Abstract
Using functional magnetic resonance imaging (fMRI), this study directly examined an issue that bridges the potential language processing and multi-modal views of the role of Broca’s area: the effects of task-demands in language comprehension studies. We presented syntactically simple and complex sentences for auditory comprehension under three different (differentially complex) task-demand conditions: passive listening, probe verification, and theme judgment. Contrary to many language imaging findings, we found that both simple and complex syntactic structures activated left inferior frontal cortex (L-IFC). Critically, we found activation in these frontal regions increased together with increased task-demands. Specifically, tasks that required greater manipulation and comparison of linguistic material recruited L-IFC more strongly; independent of syntactic structure complexity. We argue that much of the presumed syntactic effects previously found in sentence imaging studies of L-IFC may, among other things, reflect the tasks employed in these studies and that L-IFC is a region underlying mnemonic and other integrative functions, on which much language processing may rely.
Keywords: Broca’s area, sentence complexity, task demands, fMRI
INTRODUCTION
Much of the neuroimaging work on language processing has supported the concept that there is a fundamental difference in the neural tissue (and hence processes) involved in comprehending simple and complex sentential material. Most of this work has implicated left anterior frontal brain regions, specifically including Broca’s area, in the processing of sentences with complex structure (Ben-Shachar et al., 2003; Caplan et al., 1998, 1999, 2001; Cooke et al., 2001; Dapretto and Bookheimer, 1999; Friederici et al., 2000; Grossman et al., 2002; Heim et al., 2003; Inui et al., 1998; Just et al., 1996; Musso et al., 2003; Newman et al., 2001; Ni et al., 2000; Sakai et al., 2001; Stromswold et al., 1996; Waters et al., 2003; Wartenburger et al., 2004). In general, conclusions drawn from this research fit well with behavioral and lesion evidence gleaned over decades about the role of left inferior frontal cortex (L-IFC) including Broca’s area. The L-IFC has been shown to have a role in syntactic processing (e.g., Caramazza and Zurif, 1976), syntactic representation (e.g., Grodzinsky, 1986), and sustaining appropriate timing and integration subserving structural processing (e.g., Swinney et al., 1996). Overall, the vast majority of such lesion work suggests that Broca’s area underlies structurally complex language processing.
Neuroimaging research of other mental processes (other than language) has found L-IFC neural involvement. For example, Brodmann’s area (BA) 44 and BA 45 have been implicated by a number of positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies in verbal working memory functions (phonological rehearsal) (e.g., Smith et al., 1998; Gruber, 2001; Waters et al., 2003), by magnetoencephalographic (MEG) studies in the processing of music (e.g., Maess, 2001), and by fMRI studies of motion imagery (e.g., Binkofski et al., 2000), among others.
One obvious question raised by these disparate research findings concerns whether L-IFC is essentially an area largely subserving language function on which other domains draw. This is a conclusion one might easily draw based on the majority of lesion data accumulated over the years. Alternatively, the L-IFC might instead be better characterized as multi-modal processing region that subserves both language and other domains, an account in line with recent imaging findings in non-language domains (e.g., Fiebach et al., 2001). The latter account is, in fact, also given additional support from lesion evidence which has, for years demonstrated short term memory deficits in Broca’s aphasics along with language deficits (e.g., Swinney and Taylor, 1971).
In an effort to shed light on this debate and to add to our understanding of the precise role of L-IFC, this paper examines a factor which has been largely uncontrolled in the neuroimaging studies of language: the effects of task-demand in studies of language comprehension. While language comprehension is often considered a singular unified process, much evidence exists that it is actually something that can be accomplished in a number of ways (e.g., Swinney, 1999). The task demands involved in various studies of comprehension, whether they involve passive listening to lists of sentences, listening to sentences while judging their grammaticality, listening while making secondary decisions to other sentence material, etc., may all change the nature of the comprehension processes, and, hence, potentially the neural substrate underlying those processes (Hickok and Poeppel, 2000, 2004; Osterhout et al., 2002). The probable role of memory is paramount here; tasks that require decisions about entire sentences, about the syntactic or thematic role of Noun Phrases (NPs) in sentences, about the grammaticality of sentences, etc., all would appear to require additional memory and attentional components over and above those involved in basic (passive) comprehension. Investigators in these areas have often used tasks other than passive comprehension to ‘insure’ that participants are comprehending the sentential material and/or to allow presumed identification of precisely what participants are doing during imaging of comprehension. However in doing so, they have introduced potential confounds. In reporting whether complex versus simple syntactic constructions recruit L-IFC, many aspects of task demand have been ignored in the literature; by and large, all tasks have been treated as equal, and by inference, as involving ‘normal’ comprehension (with a few notable exceptions: see discussions in Carpenter et al., 2000; Meyer et al., 2000; Just et al., 1996; Sakai et al., 2001). Yet, there is no ‘task’ that does not potentially change the comprehension process itself. Thus, effects of task-demands in brain imaging studies are to be of potential concern to the general field. Indeed, Ojemann et al. (1998) notes that task demands have long been cited as prime candidates for non-reliability and non-replicability in neuroimaging across both laboratories and techniques.
While the majority of language processing studies find recruitment of L-IFC largely with complex materials, a number of neuroimaging studies (both PET and fMRI) have demonstrated activation of Broca’s area (BA 44 and/or BA 45) as well as parts of the dorsolateral prefrontal cortex, (DLPFC, BA 46) during the processing of both complex and simpler sentence structural forms in both auditory and visual paradigms. The sentence structures which have been compared include the following (NB: the more complex form is given first): center-embedded vs. right branching constructions (Stromswold et al., 1996; Caplan et al., 1998); object cleft vs. subject cleft sentences (Caplan et al., 1999); center-embedded vs. left branching sentences in Japanese (Inui et al., 1998); passive vs. active sentences (Dapretto and Bookheimer, 1999); object relative vs. conjoined active sentences (Michael et al., 2001); sentences with embedded objects vs. non-transformed sentences (Ben-Shachar et al., 2003) and object relative vs. subject-relative sentences, (Cooke et al., 2001; Grossman et al., 2002; Just et al., 1996).
Note that the tasks that have been employed in each of these studies are relatively complex, including sentence “acceptability” judgment, sentence “plausibility” judgment, “understand relationship among three characters”, same-different syntactic judgment for sentence pairs, true-false probe judgment, sentence “grammaticality” judgments, and thematic role judgment. A cursory review of the tasks that have been employed reveals that the majority requires relatively sophisticated conscious, reflective judgments about a just-presented sentence. Indeed, most require that the sentences be held in memory (at least partially) and compared or examined consciously against a task-defined criterion. Thus, the task demands in most studies demonstrating complex-sentence activation of L-IFC have been relatively high. Note that this task-demand component of the studies (which may be argued to call on memory and other extra-language processes) is in addition to any memory demands that may also be involved in comprehension of the more complex sentences, per se. For discussion of memory demands involved in comprehending complex sentences (see, e.g., Cooke et al., 2001; Fiebach et al., 2001; Just et al., 1996; Kaan and Swaab, 2002)1.
The goal of this study is to examine the effect of task demand simultaneously with that of syntactic complexity during auditory comprehension in order to better understand whether sentence syntactic complexity, requirements of the task, or an interaction of sentence complexity with task-demand underlies the activation of L-IFC (and particularly Broca’s area) which has been found for language comprehension. We explicitly manipulated both sentence complexity (specifically less complex subject relative clause sentences with more complex object relative constructions) and task complexity (passive listening, end of sentence probe verification, and thematic judgment) in auditory sentence comprehension2. Our goal is to begin to de-couple the neural representation of potential contributions of sentence complexity and task demand during sentence comprehension, and add to our understanding of the role of L-IFC in cognitive processing in general. As such, we focus particularly on analysis of L-IFC with specific focus on BA 44, BA 45 and BA 46.
METHODS
Subjects
Ten right-handed native English speaking volunteers (8 females and 2 males) were recruited from the University of California, San Diego community and paid $50 each for their participation. The participants ranged in age from 19 to 27 years (mean age 22.4 years, SD 2.6 years) with a mean of 16 years (SD = 1.5) of education. Participants reported no history of major medical illness, neurological or psychiatric disorder, head trauma, substance abuse, or auditory disorder. To document their fluency in English, all participants were administered an extensive questionnaire regarding language background and exposure. Edinburgh Handedness Inventory laterality quotients (Oldfield, 1971) ranged from 58 to 100, indicating a strong right-hand preference for all participants. The Institutional Review Board of the University of California, San Diego approved this study. Participants provided informed consent prior to participation in the experiment.
