Abstract
Adducts of reactive chemicals with hemoglobin (Hb) or human serum albumin can be used as biomarkers of internal doses of carcinogens. Since dried blood spots (DBS) are easier to collect and store than conventional venous blood samples, they encourage applications of biomarkers of exposure in large epidemiology studies. Also, neonatal DBS can be used to investigate chemical exposures in utero. Here, we report a simple method to isolate Hb from DBS with high recovery and purity using the addition of ethanol to aqueous DBS extracts. To prove the concept that DBS-derived proteins can be used to assay for adducts, we measured Hb adducts of benzene oxide, a reactive metabolite of the ubiquitous air pollutant, benzene, in 9 neonatal and 9 adult DBS (from volunteer subjects), using a gas chromatography-mass spectrometry method that we had previously developed. For comparison, benzene oxide-Hb adducts (BO-Hb) were measured in the same 9 adult subjects, using Hb that had been isolated and purified using our conventional method for venous blood. The geometric mean BO-Hb levels in all DBS samples ranged from 27.7 to 33.1 pmol/g globin. Neither of the comparisons of mean (logged) BO-Hb levels between sources (adult conventional vs. adult DBS and adult DBS vs. newborn DBS) showed a significant difference. Based upon the estimated variance of the BO-Hb levels, we had 80% power to detect a 1.7-fold difference in geometric mean levels of BO-Hb in our samples of 9 subjects.
Keywords: Hemoglobin adducts, dried blood spots, benzene oxide
Introduction
Biomarkers of internal dose can be more accurate and precise surrogates for carcinogen exposures than environmental measurements per se (1). Yet, because chemical carcinogens are usually reactive electrophiles with very short life spans in vivo (2) (e.g., alkylating and acylating agents, aldehydes, alkylnitrosamines, dialkylsulfates, oxiranes, quinones, reactive oxygen and nitrogen species), it is rarely possible to measure them in target tissues. This has motivated the use of adducts of these electrophiles with abundant blood proteins, particularly hemoglobin (Hb) and human serum albumin (HSA), as measures of carcinogen dose (3, 4). Electrophiles enter the blood from absorption in the lungs or gut (e.g., inhalation of ethylene oxide) or, more typically, via metabolism of procarcinogens in the liver or other tissues (e.g., production of benzene oxide by Cytochrome P-450 metabolism of benzene). Once in the blood, electrophiles react at varying rates with all available nucleophiles to form adducts by numerous mechanisms (5). Hemoglobin and HSA contain a myriad of nucleophilic sites, namely the free thiol groups of Cys, amine groups of His, Trp, Lys, as well as the N-termini, hydroxyl groups of Ser and Tyr, and the carboxylic acid groups of Asp, Glu, and the C-termini. Since protein adducts are not repaired and are much more abundant than DNA adducts in blood (one ml of blood contains about 150 mg Hb, 30 mg of HSA, and 0.003–0.008 mg of DNA) (5), they are potentially more useful measures of internal dose than DNA adducts, which have paradoxically received far more attention in this regard. Indeed, the kinetics of production and elimination of Hb and HSA adducts are sufficiently simple to permit straightforward estimates of systemic doses of carcinogens over the mean residence time of these proteins (28 d for HSA and 63 d for Hb in humans) (6–8).
Levels of targeted Hb and/or HSA adducts have been investigated in human blood for several environmental toxicants that are either electrophilic carcinogens or their precursors, i.e., ethylene oxide, benzene, 1,3-butadiene, acrylamide, aflatoxin B1, a variety of aromatic amines, and polycyclic aromatic hydrocarbons [reviewed in (5)]. However, the need to obtain venous blood samples has limited the utility of protein adducts (and other blood-based biomarkers) as measures of exposure in large epidemiology studies. This has motivated investigators to employ dried blood spots (DBS) that can be obtained by a simple skin prick as sources of blood biomarkers. Robert Guthrie first used DBS in the 1960s to screen newborn populations for hyper-phenylalanine associated with the genetic disease phenylketonuria (9). Neonatal DBS offer valuable opportunities for investigating chemical exposures in utero and their possible links to childhood cancers. Immunoassays have been applied to measure a variety of biomarkers in DBS from adult populations, e.g., folate (10), transferrin receptor (11, 12), immunoglobulin E (13), Epstein-Barr virus antibodies (14), leptin (15), and C-reactive protein (16). Thus, DBS can potentially be used in both prospective and retrospective studies to process large numbers of blood specimens from human subjects.
