Abstract
Store-operated Ca2+ entry (SOCE) is a universal mechanism to increase intracellular Ca2+ concentrations in non-excitable cells. It is initiated by the depletion of ER Ca2+ stores, activation of stromal interaction molecule (STIM) 1 and gating of the Ca2+ release activated Ca2+ (CRAC) channel ORAI1 in the plasma membrane. We identified a minimal activation domain in the cytoplasmic region of STIM1 (CCb9) which activated Ca2+ influx and CRAC currents (ICRAC) in the absence of store depletion similar to but more potently than the entire C-terminus of STIM1. A STIM1 fragment (CCb7) that is longer by 31 amino acids than CCb9 at its C-terminal end showed reduced ability to constitutively activate ICRAC consistent with our observation that CCb9 but not CCb7 efficiently colocalized with and bound to ORAI1. Intracellular application of a 31 amino acid peptide contained in CCb7 but not CCb9 inhibited constitutive and store-dependent CRAC channel activation. In summary, these findings suggest that CCb9 represents a minimal ORAI1 activation domain within STIM1 that is masked by an adjacent 31 amino acid peptide preventing efficient CRAC channel activation in cells with replete Ca2+ stores.
Keywords: ORAI1, STIM1, CRAC, SOCE, store-operated calcium entry, Ca2+, calcium
Introduction
Ca2+ functions as an important second messenger in many cell types including those of the immune system [1]. Many cells use store-operated Ca2+ (SOC) channels, best characterized as Ca2+ release activated Ca2+ (CRAC) channels in lymphocytes and mast cells, to regulate Ca2+ influx [2]. ORAI1 (or CRACM1) was identified as the pore forming subunit of the CRAC channel and mutations in ORAI1 in human patients abolish CRAC channel function [3; 4; 5; 6; 7; 8]. STIM1 is a single pass membrane protein located in the ER which serves as a sensor of ER Ca2+ concentrations and essential activator of CRAC channels [9; 10]. A reduction in the ER Ca2+ concentration (i.e. store depletion) results in dissociation of Ca2+ from the N-terminal EF hand Ca2+-binding domains of STIM1, unfolding of the EF-sterile alpha motif (SAM) domain and multimerization of STIM1 ultimately leading to the assembly of STIM1 in large clusters (or puncta) in the ER membrane [11; 12]. Multimerization of STIM1 in the ER is sufficient to activate SOC/CRAC channels [13]. Nonetheless, expression of the soluble cytoplasmic C terminus of STIM1 (STIM1-CT) was also shown to be able to activate CRAC channels in the absence of store depletion [14; 15]. Three studies recently described regions within STIM1-CT sufficient for multimerization of STIM1 and CRAC channel activation [16; 17; 18]. We here report the independent identification of a minimal activation domain in the C terminus of STIM1 (CCb9, for coiled-coil domain containing region b9) which is sufficient for binding to and activation of ORAI1 CRAC channels independent of store depletion. In addition, we identified a 31 aa peptide located C terminally of CCb9 that interferes with ORAI1 binding and CRAC channel activation by STIM1.
Materials and Methods
Cell culture
HEK293 cells were cultured in DMEM (Mediatech, Manassas, VA) at 37°C, 10% CO2; Jurkat T cells (clone E6-1, ATCC, Manassas, VA) were grown in RPMI 1640 medium (Mediatech) at 37°C, 5% CO2.
Plasmids and transfections
The following N-terminally Cherry-tagged STIM1 fragments were amplified by PCR and cloned into pFLAG-CMV10 (Sigma Aldrich, St.Louis, MO), numbers indicate amino acid residues of human STIM1: CT (C terminus) 251-685, ERM 251-535, S/P-K 536-685, CCa 251-422, CCb1 339-535, CCb2 351-535, CCb5 339-495, CCb7 339-475, CCb8 339-460, CCb9 339-444, CCb10 339-436). For purification of GST-tagged STIM1 fragments, CCb7, CCb9 and CCb10 were cloned into pGEX6P-2 (GE Healthcare, Piscataway, NJ). GFP-myc-ORAI1 was a gift of Dr. R.S. Lewis; ORAI1 IRES-GFP plasmid was described previously [3]. For measurements of intracellular [Ca2+] and ICRAC, HEK293 cells were transfected with STIM1 and ORAI1 in DMEM containing 1 mM EGTA to reduce extracellular [Ca2+] and cytotoxicity due to constitutive Ca2+ influx.