Stimuli
Forty-five subject relative (SR) and 45 object relative (OR) sentences were created for use in this experiment. The SR and OR forms were pair-wise matched for word length, and varied between 10 and 13 words. The SR constructions took the form illustrated in the following example:
The relevant complexity difference between these two sentence constructions hinges on the linguistic and behavioral fact that understanding SR sentences only requires that the adjacent terms (‘girl’ and ‘who’) be linked (‘girl’ is the person doing the seeing), while understanding the OR constructions requires that the referent (‘girl’) be linked to later occurring verb element while following much intervening material (‘girl’ is the person being seen) (e.g., Swinney et al., 1996). The latter OR structure is termed a ‘long distance dependency’ and has been repeatedly shown to be more difficult to process as demonstrated by both decreased accuracy and increased response times (see, among others, Nicol et al., 1997).
In this study, each sentence was paired with an auditory probe word. Digitized audio versions of the sentences were created using CoolEdit Pro on a PC-compatible computer. The words were spoken by a female native English speaker at a normal rate of speech (4.83 syllables per second). Thus, each sentence was approximately 3 sec in duration. The probe words were spoken by a male native English speaker and were presented 1000 msec after the end of the sentence. The sentences and probe words were adjusted to equate volume, saved in WAV format (16-bit, 44 kHz sampling, monaural), and recorded to CD-ROM.
Design and Procedure
Each participant was tested in six experimental conditions conducted during fMRI. Three task conditions were employed with both SR and OR sentence constructions (thus creating a 3 × 2 within subjects design, yielding six conditions).
In each of the three tasks, the participants heard a sentence followed one second later by that sentence’s associated probe word. Each of the three task conditions required a different use of this post-sentence probe word. The three task conditions were: passive comprehension (PASSIVE), post-sentential probe verification (PROBE), and thematic judgment (THEME).
In the PASSIVE condition, participants were instructed to listen and comprehend the sentences, to ignore the post-sentence probe word and to simply make alternating ‘left’ and ‘right’ (non-verbal) responses on successive sentences. The alternating responses required the same motor movement as in the other two task conditions. In the PROBE condition, participants were instructed to indicate via a ‘yes’ or ‘no’ response whether the post-sentence probe word was present in the sentence they had just heard. One half the items contained probe words requiring a ‘yes’ response, the other half were words not presented in the sentence, thus requiring a ‘no’ response. In the THEME condition, participants were instructed to indicate via a ‘yes’ or ‘no’ response if the first NP (NP1) of the sentence was the actor/agent of the probe word (note that in this latter condition, half the time the probe was the first verb in the sentence and half the time it was the second verb in the sentence).
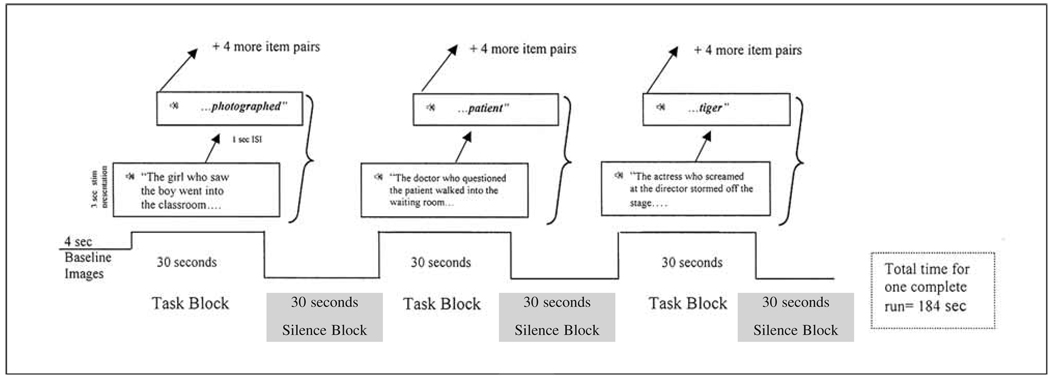
Each of the six experimental runs (one run for each condition; see above) used a blocked-design format consisting of 3 on/off cycles of alternating 30 sec sentence/probe word pairings (task) and rest (no task) blocks. The basic design of each run is shown in Figure 1. Within each task block, five sentence/probe trials were presented yielding 15 such trials within each run. A trial began with the presentation of the sentence in the female voice that lasted approximately 3000 msec. The probe word was presented in the male voice 1000 msec after the completion of the sentence. Following the probe word, there was a 2000 msec silent period before the beginning of the next sentence trial. During the rest blocks (silence), participants were instructed to remain relaxed and still. An additional 4000 msec rest block was presented at the beginning of each run. In total, each run lasted 184 sec with the total functional duration time lasting approximately 18 minutes. The presentation order of the six conditions was the same for each participant3.
Fig. 1.
Example (using subject-relative – SR – stimuli) to demonstrate the timing presentation for one block design run (or ‘epoch’) with the ‘task’ blocks and ‘silence’ blocks shown.
MRI data acquisition
The participants lay supine within the MR scanner during data collection. The participant’s head was secured with foam and their forehead taped to the head coil support to reduce motion. They were instructed to keep their eyes closed during the test runs. The lights within the scanner room were dimmed throughout the entire study. The auditory stimuli were presented via headphones (30 dB noise attenuation) from a Commander XG MRI-compatible sound system (Resonance Imaging, Inc., Northridge, CA, USA) connected to a CDROM player in the scanner console room. Intensity of the experimental stimuli was individually adjusted for each participant to ensure that stimulus presentation was both clear and comfortable. The participants indicated their yes/no responses via a custom-made device held in the right hand.
Imaging data were acquired at the Thornton Hospital at the University of California, San Diego, using a 1.5 T Siemens Symphony MR scanner (Erlangen, Germany) fitted with a three-axis local head gradient coil (Wong et al., 1992). During each of the six test runs, 92 whole-brain T2*-weighted axial images were acquired using a single-shot gradient-recalled echo-planar imaging sequence (20 contiguous slices; 6 mm slab; TR = 2000 msec; TE = 36 msec; flip angle = 90°; FOV = 256 mm; matrix = 64 × 64; in-plane resolution = 4 mm2). A high-resolution 3D magnetization prepared rapid gradient echo (MP-RAGE) scan was acquired for anatomical localization (TR = 11.4 msec; TE = 4.4 msec; flip angle = 45°; FOV = 256 mm; matrix 256 × 256; 180 slices; resolution = 1 mm3)
fMRI data analysis
fMRI analyses were conducted using the analysis of functional neuroimages (AFNI) package (version 2.5; http://afni.nimh.nih.gov/afni) (Cox and Hyde, 1997). Motion correction and three-dimensional registration within the six conditions was done with the automated alignment program 3dvolreg, which co-registered each volume in the time series to the fourth volume acquired in that series (Cox and Jesmanowicz, 1999). Registration between time series was accomplished by aligning each volume in the initial five runs to the fourth volume of the final run. After registration, each image was smoothed spatially with a Gaussian filter (FWHM = 8 mm).
fMRI analyses of individual participants
The fMRI data from individual participants were analyzed using a separate multiple regression analysis for each language condition. Nine parameters were entered into each regression analysis. One parameter was the estimated hemodynamic response function (HRF) to the stimulus presentation. The HRF estimate was calculated by convolving the stimulus presentation time series with a gamma variate function via the AFNI waver program. Six parameters were used to orthogonally remove effects of motion for rotation (i.e., roll, pitch, and yaw motion measured in degrees) and displacement (i.e., mm motion in the x, y, and z planes), and two parameters orthogonally removed the effects of the global mean and linear trend within each language condition time series. The linear contrast weights for each language type resulting from the multiple regression analysis, which estimated the BOLD signal change for each language type relative to the null trial conditions, were converted to standardized Z scores. The resulting Z-score activation maps were resampled into Talairach space using the AFNI hand land-marking procedure (resampled volumes = 3 mm3).