A single dried blood spot (DBS) contains about 50 μl of human blood (10). Assuming a protein concentration of 192 mg/ml (17), one DBS should contain about 9.6 mg of protein of which there should be about 7.7 mg of Hb (80% of total protein) and 1.2 mg HSA (12% of total protein). Since most current assays for protein adducts typically require between 1 and 10 mg of globin (from Hb) or HSA, a single DBS should contain sufficient quantities of these proteins to measure adducts. Here, we describe experiments to isolate Hb in high purity from a single DBS, to purify the resulting globin, and to measure cysteinyl adducts of benzene oxide (BO-Hb) in these proteins. We previously detected BO-Hb in globin isolated from conventional venous blood samples in both benzene-exposed and control subjects and showed that levels of BO-Hb increased with the level of benzene exposure (18). Detection of BO-Hb in control subjects points to production of adducts from environmental exposures to benzene and/or dietary and endogenous sources of benzene oxide or other precursor molecules that produce the same adduct (19). In the current study, we show that BO-Hb is present at comparable levels in adult globin, isolated either from DBS or from conventional red blood cells, and in globin obtained from neonatal DBS.
Materials and Methods
Chemicals
S-Phenylcysteine (SPC) and [2H5]SPC were kindly provided by Dr. A. Gold of the Chemistry Core of the Superfund Basic Research Program at the University of North Carolina, Chapel Hill. Hydrochloric acid (conc.), acetone (nanograde), hexanes (pesticide grade), and ethanol (100%) were purchased from Fisher Scientific (Pittsburg, PA). Methanesulfonic acid was obtained from Fluka Chemical Company (Switzerland). Trifluoroaceticanhydride (TFAA) was from Pierce (Rockford, IL) and was distilled once prior to use. Human Hb was purchased from Sigma Chemical Company (St. Louis, MO). ProteinSaver 903® specimen collection cards were purchased from Whatman (Florham Park, NJ). Safety lancets for preparation of fresh adult DBS were obtained from Fisher Scientific (Pittsburg, PA). Bio-Rad protein-assay reagents were purchased from Bio-Rad Laboratories (Hercules, CA).
Dried blood spots
Nine newborn DBS, collected via heel lancet, were obtained from the North Carolina Laboratory of Public Health, Raleigh, NC. These single DBS, which were collected on Whatman ProteinSaver 903® specimen collection cards, were obtained within 30 days of collection. Nine adult DBS were prepared using archived frozen whole blood, randomly selected from 191 adult volunteer subjects collected in the state of North Carolina in 1998 and stored at −80°C (20). After thawing to room temperature, 50-μl aliquots of these blood specimens were spotted on specimen collection cards to produce adult DBS; these DBS were stored at room temperature for 2 weeks before processing. Five of the 9 adult DBS were spotted in duplicate to assess assay precision. Fresh adult DBS, required for experiments to develop the method, were prepared on specimen collection cards with blood obtained by finger lancet from laboratory volunteer subjects.
Precipitation of hemoglobin via addition of ethanol
In order to determine optimal conditions for precipitating Hb, while leaving other blood proteins in solution, DBS specimens were precipitated from varying mixtures of ethanol/water. Eighteen individual DBS from a single volunteer subject were dried overnight and then excised with scissors and extracted with 2-ml of deionized water by agitation on a shaker table (Lab-Line 4626, Barnstead, Melrose Park, IL) at 160 rpm for 90 min. The specimen collection paper was removed using forceps and the eluted blood was concentrated to approximately 200 μl using a SpeedVac® system (Savant SC110, ThermoFisher Scientific, Pittsburg, PA). To determine the optimum concentration of ethanol/water to precipitate Hb, the eluted blood was added drop-wise to aqueous solutions containing between 15% and 66% ethanol, in increments of 3% ethanol. All samples were incubated at 4°C for one h and were centrifuged at 30,000 × g to pellet the precipitated protein. The supernatant fraction, containing soluble proteins, was decanted and discarded. Total protein concentration was measured in the precipitated protein fraction using a variation of the Bradford assay (Bio-Rad Protein Assay, Bio-Rad Laboratories, Hercules, CA), based upon absorbance at 595 nm. Hemoglobin concentrations were estimated by selective absorbance at 523 nm.