Purification and pulldown of GST tagged proteins
For expression of GST-tagged STIM1 fragments, BL21pLys cells were transfected with STIM1 fragments cloned into pGEX6p-2. Protein expression was induced by 0.1 mM IPTG at 18 °C overnight and cells were lysed in buffer containing 20 mM Tris/HCl, 1 mM EDTA, 1 mM DTT and 250 mM sucrose. GST-tagged proteins were purified on glutathione Sepharose 4B resin (GE Healthcare). FLAG-tagged ORAI1 proteins were expressed in HEK293 cells, membrane proteins solubilized by sonication and cell debris was eliminated by centrifugation at 18,000 g for 25 min. ORAI1-containing lysates were incubated with GST-tagged proteins plus GST beads, and bound proteins were detected by immunoblotting using anti-FLAG (M2) antibody (Sigma-Aldrich).
Confocal microscopy
Confocal images of GFP-ORAI1 and Cherry-STIM1 fragments expressed in HEK293 cells were taken using an inverted Zeiss LSM510 laser scanning confocal microscope (63× Plan-Neofluar). For quantifications shown in Fig. 4B, ≥200 cells were scored visually for cytoplasmic or membrane localization of STIM1 fragments.
Figure 4. STIM1 fragment CCb9 but not CCb7 efficiently colocalizes with and binds to ORAI1.
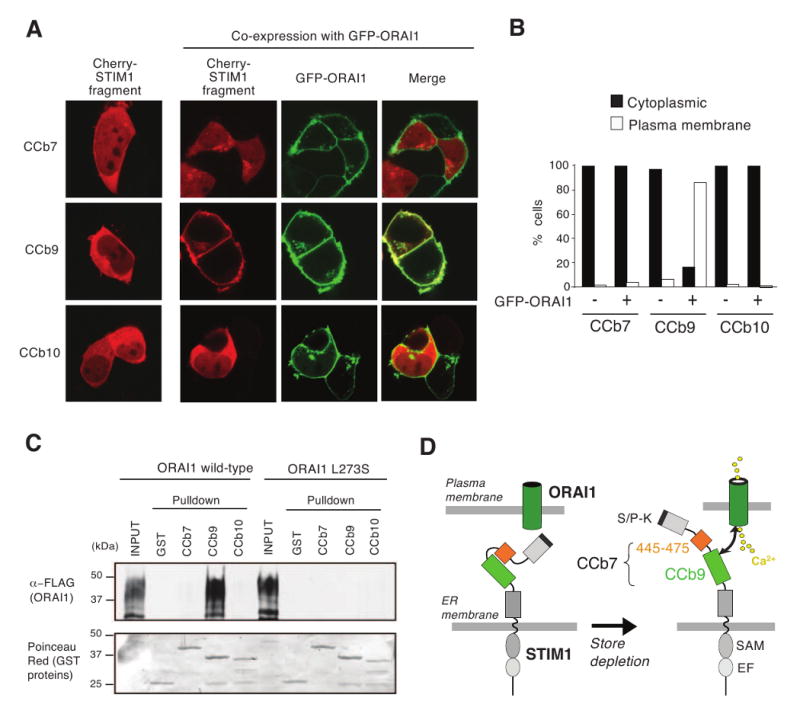
(A) CCb9 colocalizes with ORAI1 at the plasma membrane. HEK293 cells were transfected with Cherry-STIM1 fragments CCb7, CCb9 and CCb10 alone or together with GFP-ORAI1. The subcellular distribution of CCb fragments was analyzed by confocal microscopy. Representative cells from 2 independent experiments are shown. (B) Quantification of subcellular distribution of CCb fragments from the experiment shown in (A). ≥ 200 cells per condition were analyzed as described in Materials and Methods. (C) STIM1 fragment CCb9 binds to ORAI1. For pulldown experiments, HEK293 cells were transfected with FLAG-tagged wild-type ORAI1 or mutant ORAI1-L273S. Cell lysates were incubated with purified GST-tagged STIM1 fragments or GST alone followed by pull-down of protein complexes with glutathione sepharose resin. Proteins were detected by SDS-PAGE, immunoblotting with anti-FLAG antibody (top) and Poinceau Red staining (bottom). (D) Model of ORAI1 activation by STIM1. In the resting state with replete Ca2+ stores, the CCb9 ORAI1-binding domain in STIM1 is masked by the STIM1445-475 peptide. Upon store depletion, the CCb9 domain is released, binds to and activates the ORAI1 CRAC channel. For abbreviations see Fig. 1.
