characteristics of the Sample
MR scans were aborted in one control and one depressed participant when they reported anxiety. Data from two controls had significant motion artifacts. Therefore, results are presented for 32 control, 22 high-risk and 29 depressed participants. Demographic and clinical features of the sample are outlined in Table 1. The groups did not differ significantly with respect to age, gender, ethnicity/race or pubertal status. The depressed and high-risk groups had lower SES, and a higher magnitude of early-life adversity and chronic stress during adolescence, than controls. Depressed adolescents had higher BDI and HDRS scores than the control and high-risk groups. Depressed adolescents also had a lower CGAS score than controls, and the high-risk group was intermediate. Of 29 adolescents with depression, 15 (51.7%) had a history of parental depression, and only two (6.9%) had a prior history of antidepressant treatment. By definition, all high-risk subjects had a parent with a history of depression.
Table 1.
Baseline demographic and clinical characteristics by diagnosis
| Control (n = 32) | High-Risk (n = 22) | Depressed (n= 29) | Statistic | p | |
|---|---|---|---|---|---|
| Age (years) | 15.1 (1.6) | 15.0 (1.7) | 14.6 (1.9) | 0.65 | NS |
| Gender | 4.09 | NS | |||
| male | 19 (59.4) | 13 (54.5) | 10 (34.1) | ||
| female | 13 (40.6) | 9 (45.5) | 19 (65.5) | ||
| Ethnicity | 0.30 | NS | |||
| Caucasian | 15 (46.9) | 9 (40.9) | 14 (48.3) | ||
| Non-Caucasian | 17 (53.1) | 13 (59.1) | 15 (51.7) | ||
| Pubertal status | 0.03 | NS | |||
| Tanner Stage III | 4 (12.5) | 3 (13.6) | 4 (13.8) | ||
| Tanner Stage IV | 10 (31.3) | 7 (31.8) | 9 (31.0) | ||
| Tanner Stage V | 18 (56.3) | 12 (54.5) | 16 (55.2) | ||
| Socioeconomic status1 | 47.4 (8.5)a | 40.2 (10.9)b | 38.1 (9.8)b | 6.81 | .002 |
| Beck Depression Inventory | 1.6 (2.2)a | 3.1 (2.8)a | 14.3 (8.9)b | 39.11 | .0001 |
| Hamilton Depression Scale | 1.2 (1.7)a | 1.6 (2.7)a | 20.0 (5.5)b | 227.90 | .0001 |
| CGAS score1 | 85.0 (8.9)a | 74.9 (10.2)b | 57.0 (12.4)c | 36.32 | .0001 |
| Early-life adversity | 9.0 (2.2)a | 12.5 (3.9)b | 11.6 (3.8)b | 8.46 | .0001 |
| Adolescent chronic stress | 17.6 (3.2)a | 20.7 (3.4)b | 22.5 (4.6)b | 12.61 | .0001 |
CGAS = Children’s Global Assessment Scale
Higher score is associated with higher socioeconomic status or higher level of functioning
Data in parentheses reflect standard deviations or percentages
Different subscripts denote significant differences among groups
Relationship between Demographic/Clinical Variables and Hippocampal Volume
The right and left hippocampal volumes correlated highly with each other (r = .89, df = 83, p = .0001). Age, SES, BDI, HDRS and CGAS did not correlate significantly with the hippocampal volume. Gender, ethnicity/race and pubertal status also did not have a significant effect on hippocampal volume.