Isolation of globin from DBS and red blood cells
Dried blood spots were excised from specimen collection cards with scissors and placed in 4-ml glass vials with Teflon® lined caps. Two ml of deionized water were added and the vials were briefly agitated with a vortex mixer and then gently mixed on a rotary shaker (Lab-Line 4626, Barnstead, Melrose Park, IL) at 160 rpm for 90 min. The specimen collection paper was removed with forceps and the eluted blood was dried with a SpeedVac®. After dissolving the dried proteins in 570 μl of deionized water, 430 μl of ethanol (43% v/v, which was found to be optimal in the preliminary experiment described above) was added drop-wise to selectively precipitate Hb. Samples were incubated at 4°C for one h, transferred to 1.5-ml plastic vials and centrifuged at 30,000 × g to pellet the Hb. The precipitated Hb was reconstituted in 200 μl of deionized water and then added drop-wise to preweighed 4-ml glass vials containing 2 ml of 0.1% HCl in acetone (−20°C) to precipitate globin. Vials were incubated for 4 h at −20 °C, centrifuged, and the supernatant was decanted. The globin was then washed three times with ice cold acetone and was dried in a vacuum oven overnight at 37°C and 15 mm Hg. The amount of isolated globin was determined gravimetrically.
Globin had been isolated from red blood cells from the same 9 subjects who provided DBS according to our conventional method (21). Portions of these conventional globin specimens were processed in parallel with globin derived from DBS.
Analysis of globin purity from dried blood spots
Two-dimensional electrophoresis was performed using the carrier ampholine method of isoelectric focusing by Kendrick Laboratories (Madison, WI). Nonequilibrium pH gradient electrophoresis was carried out in a glass tube of inner diameter 2.0 mm at 200 volts for 13 h using 1.5% pH 3.5–10 and 0.25% 8–10.5 ampholines (GE Healthcare, Piscataway, NJ). One μg of tropomyocin (MW 33,000, pl 5.2) and 1 μg of lysozyme (MW 14,000, pl 11.5) were added as internal markers. After equilibrium in SDS sample buffer (10% glycerol, 50 mM dithiothreitol, 2.3% SDS and 0.0625 M tris, pH 6.8), each tube gel was sealed to the top of a stacking gel overlying a 12% acrylamide slab gel (0.75 mm thick) and SDS slab gel electrophoresis was carried out for 4 h at 15 mA/gel. The following proteins were added as molecular weight standards: myosin (MW 220,000), phosphorylase A (MW 94,000), catalase (MW 60,000), actin (MW 43,000) carbonic anhydrase (MW 29,000) and lysozyme (MW 14,000) (Sigma Chemical Co, St. Louis, MO). Gels were scanned with a laser densitometer (Model PDSI, Molecular Dynamics Inc, Sunnyvale, CA), which had been checked for linearity with a calibrated Neutral Density Filter Set (Melles Griot, Irvine, CA). The images were analyzed using Progenesis Discovery software (version 2005, Nonlinear Dynamics, Durham, NC). The general method of computerized analysis included automatic spot finding, background subtraction (Progenesis mathematical model), quantification and matching with detailed manual checking. Purity of the globin derived from DBS was assessed by comparing 2D-gels of globin from DBS with globin derived from red blood cells, assuming the latter to be 100% pure.
Analysis of benzene oxide-hemoglobin adducts (BO-Hb)
The cysteinyl adduct of benzene oxide with Hb was assayed using the procedure of Yeowell-O'Connell et al. (21) with minor modifications. Following protein isolation, 4.5 mg of globin (from either a DBS or a conventional red-blood-cell specimen) and one pmol of [2H5]S-phenylcysteine (internal standard) were placed in 4-ml glass vials and dried in a vacuum oven (70 – 80 °C, 15 mm Hg). Seven hundred and fifty μl of TFAA and 20 μl of methanesulfonic acid were added and the vials were capped tightly with Teflon®-lined caps. This reaction cleaves the cysteinyl adduct of benzene oxide and converts it to the volatile derivative, phenyltrifluorothioacetate (PTTA). The reaction mixture was heated at 100 °C for 40 min, cooled to room temperature, and the excess TFAA was removed under a stream of nitrogen. One ml of hexane was added and the organic extract (containing PTTA) was washed with 1 ml of 0.1 M Tris buffer, pH 7.3, and twice with 1 ml of deionized water. The hexane layer was then transferred to 1.5-ml high recovery auto sampler vials (Agilent Technologies, Santa Clara, CA) and was reduced to a final volume of about 50 μl under nitrogen for analysis of PTTA by gas chromatography-mass spectrometry (GC-MS) in negative ion chemical ionization mode. A Hewlett-Packard 5890 series II plus gas chromatograph and a Hewlett-Packard 5989B MS engine were used with a DB-5 fused silica capillary column (60 m, 0.25-mm i.d., 0.25-μm phase thickness, J & W Scientific, Inc.) operating a helium carrier gas at a flow rate of one ml/min. The injection port and source temperatures were 250 and 100°C, respectively. The oven temperature was held at 50°C for 3 min and then ramped at 5°C/min to 140°C. Late-eluting compounds were removed by increasing the oven temperature to 250°C at 50°C/min, where it was held for 10 min. Three-μl injections were made in the splitless mode. Ions of PTTA (m/z 206) and [2H5]PTTA (m/z 211) were monitored using selective ion monitoring. To estimate the precision associated with the GC-MS of the BO-Hb analyte, triplicate injections were performed for 4 DBS (three adult and one newborn) and duplicate injections were performed for one adult DBS.