Single-cell calcium imaging
Measurements of intracellular Ca2+ concentrations were done as described [19]. Briefly HEK293 cells were grown on UV-sterilized coverslips, loaded with 1 μM fura-2/AM and analyzed by time-lapse videoimaging on an IX81 epifluorescence microscope (Olympus). For single cell analysis, 340/380 nm Fura-2 emission ratios of > 20 Cherry+ cells per experiment were analyzed.
Patch-clamp measurements of CRAC channel currents
Patch-clamp experiments were performed as described [8]. Briefly, CRAC currents in HEK293 and Jurkat cells were measured in whole-cell configuration at 21-25 °C. Voltage ramps from −130 to +100 mV were delivered at a rate of 0.5 Hz from a holding potential of +30 mV. For active store depletion in cells expressing wild-type ORAI1 and STIM1, 20 μM IP3 was added to the pipette solution; for experiments with cells expressing ORAI1 and STIM1 fragments 4 mM CaCl2 was added to the pipette solution to clamp free [Ca2+]i to ∼ 150 nM. Where indicated, STIM1445-475 peptide (445-475: PGIHSLVAALNIDPSWMGSTRPNPAHFIMTD, Genscript, Piscataway, NJ) or myelin oligodendrocyte glycoprotein (MOG) control peptide (35-55: MEVGWYRSPFSRVVHLYRNGK, Anaspec, San Jose, CA) were added to the internal pipette solution at 20 μM. Purity of both peptides was >95%.
A more detailed description of the experimental procedures is available online in Supplementary Materials.
Results and Discussion
Screening for a SOC/CRAC channel-activating region in the C terminus of STIM1
ORAI1 CRAC channels have been shown to be constitutively activated in the absence of store depletion by expression of the soluble STIM1 C-terminus but the levels of [Ca2+]i and ICRAC were lower than those observed after store depletion [14; 17]. To screen for protein domains that are can mediate CRAC channel activation, we generated Cherry-tagged fragments of the STIM1 C terminus (Fig. 1A). Truncation of STIM1 did not interfere with protein expression as all STIM1 fragments were detectable at the expected sizes by SDS-PAGE and immunoblotting (Fig. 1B).
Figure 1. Identification of CRAC channel activating regions in STIM1.