Group Comparisons on Hippocampal Volume
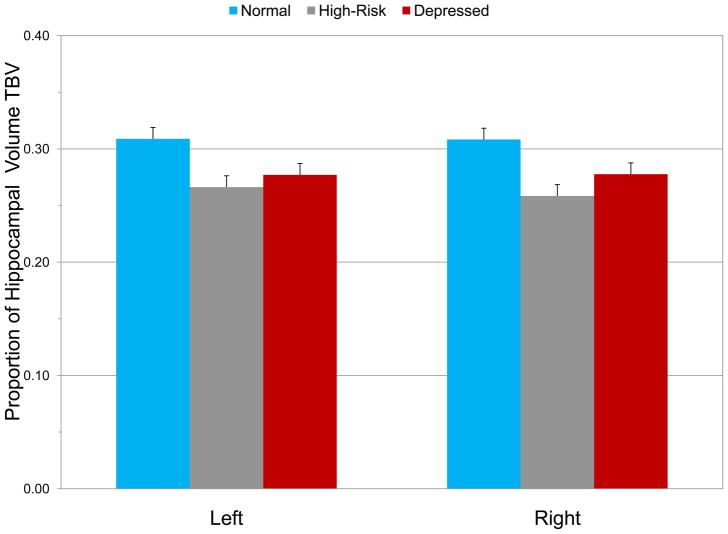
After controlling for age, gender, ethnicity/race, pubertal status and SES, diagnostic status had a significant effect on hippocampal volume (F4,148 = 5.32, p = .0001). None of the covariates had a significant effect on hippocampal volume even in the multi-variate analysis. In post-hoc analyses, the depressed and high-risk groups had smaller hippocampal volume than controls bilaterally (Left: F2,75 = 8.54, p = .0001; Right: F2,75 = 10.26, p = .0001; see Figure 2). Compared with controls, the left hippocampal volume was smaller by 4.2% and the right by 4.9% in the high-risk group, and 3.1% and 3.0%, respectively in the depressed group.
Figure 2.
Left and right hippocampal volumes (mean ± SEM) in control, high-risk and depressed groups (Significant differences in hippocampal volume after controlling for age, gender, ethnicity/race, pubertal status and socioeconomic status; Left: controls vs. high-risk, p = .0001; controls vs. depressed, p = .002; Right: controls vs. high-risk, p = .0001; controls vs. depressed, p = .005).
Effect of Early-life Adversity on Hippocampal Volume
After controlling for chronic stress during adolescence, higher scores on early-life adversity were associated with smaller hippocampal volumes in the controls (Left: r = −.29, df = 29, NS; Right: r = −.44, p = .02), and in high-risk participants (Left: r = −.52, df = 18, p = .02; Right: r = −.54, p = .02). In the depressed cohort, the association between early life-adversity and hippocampal volume was moderated by parental depression. Among adolescents with parental history of depression, early-life adversity correlated negatively with hippocampal volume (Left: r = −.68, df = 12, p = .007; Right: r = −.43, NS). In contrast, early-life adversity correlated only modestly in adolescents without family history (Left: r = .25, df = 11, NS; Right: r = .05, NS).
Follow-up Information
Follow-up information was available for 79/83 (95.2%) participants. Subjects who did not participate in follow-up assessments did not differ significantly from those with follow-up information on any baseline characteristics. Recruitment did not occur simultaneously and, therefore, not all subjects were studied longitudinally for the same period of time. Of the 79 adolescents who had follow-up information, 7.6% were followed for 1 year, 20.2% for 2 years, 22.8% for 3 years, 21.6% for 4 years and 27.8% for 5 years. The three groups were comparable on the mean follow-up interval (3.1 ± 1.3 years in controls, 3.0 ± 1.2 in the high-risk group, and 3.2 ± 1.3 in the depressed group; F2,76 = 0.39, NS).