fMRI group analyses
The group analysis was conducted by submitting the Z-score activation maps from each participant in all six language conditions to a three-way repeated-measures mixed-model analysis of variance (ANOVA) with sentence condition (2 levels, fixed effect), task condition (3 levels, fixed effect), and subject (random effect) as factors. The AFNI 3dANOVA3 program was used to conduct the analysis. Two ANOVAs were conducted. One ANOVA considered the activity within the left hemisphere frontal lobe regions encompassed by BA 44, 45, and 46 including the inferior and middle frontal gyri (see region of interest – ROI – definition below). A voxel-cluster threshold correction based on a Monte Carlo analysis was used to correct for multiple comparisons (Forman et al., 1995) resulting in an overall corrected level of significance (alpha) of .05. The cluster threshold correction for the ROI analysis required activation to be observed in 7 contiguous 3 mm3 voxels (activation cluster ≥ 189 µl) using a voxel-wise threshold of p ≤ .001 (FWHM autocorrelation estimate = 8 mm). The second ANOVA was used to examine whole-brain activation patterns (see Appendix A for results). Two cluster threshold correction criteria were used for the whole-brain analysis. The cluster threshold correction for mean activation above baseline for the whole brain required activation to be observed in 8 contiguous 3 mm3 voxels (activation cluster ≥ 216 µl) using a voxel-wise threshold of p ≤ .00001 (FWHM autocorrelation estimate = 8 mm). For the main effects of the Sentence and Task conditions, and for the Sentence × Task interaction, the cluster threshold correction required activation to be observed in 7 contiguous 3 mm3 voxels (activation cluster ≥ 189 µl) using a voxel-wise threshold of p ≤ .001 (FWHM autocorrelation estimate = 8 mm). Post-hoc analyses of main effects and interactions used paired-comparison t tests of the conditions under examination with a voxel-wise threshold correction of p < .001 (uncorrected).
ROI Definition
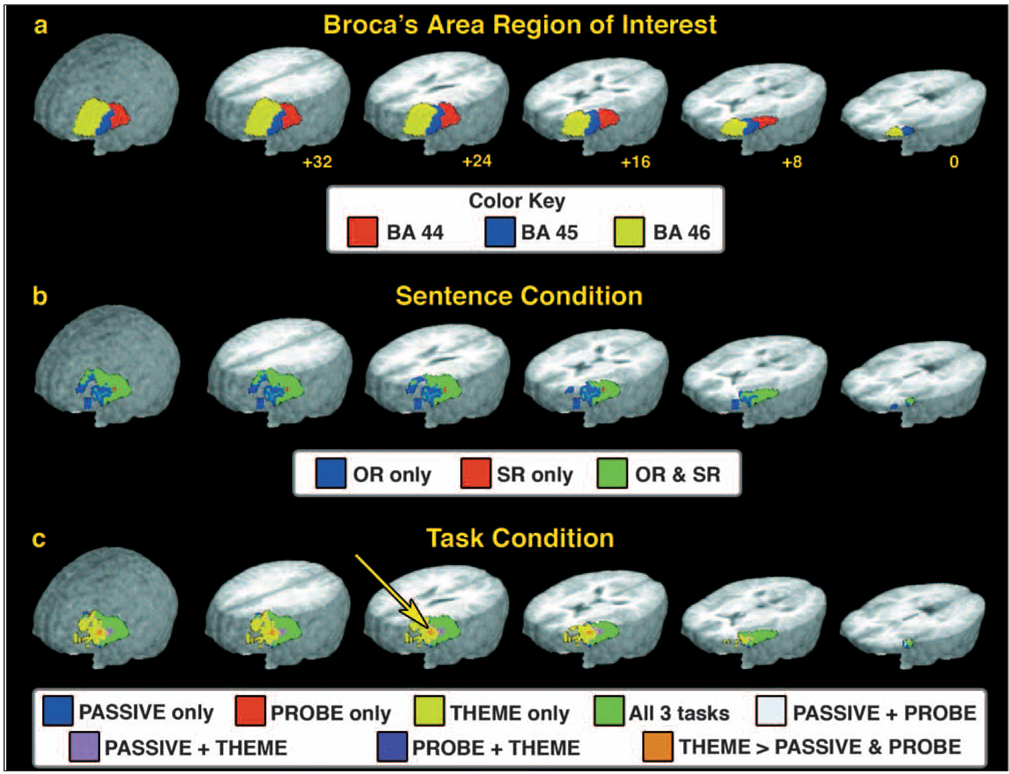
To define this ROI, mask images were produced that delineated the left hemisphere extent of BA 44, 45, and 46 in the Talairach and Tournoux (Talairach and Tournoux, 1988) coordinate system based on the AFNI (Cox and Hyde, 1997) implementation of the Talairach daemon database (Lancaster et al., 2000). The resulting mask image was manually adjusted for our research sample relative to the average magnetization prepared rapid gradient echo (MPRAGE) structural image based on all 10 participants using the AFNI drawing plug-in program. The ROIs for each region were defined as follows in mm relative to the anterior commissure. The approximate extent of the BA 44 ROI was from 38 mm to 58 mm in the in the x plane (right to left), −4 mm to 16 mm in the y plane (posterior to anterior), and 2 mm to 20 mm in the z plane (inferior to superior). This ROI contained 177 3 mm3 voxels (4779 µl). The approximate extent of the BA 45 ROI was from 38 mm to 56 mm in the in the x plane (right to left), 10 mm to 34 mm in the y plane (posterior to anterior), and 0 mm to 24 mm in the z plane (inferior to superior). This ROI contained 181 3 mm3 voxels (4887 µl). The approximate extent of the BA 46 ROI was from 28 mm to 52 mm in the in the x plane (right to left), 16 mm to 56 mm in the y plane (posterior to anterior), and 0 mm to 30 mm in the z plane (inferior to superior). This ROI contained 510 3 mm3 voxels (13,770 µl). See Figure 2a (below) for a visual depiction of the ROI used.
Fig. 2.
fMRI activation results from the ROI analysis. a) Region of interest used for the ROI analysis. The three BA in the left hemisphere lateral frontal cortex, BA 44, 45, and 46, used in the ROI are shown. Although shown here separated, the ROI analysis included all three regions as a single ROI. b) fMRI results from the analysis of the Sentence condition. Results indicate where activity exceeded the baseline condition for the OR and SR sentences either separately (‘OR only’ and ‘SR only’) or where both sentence constructions showed greater than baseline activation (‘OR and SR’). No voxels were found that significantly differed between the sentence constructions. c) fMRI results from the analysis of the Task condition. The color coding indicates regions where activity exceeded the baseline condition and which tasks were associated with that activity. The yellow arrow indicates a region in the inferior frontal gyrus (BA 46) where the activity during the THEME task was greater than that observed in both the PROBE and PASSIVE tasks. The ROI and fMRI results are displayed on a 3D rendering of the averaged brain obtained by forming a mean image from the structural MRI from each of the 10 participants. The slices displayed in the rendering are at +32 mm, +24 mm, +16 mm, +8 mm, and 0 mm superior to the line formed by anterior and posterior commissures (‘AC-PC’ line) in Talairach coordinates.
RESULTS
Behavioral Performance Findings
Data from each individual participant from the three task conditions were converted to a percent correct score (see Table I for scores). These data were analyzed with a two-way ANOVA using task condition (PROBE and THEME) and sentence complexity (SR and OR) as factors. Data from the PASSIVE task were not included in the analysis because, unlike the THEME and PROBE tasks, performance on the PASSIVE task did not require a decision based on the material presented within the sentence. Instead, participants were required to alternate ‘left’ and ‘right’ motor responses. This task was easily mastered by the participants as demonstrated by their 100% mean accuracy score.
TABLE I.