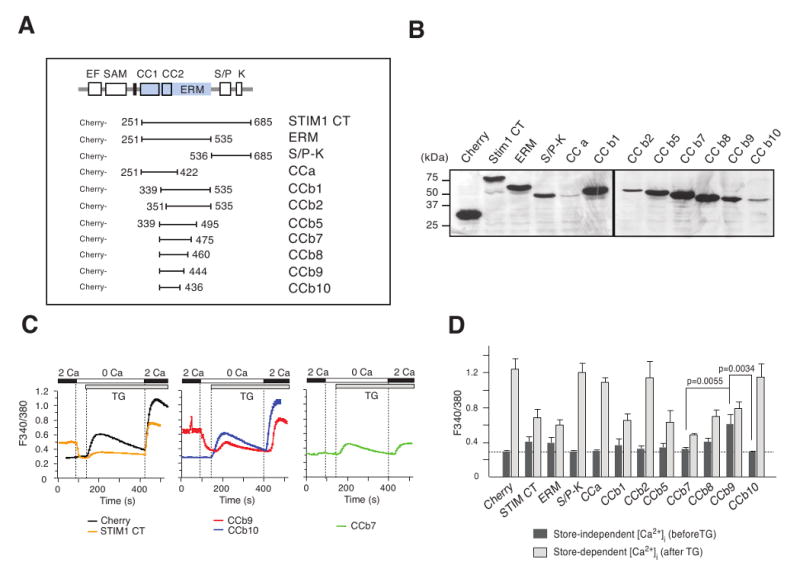
(A) Schematic representation of STIM1 protein domains and C-terminal fragments generated in this study. CC, coiled-coil; CT, STIM1 C-terminus; EF, EF hand; ERM, ezrin/radixin/moesin; SAM, sterile alpha motif; TM, transmembrane; S/P, serine-proline; K, lysine. (B) Western blot of STIM1 C-terminal fragments. HEK293 cells were transfected with FLAG-Cherry-tagged STIM1 fragments, cell lysates separated by SDS-PAGE and STIM1 fragments detected with anti-FLAG antibody. (C) Store-independent and store-dependent Ca2+ influx in cells expressing STIM1 fragments. HEK293 cells were transfected with Cherry-tagged STIM1 fragments and [Ca2+]i was measured by single cell time-lapse imaging in 2 mM Ca2+o before and after addition of 1 μM thapsigargin (TG). Traces represent mean 340/380 Fura-2 emission ratios from one representative experiment; error bars represent s.e.m. (D) Summary of experiments similar to those shown in (C). Bar graphs represent average peak [Ca2+]i in 2 mM Ca2+o before (dark gray) and after thapsigargin (light gray) addition from 3-6 independent experiments; error bars represent standard deviation.
The effects of STIM1 fragments on store-independent (i.e. constitutive) and store-dependent Ca2+ influx were evaluated by single cell Ca2+ imaging before and after addition of thapsigargin (TG), respectively, an inhibitor of the sarcoplasmic/endoplasmic reticulum ATPase (SERCA). Focusing on Ca2+ levels before store depletion, we observed constitutive Ca2+ influx in cells expressing the entire STIM1 C-terminus (CT) and the ERM domain fragment, whereas the S/P-K region or the coiled-coil domain alone (CCa) had no effect (Fig. 1C,D). Strong constitutive Ca2+ influx was detectable in cells expressing STIM1 fragments encompassing amino acids (aa) 339-460 (CCb8) and aa 339-444 (CCb9) which contain the second coiled-coil domain and part of the predicted ERM domain of STIM1. Expression of longer fragments CCb1, CCb2, CCb5 and CCb7 did not result in constitutive Ca2+ entry. Further truncation of CCb9 by 8 amino acids at its C terminus yielding fragment CCb10 abolished the ability of CCb9 to induce store-independent Ca2+ influx (Fig. 1C,D). While STIM1 fragments had variable effects on SOCE, it is of note that expression of CCb7 consistently resulted in a robust inhibition of SOCE (Fig. 1C,D). Taken together, these data suggest that the 106 aa CCb9 fragment within STIM1 represents a minimal CRAC channel activating region that promotes store-independent Ca2+ influx. It is flanked at its C-terminal end by a putative inhibitory region (aa 445-475) which is part of the 137 aa CCb7 fragment.