Depressive Episode during Follow-up
Of 31 controls with follow-up information, two (6.5%) developed a depressive episode compared to 6/21 (28.6%) high-risk subjects. In the depressed cohort, 11/27 (40.7%) developed a recurrent episode. Participants with and without recurrent episodes did not differ significantly on treatment history. The groups differed significantly on the probability of depression during follow-up (Mantel-Cox χ2 = 9.45, df = 2, p = .009), with controls differing having significantly lower risk than high-risk (χ2 = 5.67, p = .02) and depressed youth (χ2 = 10.18, p = .001). In the controls, the probability of depression was 0% in the first two years, 6.7% in the third year and 14.4% in the fourth year. In the high-risk group, the probability of depression was 4.8% in the first year, 24.8% in the second year, and 32.3% in the third year. Among depressed adolescents, the probability of a recurrent episode was 7.4% in the first year, 21.9% in the second year, 44.7% in the third year, and 57.9% in the fourth year.
Associations among Early-life Adversity, Hippocampal Volume and Depression at Follow-up
The relationships among early-life adversity, hippocampal volume and depressive disorder are depicted in Table 2 (also see Figure 1). After controlling for age, gender, ethnicity/race, pubertal status and SES, early-life adversity was associated with depressive disorder (path C) and hippocampal volume (path A). When hippocampal volume was included in the model, the effect of early-life adversity on depression was reduced (path C′). After controlling for early-life adversity, hippocampal volume predicted depression (path B) during follow-up. However, the mediational effect was not statistically significant (Left: AB = 0.08, CI = 0.00–0.16, z = 1.85, Sobel Test, p = .06, Cohen’s d = 1.11; Right: AB = 0.07, CI = −0.01–0.15, z = 1.80, Sobel Test, p = .07, Cohen’s d = 1.44). Although the Sobel Test was not statistically significant, the coefficient of path C′ for the left hippocampus (beta = 0.18) was less than the coefficient of path C (AB+C′ = 0.26). This also was true for the right hippocampus (path C′ = 0.20; AB+C′ = 0.28). Hippocampal size did not moderate the relationship between early-life adversity and depression (interaction effect: Left: β = −0.09, SE = 0.30, OR = 0.92, CI = 0.51–1.66, F = 0.01, NS; Right: β = −0.28, SE = 0.29, OR = 0.76, CI = 0.43–1.33, F = 0.94, NS).
Table 2.
Mediational analyses predicting major depressive episode at follow-up1
| Predictor | Path | β | F | OR (95% CI) | Comparable β (SE)a |
|---|---|---|---|---|---|
| Left hippocampal vol. | |||||
| Major depressive episode | |||||
| Early-life adversity | C | 0.64* | 5.74 | 1.89 (1.12–3.19) | 0.33 (0.14) |
| Hippocampal volume | |||||
| Early-life adversity | A | −0.37** | 3.05 | 0.37 (0.13–0.62) | −0.20 (0.06) |
| Major depressive episode | |||||
| Early-life adversity | C′ | 0.38 | 1.95 | 1.46 (0.86–2.47) | 0.18 (0.13) |
| Hippocampal volume | B | −0.83* | 5.24 | 0.44 (0.22–0.89) | −0.40 (0.17) |
| Right hippocampal vol. | |||||
| Major depressive episode | |||||
| Early-life adversity | C | 0.64* | 5.74 | 1.89 (1.12–3.19) | 0.33 (0.14) |
| Hippocampal volume | |||||
| Early-life adversity | A | −0.41*** | 3.38 | 0.41 (0.17–0.65) | −0.22 (0.06) |
| Major depressive episode | |||||
| Early-life adversity | C′ | 0.41 | 2.23 | 1.51(0.88–2.58) | 0.20 (0.14) |
| Hippocampal volume | B | −0.68* | 4.38 | 0.51 (0.27–0.96) | −0.33 (0.16) |
Age, gender, ethnicity/race, pubertal status and socioeconomic status served as covariates in the analyses.
β = beta; OR = odds-ratio; CI = confidence interval; SE = standard error.
C = total effects of early-life adversity on depression; A = effect of early-life adversity on hippocampal volume; B = effect of hippocampal volume on depression after controlling for early-life adversity; and C′ = direct effect of early-life adversity on depression controlling for hippocampal volume.