Mean percent scores for the two types of sentence complexity (SR and OR) for the PROBE and THEME task conditions
| Task | ||
|---|---|---|
| Sentence complexity | PROBE | THEME |
| Subject relative (SR) | 98.6% | 89.3% |
| Object relative (OR) | 96.7% | 89.2% |
Results from the 2 × 2 ANOVA demonstrated a significant main effect of task condition [F (1, 36) = 7.052, p = .012] but no effect of sentence complexity [F (1, 36) = .1, p > .10]. Collapsing across sentence constructions demonstrated that the THEME condition proved to be more challenging than the PROBE task, insofar as mean accuracy scores were significantly lower for THEME than for the PROBE task (mean score = 89.7% and 97.7% correct, respectively) [t9 = 3.074, p = .012].
fMRI Findings
The major focus of this study was on activity within the left hemisphere inferior frontal region including BA 44, 45, and 46 within the inferior and middle frontal gyri. This activity was analyzed using a region of interest approach and is described below. Although not the primary focus, we also evaluated activity within the rest of the brain. Those results are described in Appendix A.
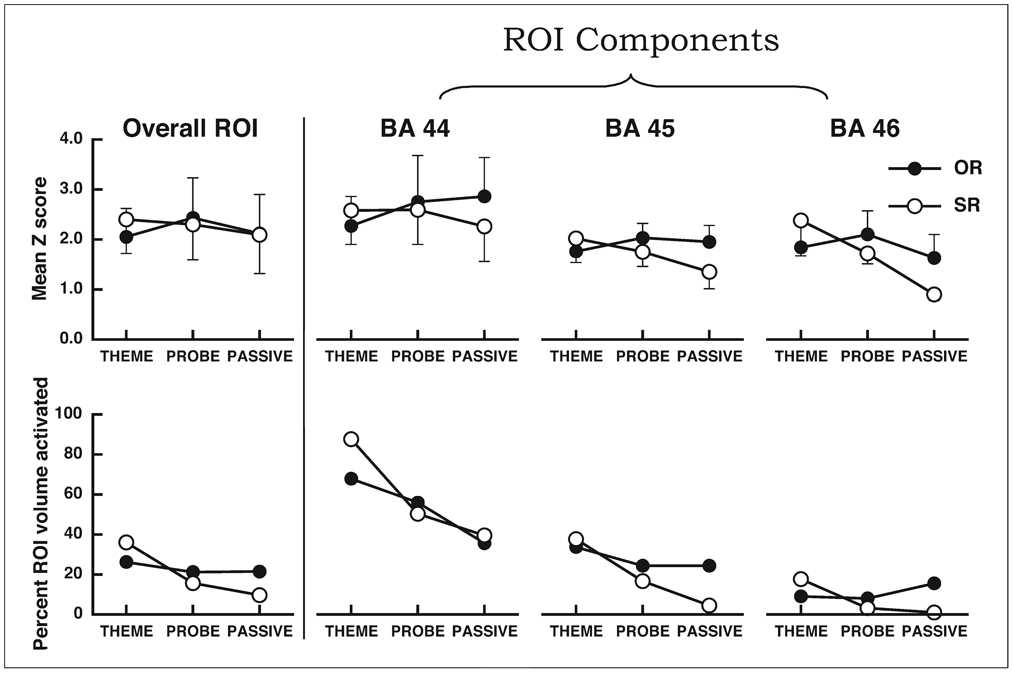
The L-IFC ROI used in the analysis is shown in Figure 2a. The ROI included BA 44, 45, and 46 in the middle and inferior frontal gyri (see ROI description above). The analysis revealed no reliable activity in this ROI associated with the sentence × task interaction. The similarity of activity in both intensity and extent across both sentence conditions in each of the three tasks are appreciated by the results presented in Figure 3. The left panel of Figure 3 shows the overall intensity as measured by the mean Z score of the group (top graph) and the percentage of the ROI that was determined activated following the cluster-threshold correction (bottom graph). Overall, performance in each of the six sentence-task combinations produced robust and similar activation within the region of interest, consistent with the null sentence × task interaction effect. The intensity and extent of activation were also calculated for each of the separate BA regions within the ROI and are shown in Figure 3. These results suggested that the overall ROI pattern was mirrored in each of the three BA. Specifically, the mean Z scores (intensity) and extent of activation were similar in each of the six sentence-task conditions. In general, the higest intensity and most extensive activity was observed within BA 44, but robust activation was observed across the three regions.
Fig. 3.
Mean intensity and volume measures from the ROI analysis. The top panels display the mean Z scores for each of the six separate sentence-task combinations for the overall ROI (leftmost graph) and the three subregions of the ROI (BA 44, 45, and 46, rightmost graphs, respectively) for voxels that were identified as significantly active in the ROI analysis following the cluster-threshold correction. OR = object relative sentence constructions, SR = subject relative sentence constructions. Error bars indicated the standard deviation. The bottom panels display the percentage of the ROI that contained significantly active voxels.
The fMRI activation results from the sentence conditions collapsed across the three task conditions, and the task conditions collapsed across the two sentence conditions are shown in Figures 2b and 2c, respectively. These effects were color-coded to identify the separate and combined activation effects across conditions (for explanation of the color-coding rationale, see Haist et al., 2004). Within the sentence condition, there was no main effect of sentence type, indicating that both OR and SR sentences produced similar levels of activation. As can be seen in Figure 2b, a large proportion of the ROI that was active in the sentence condition was activated by both the OR and SR sentence constructions. However, we do wish to note that the OR sentence condition, upon visual inspection, did produce hints to more activity in the anterior aspects of the ROI included in BA 45 and BA 46 than that observed in the SR sentence condition. Nevertheless, those regions of OR sentence activity were not reliably greater than that observed in the SR sentence condition. Over the entire ROI, the mean Z score for active voxels in the OR condition was 1.75 (SD = .7; mean t9 = 9.59, p < .001; volume = 10,908 µl), and for the SR condition was 1.70 (SD = .6; mean t9 = 9.18, p < .001; volume = 13,635 µl).
The analysis of the task conditions revealed a main effect of task condition within a cluster of voxels located in the superior aspects of the inferior frontal gyrus within BA 46. This is identified by the arrow in Figure 2c. In this region, the activation associated with the THEME condition was significantly greater than that observed in either the PROBE or PASSIVE conditions (THEME: mean Z = 1.69, SD = .1, mean t18 = 7.34, p < .001). No voxels were active within this region for either the PROBE or PASSIVE conditions. In addition, as can be seen in Figure 2c, the THEME condition produced significant activation within the BA 46 region, which was not shared by the PROBE and PASSIVE conditions, but this additional activation was not reliably different between the three conditions. Within the BA 44 and 45 aspects of the ROI, all three task conditions produced robust activation. Over the entire ROI, the mean Z score for active voxels in the THEME condition was 1.81 (SD = .6; mean t18 = 7.43, p < .001; volume = 15,930 µl), the mean Z score for the PROBE condition was 1.85 (SD = .7; mean t18 = 7.78, p < .001; volume = 10,287 µl), and the mean Z score for the PASSIVE condition was 1.63 (SD = .6; mean t18 = 6.80, p < .001; volume = 10,800 µl).
In summary, when looking at the overall L-IFC ROI, both the OR and SR sentence constructions produced robust activation resulting in no reliable differences in activation patterns between the sentence complexity types. When looking at the sub-regions included in this ROI analysis, the OR sentence constructions produced more extensive activation of the anterior aspects of BA 46. Collapsing across sentence complexity- looking specifically at task condition- performance during the THEME task produced reliably greater activity within the posterior aspects of BA 46. The greater activation observed during the THEME task is consistent with the proposal that this task taxed complex cogntive operations to a greater extent than either the PROBE or PASSIVE tasks.
DISCUSSION
The key findings in this study are those focused on the contribution of L-IFC to the processing of sentences (particularly, complex constructions) and to different task-demand conditions. We found, contrary to much prior literature, that both SR and OR sentence engaged L-IFC robustly and, importantly, to the same degree. In addition, we found that as task demands increased (from passive listening to thematic specification) there was successively more engagement of these frontal systems whereby the most complex task implicates the more anterior region (BA 46, in addition to others). Interestingly, the sentence complexity and task-demand conditions did not interact (i.e., the task effect was equally robust for SR and OR sentences). We note that this finding of no different localization of subject and object relative constructions in terms of L-IFC activation is in line with the current work of Fiebach et al. (2004) and Wartenburger et al. (2004) who investigated canonical and non canonical forms.