Constitutive CRAC channel activation by STIM1 domains CCb9 and CCb7
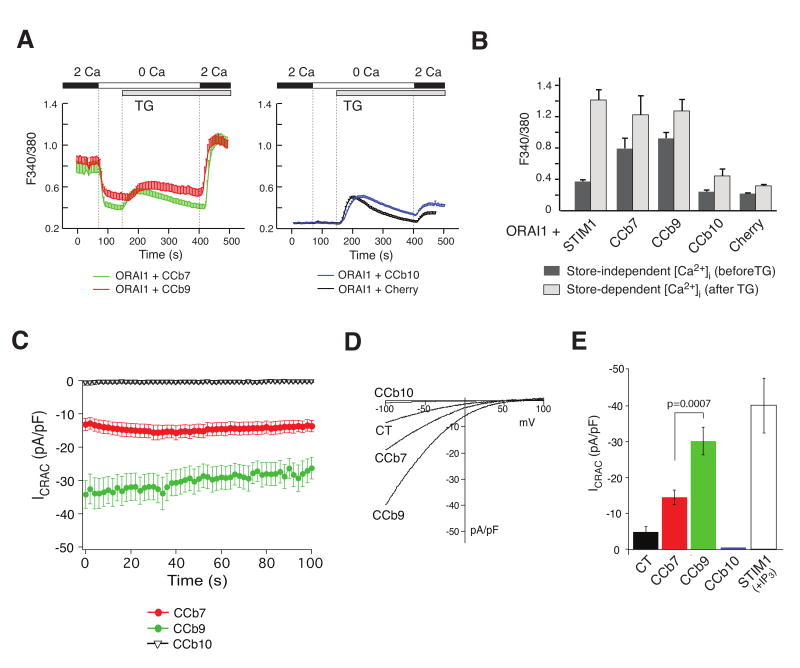
Ectopic expression of ORAI1 and STIM1 together dramatically amplifies CRAC channel currents (ICRAC) and robustly increases Ca2+ entry [4; 20]. We used this system to study the effects of the STIM1 fragments on store-independent Ca2+ influx and CRAC channel activation. First, we measured [Ca2+]i in HEK293 cells transfected with ORAI1 and the Cherry-tagged STIM1 fragments CCb7, CCb9 or CCb10. Combined expression of ORAI1 with CCb7 or CCb9 resulted in constitutive, store-independent Ca2+ influx whereas expression of CCb10 did not (Fig. 2A,B). In addition, CCb7 and CCb9 were as efficient as full-length STIM1 to induce SOCE whereas CCb10 failed to enhance SOCE significantly above levels seen with the Cherry control alone (Fig. 2B). CCb7 and CCb9 enhance SOCE although they do not possess an EF hand domain and are not located in the ER, most likely because they bind to endogenous full-length STIM1 and enhance its function.
Figure 2. Constitutive Ca2+ influx and CRAC channel activation by STIM1 fragments CCb7 and CCb9.
(A) CCb7 and CCb9 fragments induce store-independent, constitutive Ca2+ influx when co-expressed with ORAI1. HEK293 cells were transfected with ORAI1-IRES-GFP and Cherry-tagged STIM1 fragments and [Ca2+]i was measured as described in Fig 1. Traces are representative of > 3 similar experiments; error bars represent s.e.m. (B) Summary of experiments similar to those shown in (A). Bar graphs represent peak [Ca2+]i before (dark gray) and after (light gray) addition of thapsigargin (TG) in the presence of 2 mM Ca2+o. (C) Constitutive CRAC channel activation by CCb7 and CCb9. HEK293 cells were transfected as described above. Shown are the time courses of currents obtained after establishing whole-cell configuration. To prevent passive store depletion during ICRAC recordings, [Ca2+]I was clamped to ∼ 150 nM by addition of 10 mM BAPTA and 4 mM Ca2+ to the internal pipette solution. (D) Representative current-voltage relationships (I-V) extracted from currents shown in (C). (E) Averages of current amplitudes at -80 mV obtained in the first 100 seconds after break-in from experiments shown in (C,D). STIM1-CT (n=10), CCb7 (n=21), CCb9 (n=21), CCb10 (n=6), full-length STIM1 + IP3 (n=8); error bars represent s.e.m.