Due to the scale differences between coefficients from ordinary least-squares regression and Cox regression, comparable coefficients were calculated by multiplying each coefficient by the standard deviation of the predictor variable and dividing by the standard deviation of the criterion variable.
p ≤ .05;
p ≤ .005;
p ≤ .001.
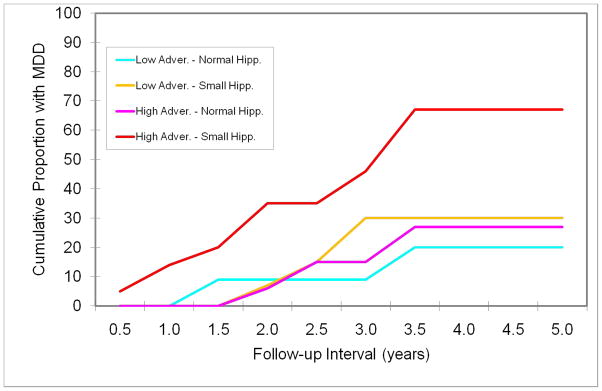
For the purpose of graphical representation, the sample was stratified into four groups based on a median split of early-life adversity and right hippocampal size: (1) low adversity-normal hippocampus (n = 24); (2) low adversity-small hippocampus (n = 16); (3) high adversity-normal hippocampus (n = 17); and (4) high adversity-small hippocampus (n = 21). The groups then were compared on the probability of a depressive episode during follow-up (see Figure 3). Among adolescents with low-adversity and normal hippocampal volume, 20.3% were likely to develop a depressive episode by 3.2 years (mean follow-up period), whereas 56.6% of those with high adversity and small hippocampal volume were likely to develop a depressive episode during the corresponding period (Mantel-Cox χ2 = 8.79, df = 3, p = .03). The probability of depression during that period was 30.4% in subjects with low adversity and small hippocampal volume, and 21.4% in those with high adversity and normal hippocampal volume.
Figure 3.
Probability of a major depressive disorder (MDD) episode during follow-up, stratified by early-life adversity and hippocampal volume (right) measured during the initial evaluation (high adversity-small hippocampal volume vs. low adversity-normal hippocampal volume: χ2 = 5.74, p = .02; high adversity-small hippocampal volume vs. low adversity-small hippocampal volume: χ2 = 3.03, p = .08; high adversity-small hippocampal volume vs. high adversity-normal hippocampal volume: χ2 = 4.15, p = .04).
Although the interaction of early-life adversity and hippocampal volume was not statistically significant, these data suggest that the combination of early-life adversity and smaller hippocampal size significantly increases the vulnerability to depressive episodes. Examination of the data separately in control and high-risk groups (new episode of depression) and depressed youth (recurrent episode) showed the same pattern.
Site Effects
There were no significant group differences between the two sites on any of the major variables of interest. Additionally, the analyses were repeated separately at both sites, and the same pattern of results emerged.
DISCUSSION
The findings of this study replicate and extend prior work (19–21). Smaller hippocampal volume was observed in depressed adolescents (20–21). The results also indicated that morphological changes in the hippocampus might be evident before clinical manifestation of the illness in individuals at high-risk for the disorder (27). Higher levels of adversity during childhood increased the likelihood of smaller hippocampal volume in at-risk youth even after controlling for chronic stress during adolescence (18–19, 54). Familial depression moderated the relationship early-life adversity and hippocampal volume in the depressed youth such that smaller hippocampal volume was observed only in adolescents who had familial depression and high levels of early-life adversity. Smaller hippocampal volume partially mediated the effect of early-life adversity on depression during longitudinal follow-up. Taken together, these findings suggest that genetic differences might make the hippocampus more vulnerable to the effects of early-life adversity (55), potentially increasing the risk for depressive disorder.