The finding of a similar magnitude of activation in L-IFC for SR and OR constructions warrants some further discussion given that many prior studies have reported that complex constructions elicit relatively greater engagement of this brain region. Some of these studies have reported some L-IFC activation for the ‘simpler’ constructions they present (e.g., Stromswold et al., 1996; Just et al., 1996) accompanied by greater L-IFC activation for the more complex constructions, other studies have shown absolutely no L-IFC activation with the simple forms (e.g., Cooke et al., 2001).
We can think of three reasons for the discrepancy between our findings and previous reports. One reason is a difference in the materials being used. Our sentences (both SR and OR) were relatively long (on average, 13 words) compared to the average sentence length presented in prior sentence complexity studies (ranging from 5 to 12 words). This may have pushed the memory demands even for our simpler SR sentences to a point that encouraged more L-IFC recruitment than in these other studies. Importantly, however, we note that even simple constructions can and do recruit this cortical area.
A second reason is methodological in nature. In particular, most prior studies using these types of SR/OR materials involved visual presentation, which brings different processing demands and task demands than our auditory listening task. It may be the case that processing object relative constructions is simply easier with auditory presentation than in with visual presentation. In addition, some of the prior reading studies employed word by word reading (see for example, Cooke et al., 2001). This paradigm arguably makes a difficult situation even harder in that the reader cannot rely on backtracking to re-read or re-analyze difficult passages.
A third and final critical reason is differences in reporting conventions. Information regarding the magnitude of activation in the L-IFC region by both constructions is necessary for the interpretation of the role of Broca’s area and DLPFC in language processing. What is typically reported is the location and extent of peak activation for various constructions (e.g., Cooke et al., 2001) or areas that have significantly increased regional cerebral blood flow associated with a difference score (see, for example, Caplan et al., 1999). What we would like to suggest is that it is very likely, and a more probable scenario, that the regions that have been implicated thus far in the literature (Broca’s area and DLPFC) are both called upon during language processing, but they engaged different cognitive systems. This information can be lost with these varying reporting techniques.
The central focus of the research in this paper was to investigate the effects of task-demands on activation patterns in L-IFC. We show significant task effects associated with this neural region. As can be seen in Figure 2, increases in task demands result in increases in frontal activations, independently of the type of sentences being processed. All tasks engage aspects of BA 44 and BA 45, but the most complex task we employed (theme specification) also engages aspects of BA 46 (DLPFC). This latter task is potentially most similar in complexity to the average type of task that has been employed in neuroimaging studies of sentence comprehension, as described above. It has been argued that the DLFPC plays a major role in memory, in particular in relation to the active manipulation and monitoring of information (Owen, 1997; Petrides, 1995, 1996; Smith and Jonides, 1997, 1999). The THEME task appears to fit the criterion of requiring the maintenance and manipulation of constituents during the processing of sentences: listeners must remember the first NP and verb of a sentence and after understanding the sentence, decide if that NP was the agent/actor of the first or second verb (see Nicol and Love, 2000). Clearly this is a high load task that requires much more than simple comprehension. These results fall in line comfortably with Owen (1997) and Smith and Jonides’ (1997, 1999) proposed functional role of this neural region. The overall main effect of task in activation of L-IFC suggests yet another critical factor to be considered in describing the role of L-IFC tissue in cognitive processing in general and, more specifically, in language processing.
CONCLUSIONS
Overall, our core findings of significant L-IFC activations as a function of task-demand effects (and independent of sentence complexity effects) lends support to the view that L-IFC is a multi-modal region, highly attuned to memorial (and likely, other) demands of processing (see e.g., Fiebach et al., 2001). While this does not preclude a language-specific role for this cortical area (or, even, a language specific memory role) the more parsimonious view from these data is that this area provides a memorial (and likely other forms) of processing support for complex analysis, including that involved in language tasks. Our findings also underscore the critical nature of methodological details often taken for granted in language studies. High task demands appear to require additional processes besides the basic comprehension routines and these processes are subserved by different neural substrates.
Acknowledgments
The authors wish to express their appreciation for support for this work from National Institutes of Health (NIH) grants DC 03681 and DC 02984. Special thanks to Lisa Vance Trup and Beth Enchelmayer for their assistance in the preparation of this paper.
APPENDIX A
Whole brain findings
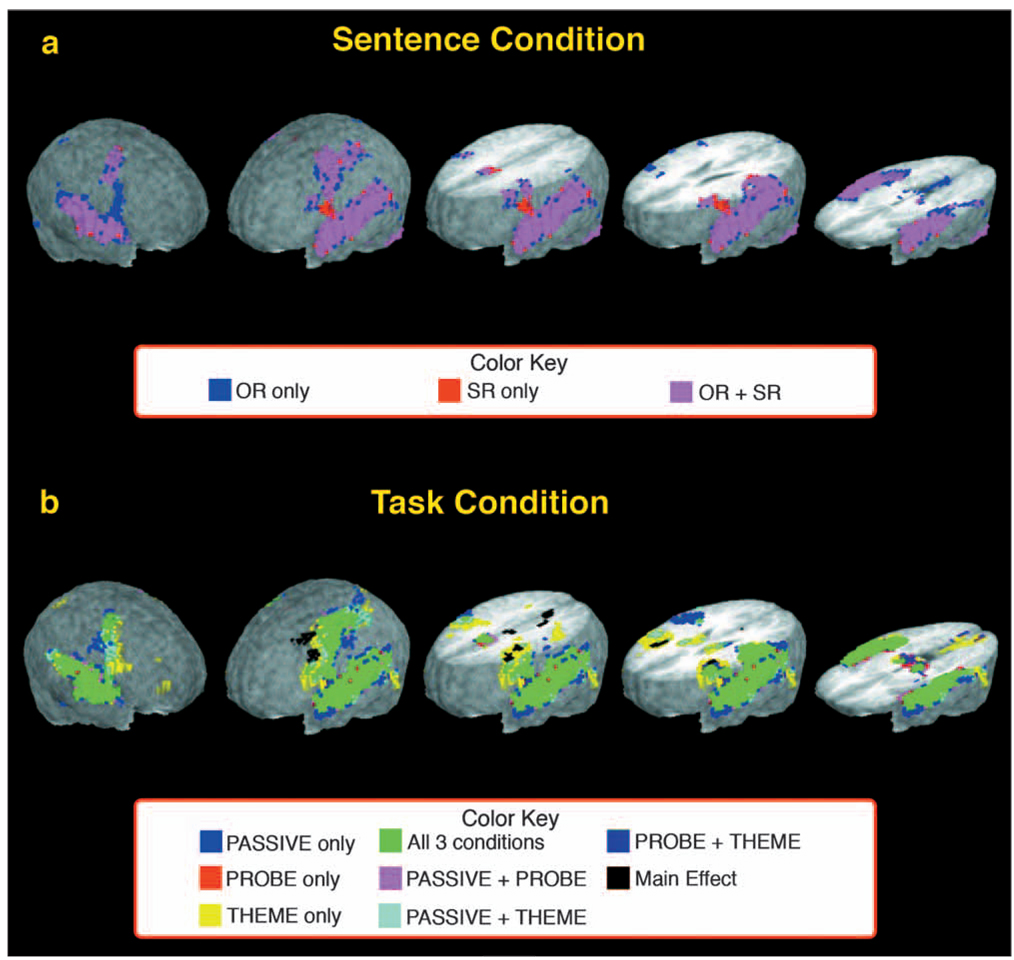
The focus of this study was targeted at activation for the sentence and task conditions within the left inferior frontal cortex (L-IFC). Nevertheless, activation throughout the rest of the brain provides important information about the neural milieu within which the L-IFC region activates, which in turn may have implications for the information processing systems contributing to L-IFC activity. Here the findings from the whole brain analysis are presented. The results for the Sentence and Task condition effects are shown in Figure 4, with the description of regional activations provided in Table II and Table III.
Fig. 4.
Summary of whole brain activation in the Sentence and Task conditions (panels a and b, respectively). The color coding identifies voxels that were significantly active following the cluster-threshold correction and the conditions in which they were associated. Within each panel, the three rendered brains on the right side of the panel are cut 36 mm, 24 mm, and −2 mm relative to a plane formed by the anterior commissure and posterior commissure (AC-PC line), respectively.
TABLE II.