To confirm that constitutive Ca2+ influx mediated by CCb7 and CCb9 is due to CRAC channel activation, we directly measured CRAC channel currents in whole-cell patch clamp recordings. To prevent ICRAC activation by passive store depletion, [Ca2+]i was clamped to ∼ 150 nM free [Ca2+]i [4]. We observed constitutive CRAC channel currents immediately after break-in in the absence of store depletion in cells expressing CCb7 and CCb9 but not CCb10 consistent with measurements of [Ca2+]i (Fig. 2C). The currents observed in the presence of CCb7 and CCb9 showed key characteristics of ICRAC, namely a strong inwardly rectifying current-voltage relationship, a positive reversal potential (Fig. 2E) and inhibition by La3+ (Suppl. Fig. 2). While CCb9, CCb7 and the C terminus of STIM1 were all able to induce store-independent ICRAC (Fig. 2E), STIM1-CT did so much less efficiently than CCb9 or CCb7 consistent with a moderate constitutive increase in [Ca2+]i in cells expressing STIM1-CT (Fig. 1C,D) and the inability of STIM-1 CT to activate ICRAC reported by others [17].
CCb7, like CCb9, was able to induce store-independent Ca2+ influx (Fig. 2 A,B) and CRAC channel activation (Fig. 2C-E) when co-expressed with ORAI1, although ICRAC activation was less robust in cells expressing ORAI1 and CCb7 (average current density 14.5 ± 1.9 pA/pF) compared to cells expressing ORAI1 and CCb9 (average current density 30.2 ± 3.8 pA/pF) (Fig. 2E). By contrast, ectopic CCb7 expression by itself without ORAI1 failed to induce constitutive Ca2+ influx (Fig. 1C,D). We speculate that CCb7 has a lower binding affinity for ORAI1 than CCb9 and that CCb7-mediated constitutive activation of ICRAC is only observed when ORAI1 is ectopically expressed at high concentrations.
STIM1445-475 peptide interferes with CRAC channel activation
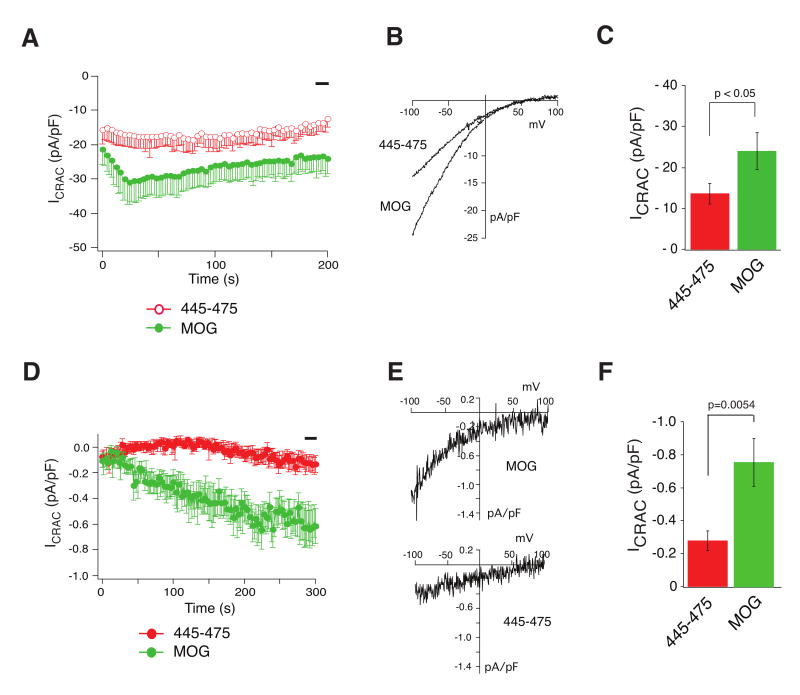
CCb7 and CCb9 only differ by 31 amino acid residues (445-475) at the C terminal end of CCb7. To investigate whether this peptide is responsible for the attenuated CRAC channel activation of CCb7 compared to CCb9 and may have an inhibitory effect on STIM1-mediated CRAC channel activation, we measured ICRAC in cells expressing ORAI1 and CCb9 followed by intracellular perfusion with synthetic STIM1445-475 peptide. Constitutive CRAC channel activation was observed upon break-in under these conditions, but current amplitudes were markedly reduced in the presence of STIM1445-475 peptide (∼ 13.5 ± 2.4 pA/pF) compared to control peptide (∼ 23.9 ± 4.5 pA/pF) (Fig 3A-C). A similar inhibitory effect on native CRAC currents was observed in untransfected Jurkat T cells in which calcium stores had been passively depleted in the presence of synthetic STIM1445-475 (∼ 0.28 ± 0.06 pA/pF) or control peptide (∼ 0.76 ± 0.14 pA/pF) (Fig 3D-F). Taken together, these data show that while both CCb7 and CCb9 are able to activate ICRAC, CCb7 contains an inhibitory region (445-475) that curtails full CRAC channel activation by the CCb9 domain in STIM1.