These results should be interpreted with caution for the following reasons. The participants were recruited from a group of volunteers based on specific inclusion/exclusion criteria, and the findings might not be generalizable to community samples of adolescents. The sample sizes were modest, and the follow-up interval was relatively short for documenting the onset and clinical course of depression. Information on early-life adversity was obtained retrospectively and, therefore, is subject to reporting bias. Also, the participants were not recruited based on early-life adversity, and the sample was heterogeneous with respect to adversity type and severity. In particular, there were only a few controls with high adversity scores. Nevertheless, the variability in exposure to early-life adversity facilitated the examination of associations among adversity level, hippocampal morphometry and vulnerability to depression. Moreover, the co-occurrence of multiple types of early-life adversity is common, and previous research has shown that combined exposure to less severe forms of adversity might be just as deleterious as the most severe form of a single type of trauma (8, 56–57).
Pubertal status was assessed only from physical characteristics (29–30), and gonadal steroid levels were not obtained. Gonadal steroids can potentially influence depressive symptoms and hippocampal volume (58–59). It is not known whether the observed hippocampal changes were the sequalae of early-life adversity or due to genetic variations in hippocampal morphology. Genetic factors have been shown to influence hippocampal size (55,60). In an investigation of adult monozygotic twins discordant for trauma exposure, hippocampal volumes were smaller in both exposed and unexposed twin pairs in whom the combat-exposed twin developed severe symptoms of post-traumatic stress disorder (60). This finding suggests that smaller hippocampal size might be an inherited trait that predisposes individuals towards the development of psychiatric disorders that are triggered or aggravated by stress (55,60).
Although early-life adversity and smaller hippocampal volume were associated with vulnerability to depressive illness, their effects were modest. Simultaneously examining other factors, such as genetic polymorphisms, hypothalamic-pituitary-adrenal (HPA) activity, coping mechanisms and social support, will be helpful in explaining a greater part of the variance associated with vulnerability to depressive disorder (7, 13–14, 61–64). Also, it is not known whether depressive episodes would induce further changes in hippocampal morphometry. In a recent study, adult patients with depression showed a greater decline in gray matter density of the hippocampus than controls after 3 years, particularly in those who failed to remit from the index depressive episode (23).
Although morphological changes in the hippocampus have been associated with unipolar depression, not all studies replicated these findings (20–21). The variability in findings might be attributed to sample size, developmental stage of the sample, number of depressive episodes, duration of illness, family history of depression, history of early-life adversity, and the inclusion of amygdala while performing morphometry (20–27).
The hippocampus continues to develop after birth and neurogenesis also continues to some degree after birth (15–16, 18). The hippocampus also has relatively high concentrations of glucocorticoid and mineralocorticoid receptors, both of which are involved in HPA regulation (17, 65). The density of synaptic connections in the hippocampus also fluctuates with age (66). These factors might contribute to its vulnerability to stress, particularly during the early developmental period (18, 67), although not all studies have demonstrated this effect (55, 68).
If, indeed, early-life adversity and/or protracted stress cause hippocampal atrophy, there is a potential opportunity to mitigate the hippocampal volume deficit through pharmacological or psychotherapeutic interventions. For example, if high levels of corticosteroids are neurotoxic (17, 69), antiglucocorticoid agents and antagonists of the corticotropin-releasing hormone (CRH) can be helpful in reducing glucocorticoid levels. Anti-glucocorticoid agents and CRH antagonists might have anti-depressant properties, and have been tested in humans for the treatment of depression and other psychiatric disorders (70–72). Also, data from clinical and preclinical studies suggest that treatment with antidepressant drugs reduces physiological responsivity to stress and increases neurogenesis (15, 73). However, in a recent report, adult patients with chronic major depression and childhood trauma responded poorly to antidepressant medication, whereas a better response to combined treatment with antidepressant and psychotherapy was observed (74).