Selective description of activation observed in the Sentence condition. Hemi: L = left hemisphere, R = right hemisphere. The x, y, and z coordinates of Talairach-space are defined with positive indicating the mm to the left, anterior, and superior of the anterior commissure, respectively. All region labels and Brodmann’s areas (BA) were determined using the AFNI implementation of the Talairach daemon (Lancaster et al., 2000). Cerebellar nomenclature according to Schmahmann et. al. (2000). Shared activity Z scores are the mean of the OR and SR sentence conditions
| Talairach coordinates |
||||||
|---|---|---|---|---|---|---|
| Location | Hemi | BA | x | y | z | Z score |
| OR and SR common activation | ||||||
| Precentral gyrus | L | 4 | 39 | − 14 | 57 | 3.16 |
| Inferior parietal lobule | L | 40 | 37 | − 41 | 53 | 2.42 |
| Postcentral gyrus | L | 3 | 37 | − 29 | 55 | 2.69 |
| Medial frontal gyrus | L | 6 | 4 | 0 | 53 | 3.24 |
| Transverse gyrus | L | 41 | 43 | − 31 | 10 | 4.62 |
| Superior temporal gyrus | L | 22 | 59 | − 17 | 1 | 5.74 |
| Thalamus | L | 14 | − 21 | 2 | 1.90 | |
| Fusiform gyrus | L | 37 | 41 | − 54 | − 15 | 1.85 |
| Cerebellum hemisphere VI | L | 31 | − 56 | − 24 | 2.12 | |
| Medial frontal gyrus | R | 6 | − 8 | 4 | 50 | 3.11 |
| Superior parietal lobule | R | 7 | − 31 | − 58 | 49 | 1.56 |
| Transverse gyrus | R | 41 | − 46 | − 31 | 10 | 3.74 |
| Insula | R | 13 | − 32 | 18 | 6 | 1.60 |
| Superior temporal gyrus | R | 22 | − 56 | − 16 | 1 | 4.66 |
| Fusiform gyrus | R | 19 | − 27 | − 58 | − 14 | 2.30 |
| Cerebellum hemisphere VI | R | − 30 | − 56 | − 24 | 2.09 | |
| OR specific activation | ||||||
| Inferior frontal gyrus | R | 44 | − 53 | 16 | 14 | 1.86 |
| Thalamus | R | − 8 | − 14 | 8 | 2.14 | |
| Precuneus | R | 7 | − 8 | − 69 | 49 | 1.77 |
| SR specific activation | ||||||
| No regions identified | ||||||
TABLE III.
Selective description of activation observed in the Task condition. See Table II for description. Shared activity Z scores are the mean of the PASSIVE, PROBE, and THEME conditions. Activity within the left hemisphere lateral frontal lobe is included in the ROI analysis (see Figure 2 and Figure 3). Task specific activation reflects regions that are not merely extensions of shared regions
| Talairach coordinates | ||||||
|---|---|---|---|---|---|---|
| Location | Hemi | BA | x | y | z | Z score |
| All tasks common activation | ||||||
| Precentral gyrus | L | 4 | 35 | 19 | 57 | 2.88 |
| Middle frontal gyrus | L | 6 | 43 | − 3 | 51 | 3.13 |
| Medial frontal gyrus | L | 6 | 2 | 3 | 50 | 3.45 |
| Postcentral gyrus | L | 3 | 35 | − 30 | 54 | 1.85 |
| nferior parietal lobule | L | 40 | 39 | − 35 | 46 | 2.41 |
| Cingulate gyrus | L | 24/32 | 7 | 5 | 43 | 2.33 |
| Inferior frontal gyrus | L | 9 | 51 | 15 | 25 | 2.56 |
| Superior temporal gyrus | L | 42 | 62 | − 29 | 16 | 4.79 |
| Transverse gyrus | L | 41 | 48 | − 28 | 13 | 4.62 |
| Superior temporal gyrus | L | 22 | 63 | − 32 | 8 | 5.26 |
| Thalamus | L | 13 | − 16 | 10 | 2.13 | |
| Fusiform gyrus | L | 19 | 31 | − 66 | − 13 | 1.32 |
| Cerebellum hemisphere VI | L | 28 | − 58 | − 22 | 2.48 | |
| Medial frontal gyrus | R | 6 | − 24 | − 10 | 50 | 2.35 |
| Middle frontal gyrus | R | 6 | − 42 | 0 | 50 | 2.53 |
| Cingulate gyrus | R | 24/32 | −8 | 5 | 39 | 1.94 |
| Inferior frontal gyrus | R | 9 | − 52 | 9 | 25 | 1.62 |
| Superior temporal gyrus | R | 42 | − 61 | − 28 | 16 | 3.13 |
| Transverse gyrus | R | 41 | − 52 | − 26 | 13 | 3.28 |
| Insula | R | 13 | − 38 | 12 | 13 | 1.46 |
| Superior temporal gyrus | R | 22 | − 57 | − 27 | 5 | 3.42 |
| Thalamus | R | − 6 | − 16 | 8 | 1.69 | |
| Superior temporal gyrus | R | 21 | − 56 | − 10 | − 5 | 4.37 |
| Cerebellum hemisphere VI | R | − 19 | − 54 | − 22 | 3.01 | |
| PASSIVE specific activation | ||||||
| Precuneus | L | 7 | 20 | − 55 | 56 | 1.63 |
| Inferior parietal lobule | R | 40 | − 30 | − 42 | 52 | 1.63 |
| Fusiform gyrus | R | 19 | − 30 | − 81 | − 17 | 1.45 |
| PROBE specific activation | ||||||
| Parahippocampal gyrus | L | 20 | 40 | − 8 | − 18 | 1.29 |
| Lingual gyrus | R | 17 | − 9 | − 85 | 5 | 1.79 |
| THEME specific activation | ||||||
| Superior parietal lobule | L | 7 | 14 | − 63 | 54 | 2.15 |
| Inferior parietal lobule | L | 40 | 35 | − 52 | 43 | 2.54 |
| Supramarginal gyrus | L | 40 | 45 | − 39 | 35 | 2.05 |
| Superior parietal lobule | R | 7 | − 30 | − 61 | 51 | 2.26 |
| Middle frontal gyrus | R | 46 | − 48 | 20 | 25 | 2.05 |
| Superior frontal gyrus | R | 10 | − 31 | 42 | 26 | 1.86 |
| Caudate | R | − 13 | − 3 | 16 | 1.68 | |
No significant voxel clusters were associated with a Sentence × Task condition interaction, nor with a main effect of Sentence condition. This suggested that, in general, both sentence conditions produced similar levels of activation within similar regions throughout the brain (see Figure 4a). Table II describes the regions where SR and OR sentence constructions produced brain activation significantly above baseline levels either alone or co-jointly. Overall, the extent of activation was greater in the OR condition than in the SR condition (7427 voxels vs. 6367 voxels, respectively). In summary, the two sentence conditions activated similar areas throughout the brain suggesting an overall communality in the neural networks engaged.
In contrast to the Sentence condition, four regions were identified that produced a significant main effect of task (see Figure 4b). These four regions included (i) the left hemisphere lateral inferior and middle frontal gyri encompassing BA 9, 8, and 6, (ii) bilateral medial frontal gyrus (BA 6) extending ventral to bilateral cingulate gyrus (BA 24), (iii) the left hemisphere posterior cingulate region (BA 31) extending dorsal to the medial precuneus (BA 7), and (iv) the right hemisphere middle frontal gyrus encompassing BA 46 extending ventral to the anterior aspects of the insula. The specific task activations within these regions are described below. A complete description of Task condition activity is provided in Table III.
Left hemisphere lateral frontal gyrus
Greater activation in terms of intensity and extent were observed for the THEME condition relative to the PROBE and PASSIVE conditions within the cluster encompassing the left hemisphere inferior and middle frontal gyri (BA 9, 8, and 6). Across the cluster, the mean Z score for significantly activated voxels for the THEME, PROBE, and PASSIVE conditions, which reflects the average effect size for each task above baseline activity in those voxels that exceeded the cluster-threshold correction, was 2.03 (SD = .55; volume = 3,132 µl), 1.21 (SD = .27; volume = 1,134 µl), and 1.06 (SD = .22; volume = 1,917 µl), respectively. Post-hoc paired t tests revealed that the activation for THEME was greater than that for PROBE (most intense voxel: Talairach coordinates, x = 45, y = 12, z = 39; t18 = 5.15, p < .001) and PASSIVE (most intense voxel: x = 35, y = 5, z = 28; t18 = 3.91, p < .001). The activation for the PASSIVE condition was significantly greater than the PROBE condition in the superior aspects of the cluster (BA 8) (most intense voxel: x = 28, y = 19, z = 44; t18 = 4.10, p < .001). The THEME and PASSIVE conditions did not differ significantly within this region of the cluster (p > .001).