Figure 3. STIM1445-475 peptide interferes with store-dependent and independent CRAC channel activation.
(A-C) Synthetic STIM1445-475 peptide inhibits constitutive ICRAC HEK293 cells were transfected with ORAI1 and CCb9 and ICRAC was recorded in the presence of 20 μM STIM1445-475 peptide or control myelin oligodendrocyte glycoprotein (MOG) peptide in the internal pipette solution. (A) Averages of time-dependent current development (STIM1445-475, n=17; MOG, n=18). (B) Representative I-Vs from the same experiments shown in A extracted at 200 sec. (C) Averages of current values recorded during the time indicated by the black bar in (A). Error bars represent s.e.m. (D-F) Store-dependent activation of native CRAC channel currents in T cells is inhibited by STIM1445-475 peptide. Endogenous ICRAC was activated in Jurkat T cells by passive store depletion with 10 mM BAPTA in the pipette solution in the presence of 20 μM STIM1445-475 or MOG control peptide. (D) Averages of time-dependent current development extracted at -80 mV. (E) Representative I-Vs from the same experiments shown in (D) at 275 sec. (F) Averages of ICRAC values recorded from 280-300 sec (black bar) in experiments shown in (D). STIM1445-475, n=13; MOG, n=13. Error bars represent s.e.m.
CCb9 colocalizes with and binds to ORAI1 more efficiently than CCb7
To understand the mechanism of CRAC channel activation by CCb7 and CCb9 and the distinct efficiencies of these fragments, we investigated their colocalization with and binding to ORAI1 proteins. Cherry-tagged STIM1 fragments were expressed in HEK293 cells either alone or together with GFP-ORAI1 and their localization was observed by confocal microscopy. In the absence of ORAI1, all STIM1 fragments – CCb7, CCb9 and CCb10 – were distributed throughout the cytoplasm (Fig. 4A, left panels). Co-expression of ORAI1 resulted in robust colocalization of CCb9 with ORAI1 at the plasma membrane; no such colocalization with ORAI1 was detectable for either CCb7 or CCb10 (Fig. 4A, right panels; Fig. 4B). These findings suggest that CCb9 can bind to ORAI1 with higher affinity than CCb7 and CCb10. To test this hypothesis, we conducted pull-down experiments in which purified GST-tagged CCb7, CCb9 and CCb10 fragments were incubated with lysates of cells expressing FLAG-tagged wild-type ORAI1 or FLAG-tagged ORAI1-L273S mutant. Substitution of L273 with serine in the putative coiled-coil domain in the C terminus of ORAI1 was shown to abolish FRET between STIM1 and ORAI1 as well as CRAC channel activation [15]. Consistent with the colocalization of CCb9 with ORAI1 (Fig. 4A) wild-type ORAI1, but not mutant ORAI1-L273S strongly interacted with CCb9 suggesting that CCb9 binds to the C terminus of ORAI1 with high affinity (Fig. 4C). By contrast, we failed to detect binding of CCb7 and CCb10 to ORAI1 (Fig. 4C), although weak binding was observed for CCb7 when ORAI1 pulldowns were conducted using an increased amount of GST-tagged CCb7 and immunoblots were exposed for a prolonged period of time (Suppl. Fig. 3). Weak binding of CCb7 to ORAI1 is consistent with its reduced ability to activate ICRAC when coexpressed with ORAI1 (Fig. 2C-E) and its failure to promote store-independent Ca2+ influx when expressed by itself (Fig. 1C,D). The weak interaction of CCb7 with ORAI1 compared to CCb9 indicates that the 31 aa STIM1445-475 peptide in CCb7 may serve as an inhibitory region by masking the interaction of the activating CCb9 domain with ORAI1 (Fig. 4D).