In summary, smaller hippocampal volume was observed before the manifestation of clinical symptoms of depression in at-risk adolescents, particularly in those who experienced high levels of adversity during childhood. Smaller hippocampal volume partially mediated the effect of early-life adversity in increasing the probability of depressive episodes during longitudinal follow-up. These findings have potential clinical implications for identifying youngsters at highest risk for the disorder.
Supplementary Material
Acknowledgments
This work was supported in part by grants DA14037, DA15131, DA17804, DA17805, MH01419, MH62464 and MH68391 from the National Institutes of Health, from the National Alliance for Research on Schizophrenia and Affective Disorders, and by the Sarah M. and Charles E. Seay Endowed Chair in Child Psychiatry at UT Southwestern Medical Center. The authors would like to express gratitude to Evelyn Babcock, Ph.D., for assistance with MRI scans, Michael Coffey, Thien Do, Diana Huang and Srirangam Muddasani for assistance with brain volumetric measures, and Karen Harker, M.P.H., for the management of statistical database.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The World Bank. The Global Burden of Disease and Risk Factors. Washington, D.C: The international Bank for Reconstruction and Development/The World Bank; New York: Oxford University Press; 2006. [Google Scholar]
- 2.Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 4.Rao U, Chen LA. Characteristics, correlates, and outcomes of childhood and adolescent depressive disorders. Dialogues Clin Neurosci. 2009;11:45–62. doi: 10.31887/DCNS.2009.11.1/urao. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics; Stirling J, Jr, Amaya-Jackson L The Committee on Child Abuse and Neglect and Section on Adoption and Foster Care, American Academy of Child and Adolescent Psychiatry; National Center for Child Traumatic Stress. Understanding the behavioral and emotional Consequences of child abuse. Pediatrics. 2008;122:667–673. doi: 10.1542/peds.2008-1885. Erratum in: Pediatrics. 2009 123(1):197. [DOI] [PubMed] [Google Scholar]
- 6.Brown GW, Harris TO. Depression and the serotonin transporter 5-HTTLPR polymorphism: a review and a hypothesis concerning gene-environment interaction. J Affect Disord. 2008;111:1–12. doi: 10.1016/j.jad.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 8.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 9.Espejo EP, Hammen CL, Connolly NP, Brennan PA, Najman JM, Bor W. Stress sensitization and adolescent depressive severity as a function of childhood adversity: a link to anxiety disorders. J Abnorm Child Psychol. 2007;35:287–299. doi: 10.1007/s10802-006-9090-3. [DOI] [PubMed] [Google Scholar]
- 10.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman J, Charney D. Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Dev Psychopathol. 2001;13:451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- 13.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bansar M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6:311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- 16.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 17.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 19.Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18:729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- 20.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 21.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 22.Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frodl TS, Koutsouleris N, Bottlender R, Born C, Jager M, Scupin I, et al. Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry. 2008;65:1156–1165. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- 24.Kronmuller KT, Pantel J, Kohler S, Victor D, Giesel F, Magnotta VA, et al. Hippocampal volume and 2-year outcome in depression. Br J Psychiatry. 2008;192:472–473. doi: 10.1192/bjp.bp.107.040378. [DOI] [PubMed] [Google Scholar]
- 25.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacMaster FP, Mirza Y, Szeszko PR, Kmiecik LE, Easter PC, Taormina SP, et al. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol Psychiatry. 2008;63:385–390. doi: 10.1016/j.biopsych.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park Y, Priebe CE, Miller MI, Mohan NR, Botteron KN. Statistical analysis of twin populations using dissimilarity measurements in hippocampus shape space. J Biomed Biotechnol 2008. 2008:694297. doi: 10.1155/2008/694297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: 1994. [Google Scholar]
- 32.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-aged Children-Present and Lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997:36. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;25:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 35.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 36.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 37.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University Department of Sociology; 1975. [Google Scholar]