Bilateral medial frontal gyrus
The main effect of task within the medial frontal gyrus (BA 6) extending to the cingulate gyrus (BA 24) was due to activity in the PASSIVE condition exceeding that observed in either the THEME or PROBE condition in intensity and extent. Across the cluster, the mean Z score for significantly activated voxels for the THEME, PROBE, and PASSIVE conditions was 1.03 (SD = .14; volume = 162 µl), 1.11 (SD = .25; volume = 810 µl), and 1.58 (SD = .47; volume = 3,078 µl), respectively. Post-hoc paired t tests revealed that the activation for PASSIVE was greater than that for the THEME (most intense voxel: Talairach coordinates, x = 1, y = −11, z = 43; t18 = 5.20, p < .001) and PROBE conditions (most intense voxel: x = 1, y = −11, z = 45; t18 = 5.54, p < .001). No voxels were identified that indicated a difference between the THEME and PASSIVE conditions within this cluster (p > .001).
Left hemisphere posterior cingulate and precuneus
The main effect within the cluster in the left hemisphere posterior cingulate (BA 31) and the medial precuneus (BA 7) was to activation occurring in the PASSIVE and THEME conditions compared to no significant activation in the PROBE condition. Overall, activation during the PASSIVE condition was greater than in the THEME. Post-hoc paired t tests revealed that the activation for PASSIVE was greater than that for THEME (most intense voxel: Talairach coordinates, x = 13, y = −46, z = 36; t18 = 5.31, p < .001) and PROBE conditions (most intense voxel: x = 4, y = −46, z = 26; t18 = 5.19, p < .001). The activation for the THEME condition was significantly greater than the PROBE condition (most intense voxel: x = 2, y = −52, z = 32; t18 = 5.88, p < .001).
Right hemisphere middle frontal gyrus
Greater activation was observed for the THEME condition relative to the PROBE and PASSIVE conditions within the cluster encompassing the right hemisphere inferior and middle frontal gyrus (BA 46). Across the cluster, the mean Z score for significantly activated voxels for the THEME, PROBE, and PASSIVE conditions was 1.97 (SD = .24; volume = 1,998 µl), 1.24 (SD = .40; volume = 1,890 µl), and .96 (SD = .17; volume = 1,701 µl), respectively. Post-hoc paired t tests revealed that the activation for THEME was greater than that for PROBE (most intense voxel: Talairach coordinates, x = −32, y = 16, z = 26; t18 = 6.60, p < .001) and PASSIVE (most intense voxel: x = −38, y = 22, z = 20; t18 = 5.37, p < .001). The activation for the both the THEME and PROBE conditions was significantly greater than the PASSIVE condition in the inferior aspects of the cluster that extended to the right insula (THEME most intense voxel: x = −32, y = 17, z = 14; t18 = 5.97, p < .001; PROBE most intense voxel: x = −32, y = 17, z = 14; t18 = 6.28, p < .001). The THEME and PROBE conditions did not differ significantly within this region of the cluster (p > .001).
Summary of whole brain findings
Consistent with both the behavioral and L-IFC ROI findings, the whole brain analysis indicated that the OR and SR sentence constructions produced substantially similar activation patterns throughout the brain. Both sentence constructions produced robust activity within widespread regions in cortical and subcortical structures (see Figure 4a and Table II). Although some regional disparity was noted where either one or the other construction showed significant activity above baseline, the regional differences failed to produce reliable differences between the sentence constructions. Therefore, these findings provide evidence that both sentence constructions activated similar brain regions, and by extension similar brain networks, that resulted in behavior that was indistinguishable between OR and SR sentence constructions.
The analysis of Task effects indicated that all three task conditions produced robust activation throughout cortex and subcortical regions (see Figure 4b and Table III). In contrast to the Sentence effects, significant differences between task conditions were found in four regions of the brain, in addition to the differences described previously in the ROI analysis. Performance during the THEME task preferentially recruited activity within the left hemisphere lateral frontal cortex including the middle and inferior frontal gyri encompassing BA 9, 6, and 8. The THEME condition also produced greater activation within the right hemisphere middle and inferior frontal gyri including BA 46, thus showing bilateral activation of this region during performance of this task. Performance on the PASSIVE task did not require explicit analysis of the verbal material, but rather depended on a alternative motor response program in response to the cue stimulus. This task preferentially engaged the medial frontal gyrus (BA 6) and the posterior parietal regions of the posterior cingulate gyrus (BA 31) and medial precuneus (BA 7). There were no regions observed where activity during the PROBE task exceeded that of the THEME task. This finding is consistent with the behavioral findings that the THEME task was more cognitively demanding, as measured by the lower performance achieved, and thus placed a greater emphasis on neural processing. Overall, the whole brain findings suggest that whereas there was substantial overlap in the regional activation during the various tasks, consistent with the similarity of the stimuli, motor responses, and verbal encoding and analysis requirements across tasks, differences in activation were observed. These differences suggest that alternative information processing steps were employed, particularly between the THEME and PROBE tasks relative to the PASSIVE task.
Footnotes
Note of these studies that Cooke et al. (2001) explicitly manipulated the memory demands of structural processing by varying the number of intervening words between two linked elements, finding only the most complex forms with the greatest language-memory requirements activating L-IFC.
We have labeled what might simply be termed an ‘agent hood’ task (‘who did the action’) as thematic judgment. But, the general issue of deciding on thematic roles is captured by the term thematic judgment.
In order to ensure that participants would perform the PASSIVE task correctly, it was critical that the PASSIVE task be presented first, in an untainted manner. In pilot work it had been discovered (in participant interviews) that when the theme task was given first, participants couldn’t easily disengage from the harder tasks. That is, they actually couldn’t help but do some level of this task when they were instructed to passively listen. It appeared as though participants had difficulty breaking out of task mode when they were exposed to the most complex task first. Therefore it was felt that the safest design to maintain the integrity of the individual tasks was to go from the simplest task (PASSIVE) to the most complex task (THEME).