We here describe the identification of the 106 amino acid CCb9 domain (aa 339-444) in the C terminus of STIM1 which binds to the C terminus of ORAI1 and is sufficient for CRAC channel activation in the absence of store depletion. This minimal activation domain is almost identical to STIM1 regions described very recently and alternatively termed SOAR (STIM1 ORAI1 activation region, aa 344-442)[16], CAD (CRAC activation domain, aa 342-446)[17] or OASF (ORAI1 activating small fragment, aa 233-450/474)[18]. Truncation of the CCb9 domain by 8 amino acids at its C terminus completely abolished the ability of the resulting CCb10 fragment (aa 339-436) to activate CRAC channels and to bind to ORAI1. A STIM1 fragment that is 31 aa longer than CCb9 called CCb7 (aa 339-475) lacked the ability to activate store-independent Ca2+ influx when expressed in the absence of ORAI1 but induced constitutive Ca2+ influx and ICRAC when co-expressed with ORAI1, albeit less efficiently than CCb9. Because CCb7 bound only weakly to ORAI1 in pull-down assays and showed no detectable colocalization with ORAI1 at the plasma membrane we hypothesize that the ORAI1-binding affinity of CCb7 is lower than that of CCb9. This would explain why store-independent activation of CRAC channels is observed only at high ORAI1 plasma membrane concentrations following ectopic expression of ORAI1 but not in cells with native ORAI1 expression.
Direct intracellular perfusion of cells with a synthetic STIM1445-475 peptide encompassing the 31 amino acid residues at the C terminus of CCb7 which distinguish CCb7 from CCb9 impaired CRAC channel activation (Fig. 3). We speculate that the STIM1445-475 peptide interferes with the interaction of STIM1 and ORAI1 by masking the high affinity ORAI1 binding site in CCb9 (Fig. 4D). Under physiological conditions in non-transfected cells, inhibition of the CCb9 domain by the STIM1445-475 peptide may be released and the CCb9 domain exposed when Ca2+ stores are depleted and STIM1 undergoes a conformational change and multimerization. Inhibition mediated by the STIM1445-475 peptide may also explain why expression of the entire C terminus of STIM1 results in significantly weaker CRAC channel activation than expression of CCb9 or similar minimal activation domains in other studies. Identification and targeting of activating and inhibitory domains in STIM1 could be the basis for specific modulation of SOCE, for instance in the context of inflammatory or autoimmune diseases, in the future.
Supplementary Material
Acknowledgments
This work was funded by NIH grant AI066128 to S.F. and a Uehara memorial foundation fellowship to T.K. We thank M. Prakriya for critical reading of the manuscript, R.S. Lewis for GFP-ORAI1 and Cherry-STIM1 plasmids, I. Ahearn for help with confocal microscopy and W. Coetzee for generous support with patch-clamp equipment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 2.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–7. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 3.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 4.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–3. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–62. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 7.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–9. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104:9301–6. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–22. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–42. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–10. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 15.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–22. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 16.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nature Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 Clusters and Activates CRAC Channels via Direct Binding of a Cytosolic Domain to Orai1. Cell. 2009;136:876–90. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A cytosolic homomerization and a modulatory domain within STIM1 C-terminus determine coupling to ORAI1 channels. J Biol Chem. 2009;284:8421–6. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feske S, Prakriya M, Rao A, Lewis RS. A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J Exp Med. 2005;202:651–62. doi: 10.1084/jem.20050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–5. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.