- 38.Dienes KA, Hammen C, Henry RM, Cohen AN, Daley SE. The stress sensitization hypothesis: understanding the course of bipolar disorder. J Affect Disord. 2006;95:43–49. doi: 10.1016/j.jad.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Hammen CL, Burge D, Daley SE, Davila J, Paley B, Rudolph KD. Interpersonal attachment cognitions and prediction of symptomatic responses to interpersonal stress. J Abnorm Psychol. 1995;104:436–443. [PubMed] [Google Scholar]
- 40.Hammen C, Brennan PA. Depressed adolescents of depressed and nondepressed mothers: tests of an interpersonal impairment hypothesis. J Consult Clin Psychol. 2001;69:284–294. doi: 10.1037//0022-006x.69.2.284. [DOI] [PubMed] [Google Scholar]
- 41.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 42.Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 43.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shenton ME, Gerig G, McCarley RW, Szekely G, Kikinis R. Amygdala-hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data. Psychiatry Res. 2002;115:15–35. doi: 10.1016/s0925-4927(02)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duvernoy HM. The Human Hippocampus: Functional Anatomy Vascularization and Serial Sections with MRI. 3. New York: Springer; 2005. [Google Scholar]
- 46.Cook MJ, Fish DR, Shorvon SD, Straughan K, Stevens JM. Hippocampal volumetric and morphometric studies in frontal and temporal lobe epilepsy. Brain. 1992;115 (Pt 4):1001–1015. doi: 10.1093/brain/115.4.1001. [DOI] [PubMed] [Google Scholar]
- 47.Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158:618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro R, Keller M. Longitudinal Interval Follow-Up Evaluation (LIFE) Boston: Massachusetts General Hospital; 1979. [Google Scholar]
- 49.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 50.Kaufman J, Martin A, King RA, Charney D. Are child-, adolescent-, and adult-onset depression one and the same disorder? Biol Psychiatry. 2001;49:980–1001. doi: 10.1016/s0006-3223(01)01127-1. [DOI] [PubMed] [Google Scholar]
- 51.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackinnon DP, Dwyer JH. Estimating Mediated Effects in Prevention Studies. Eval Rev. 1993;17:144–158. [Google Scholar]
- 53.MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav Res Methods. 2007;39:384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen RA, Paul RH, Stroud L, Gunstad J, Hitsman BL, McCaffery J, et al. Early life stress and adult emotional experience: an international perspective. Int J Psychiatry Med. 2006;36:35–52. doi: 10.2190/5R62-9PQY-0NEL-TLPA. [DOI] [PubMed] [Google Scholar]
- 55.Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF. Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry. 2001;58:1145–1151. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- 56.Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am J Psychiatry. 2006;163:993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- 57.Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- 58.Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- 59.Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- 60.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 63.Rao U, Hammen CL, Poland RE. Longitudinal course of adolescent depression: neuroendocrine and psychosocial predictors. J Am Acad Child Adolesc Psychiatry. doi: 10.1097/00004583-201002000-00008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res. 2000;34:383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 66.Eckenhoff MF, Rakic P. A quantitative analysis of synaptogenesis in the molecular layer of the dentate gyrus in the rhesus monkey. Brain Res Dev Brain Res. 1991;64:129–135. doi: 10.1016/0165-3806(91)90216-6. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 68.Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early-life stress induces long-term morphologic changes in primate brain. Arch Gen Psychiatry. 2009;66:658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119:509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- 70.Gallagher P, Malik N, Newham J, Young AH, Ferrier IN, Mackin P. Antiglucocorticoid treatments for mood disorders. Cochrane Database Syst Rev. 2008:CD005168. doi: 10.1002/14651858.CD005168.pub2. [DOI] [PubMed] [Google Scholar]
- 71.Seymour PA, Schmidt AW, Schulz DW. The pharmacology of CP-154,526, a non-peptide antagonist of the CRH1 receptor: a review. CNS Drug Rev. 2003;9:57–96. doi: 10.1111/j.1527-3458.2003.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holsboer F, Ising M. Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 73.Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- 74.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.