REFERENCES
- Ben-Shachar M, Hendler T, Kahn I, Ben-Bashat D, Grodzinsky Y. The neural reality of syntactic transformations: Evidence from fMRI. Psychological Science. 2003;14:5. doi: 10.1111/1467-9280.01459. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Amunts K, Stephan KM, Posse S, Schorman T, Freund H-J, Ziles K, Seitz RJ. Broca’s region subserves imagery of motion: A combined cytoarchitectonic and fMRI study. Human Brain Mapping. 2000;11:273–285. doi: 10.1002/1097-0193(200012)11:4<273::AID-HBM40>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G. Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. Journal of Cognitive Neuroscience. 1998;10:541–552. doi: 10.1162/089892998562843. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G. PET studies of syntactic processing with auditory sentence presentation. NeuroImage. 1999;9:343–351. doi: 10.1006/nimg.1998.0412. [DOI] [PubMed] [Google Scholar]
- Caplan D, Vijayan S, Kuperberg G, West C, Waters G, Greve D, Dale AM. Vascular responses to syntactic processing: Event-related fMRI study of relative clauses. Human Brain Mapping. 2001;15:26–38. doi: 10.1002/hbm.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, Zurif EB. Dissociation of algorithmic and heuristic processes in language comprehension: Evidence from aphasia. Brain and Language. 1976;3:572–582. doi: 10.1016/0093-934x(76)90048-1. [DOI] [PubMed] [Google Scholar]
- Carpenter P, Just M, Reichle E. Working memory and executive function: Evidence from neuroimaging. Current Opinion in Neurobiology. 2000;10:195–199. doi: 10.1016/s0959-4388(00)00074-x. [DOI] [PubMed] [Google Scholar]
- Cooke A, Zurif E, DeVita C, Alsop D, Koenig P, Detre J, Gee J, Pinango M, Balogh J, Grossman M. Neural basis for sentence comprehension: Grammatical and short-term memory components. Human Brain Mapping. 2001;15:80–94. doi: 10.1002/hbm.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR in Biomedicine. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Bookheimer A. Form and content: Dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Fiebach C, Schlesewsky M, Friederici A. Syntactic working memory and the establishment of filler gap dependencies: Insights from ERPs and fMRI. Journal of Psycholinguistic Research. 2001;30:321–338. doi: 10.1023/a:1010447102554. [DOI] [PubMed] [Google Scholar]
- Fiebach C, Vos S, Friederici A. Neural correlates of syntactic ambiguity in sentence comprehension for low and high span readers. Journal of Cognitive Neuroscience. 2004;16:1562–1575. doi: 10.1162/0898929042568479. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friederici A, Wang Y, Herrmannm C, Maess B, Oertel U. Localization of early syntactic processes in frontal and temporal cortical areas: Magnetoencephalographic study. Human Brain Mapping. 2000;11:1–11. doi: 10.1002/1097-0193(200009)11:1<1::AID-HBM10>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinsky Y. Language deficits and the theory of syntax. Brain and Language. 1986;27:135–159. doi: 10.1016/0093-934x(86)90009-x. [DOI] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Chen W, Moore P, Detre J, Alsop D, Gee J. Sentence processing strategies in healthy seniors with poor comprehension: An fMRI study. Brain and Language. 2002;80:296–313. doi: 10.1006/brln.2001.2581. [DOI] [PubMed] [Google Scholar]
- Gruber O. Effects of domain-specific interference on brain activation associated with verbal working memory task performance. Cerebral Cortex. 2001;11:1047–1055. doi: 10.1093/cercor/11.11.1047. [DOI] [PubMed] [Google Scholar]
- Haist F, Adamo M, Westerfield M, Courchesne E, Townsend J. The functional neuroanatomy of spatial attention in autism spectrum disorders. Developmental Neuropsychology. 2004;27:425–458. doi: 10.1207/s15326942dn2703_7. [DOI] [PubMed] [Google Scholar]
- Heim S, Opitz B, Friederici A. Distributed cortical networks for syntax processing: Broca’s area as the common denominator. Brain and Language. 2003;85:402–408. doi: 10.1016/s0093-934x(03)00068-3. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Science. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Inui T, Otso Y, Tanaka S, Okada T, Nishizawa S, Konishi J. A functional MRI analysis of comprehension processes of Japanese sentences. NeuroReport. 1998;9:3325–3328. doi: 10.1097/00001756-199810050-00032. [DOI] [PubMed] [Google Scholar]
- Just M, Carpenter P, Keller T, Eddy W, Thulborn K. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kaan E, Swaab T. The brain circuitry of syntactic comprehension. Trends in Cognitive Science. 2002;6:350–356. doi: 10.1016/s1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maess B, Koelsch S, Cunter TC, Friederici AD. Musical syntax is processed Broca’s area: An MEG study. Nature Neuroscience. 2001;4:540–545. doi: 10.1038/87502. [DOI] [PubMed] [Google Scholar]
- Meyer M, Friederici A, Cramon V. Neurocognition of auditory sentence comprehension: Event related fMRI reveals sensitivity to syntactic violoations and task demands. Cognitive Brain Research. 2000;9:19–33. doi: 10.1016/s0926-6410(99)00039-7. [DOI] [PubMed] [Google Scholar]
- Michael E, Keller T, Carpenter P, Just M. fMRI investigations of sentence comprehension by eye and by ear: Modality fingerprints on cognitive processes. Human Brain Mapping. 2001;13:239–252. doi: 10.1002/hbm.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso M, Moro A, Glauche V, Rijntjes M, Reichenbach J, Buchel C, Weiller C. Broca’s area and the language instinct. Nature Neuroscience. 2003;6:774–781. doi: 10.1038/nn1077. [DOI] [PubMed] [Google Scholar]
- Newman A, Pancheva R, Ozawa K, Neville H, Ullman M. An event-related fMRI study of syntactic and semantic violations. Journal of Psycholinguistic Research. 2001;30:339–364. doi: 10.1023/a:1010499119393. [DOI] [PubMed] [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fulbright RK, Shaywitz SE, Shaywitz BA, Gore JC, Shankwiler D. An event-related neuroimaging study distinguishing form and content in sentence processing. Journal of Cognitive Neuroscience. 2000;12:123–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Nicol J, Forster K, Veres C. Subject-verb agreement processes in comprehension. Journal of Memory and Language. 1997;36:569–587. [Google Scholar]
- Nicol J, Love T. Overarching agrammatism: When comprehension involves production. In: Grodzinsky Y, Shapiro L, editors. Language and the Brain: Representation and Processing. New York: Academic Press; 2000. [Google Scholar]
- Ojemann JG, Buckner RL, Akbudak E, Snyder AZ, Ollinger JM, McKinstry RC, Rosen BR, Petersen SE, Raichle ME, Conturo TE. Functional MRI studies of word stem completion: Reliability across laboratories and comparison to blood flow imaging with PET. Human Brain Mapping. 1998;6:203–215. doi: 10.1002/(SICI)1097-0193(1998)6:4<203::AID-HBM2>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–114. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Allen M, McLaughlin J, Inoue K. Brain potentials elicited by prose-embedded linguistic anomalies. Memory and Cognition. 2002;30:1304–1312. doi: 10.3758/bf03213412. [DOI] [PubMed] [Google Scholar]
- Owen AM. The functional organisation of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. European Journal of Neuroscience. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Functional organization of the human frontal cortex for mnemonic processing. Annals of the New York Academy of Sciences. 1995;769:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Specialised systems for the processing of mnemonic information within the primate frontal cortex. Philosophical Transactions of the Royal Society of London B. 1996;351:1455–1462. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hasimoto R, Homae F. Sentence processing in the cerebral cortex. Neuroscience Research. 2001;39:1–10. doi: 10.1016/s0168-0102(00)00205-4. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI Atlas of the Human Cerebellum. San Diego: Academic Press; 2000. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working memory: A view from neuroimaging. Cognitive Psychology. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marchuetz C, Koeppe RA. Components of verbal working memory: Evidence from neuroimaging. Proceedings of the National Academy of Sciences of the USA. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rauch S. Localization of syntactic comprehension by positron emisson tomography. Brain and Language. 1996;52:452–473. doi: 10.1006/brln.1996.0024. [DOI] [PubMed] [Google Scholar]
- Swinney D. Parameters and modes of language processing: Evidence from on-line comprehension studies. In: Witruk T, Lachmann E, editors. Basic Mechanisms of Language and Language Disorders. Leipzig: Leipziger Universitatsverlag; 1999. [Google Scholar]
- Swinney D, Zurif E, Prather P, Love T. Neurological distribution of processing resources underlying language comprehension. Journal of Cognitive Neuroscience. 1996;8:174–184. doi: 10.1162/jocn.1996.8.2.174. [DOI] [PubMed] [Google Scholar]
- Swinney D, Taylor O. Short-term memory recognition search in aphasics. Journal of Speech and Hearing Research. 1971;14:578–588. doi: 10.1044/jshr.1403.578. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. In: Co-Planar Stereotaxic Atlas of the Human Brain. Rayport M, translator. New York: Thieme Medical; 1988. [Google Scholar]
- Wartenburger I, Heekeren H, Burchert F, Heinemann S, Deblesser R, Villringer A. Neural correlates of syntactic transformations. Human Brain Mapping. 2004;22:72–81. doi: 10.1002/hbm.20021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters G, Caplan D, Alpert N, Stanczak L. Individual differences in rCBF correlates of syntactic processing in sentence comprehension: Effects of working memory and speed of processing. NeuroImage. 2003;19:101–112. doi: 10.1016/s1053-8119(03)00007-7. [DOI] [PubMed] [Google Scholar]
- Wong EC, Bandettini PA, Hyde JS. Echo-Planar imaging of the human brain using a three axis local gradient coil; Paper presented at the 11th Annual Meeting of The Society of Magnetic Resonance in Medicine; Berlin. 1992. [Google Scholar]