Statistical analyses
Adduct data were transformed to natural logarithms prior to statistical analyses to satisfy assumptions regarding homogeneity of variance and normality. Sources of random variation of adduct levels, from errors associated with the assay and GC-MS injections, were estimated using a nested random effects model where injections were nested within assays and assays within DBS specimens (Proc NESTED of SAS). The corresponding coefficients of variation (CV) were estimated as follows: , , and where and represent the estimated variance components (of logged data) for assays and injections, respectively, from the random effects model (18). Comparisons of mean (logged) adduct levels between sources of 9 blood specimens, namely, adult conventional versus adult DBS and adult DBS versus newborn DBS, employed two-tailed Student's t-tests with unequal variances and a significance level of α = 0.05. Pearson correlation coefficients were estimated for (logged) adduct levels from adult-conventional and adult-DBS specimens. Blood specimens with multiple assays and/or injections were aggregated (logged levels: first by injection and then by assay) prior to testing of mean values and estimation of correlation coefficients. Statistical analyses employed SAS software (version 9.1 for Windows, SAS System Software, Cary, NC) for random effects models and Microsoft Excel for t-tests and correlation.
Results
Hemoglobin precipitation with addition of ethanol
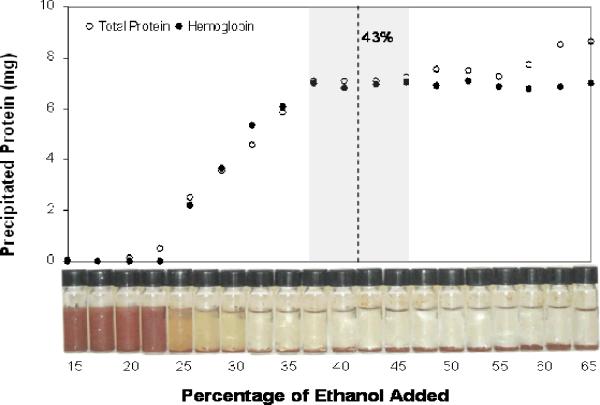
As shown in Figure 1, Hb in blood from extracted DBS began to precipitate at an ethanol concentration of approximately 20%, and reached a plateau at approximately 37%. Human serum albumin and the majority of the other plasma proteins remained soluble until an ethanol concentration of approximately 47% was reached. Since Hb and total protein were measured using different methods (Bio-Rad assay for total protein and absorbance at 523 nm for Hb), and since a combination of Hb and HSA was used as a standard in the Bio-Rad assay to approximate all of the proteins in blood, total precipitated protein was normalized to the Hb measurements. This adjustment was based on 2D-gel electrophoresis results (described below) which showed that, following precipitation with 43% ethanol, Hb represented 96% of the total protein. Based on these results, an ethanol percentage of 43% was chosen to optimize Hb recovery from the DBS, while preventing other proteins from precipitating.
Figure 1.
Selective precipitation of hemoglobin from dried blood spots (DBS) by addition of ethanol. Total protein measurements were normalized to hemoglobin levels, using results from the 2D-gel electrophoresis analysis which showed that DBS-derived globin was 96% pure when hemoglobin was precipitated with 43% ethanol.
Globin isolation from DBS
The estimated mean globin isolated from adult and newborn DBS were 6.4 mg (SD = 0.96) and 7.2 mg (SD = 2.20) of globin, respectively. This corresponds to a recovery of greater than 80%, based on previously reported Hb levels in whole blood (adults: 140 mg/ml, newborns: 193 mg/ml) (22). The purity of the isolated globin, assessed by 2-D gel electrophoresis, was determined by laser densitometry to be approximately 96%.
Precision of BO-Hb measurements in DBS
The estimated components of variance of (logged) BO-Hb levels for assays and GC-MS injections were and and 7.25×10−3, respectively. The corresponding CV values are as follows: CVassay = 0.128, CVinjection = 0.085, and CVmethod = 0.154. Using conventional samples of globin derived from red blood cells, we previously reported values of CVassay = 0.28 and CVinjection = 0.10 (18).
Comparisons of BO-Hb levels across sources of specimens
Table 1 lists the estimated means, SDs, and ranges of logged BO-Hb levels and the corresponding geometric mean BO-Hb levels for samples of 9 globin specimens from different sources. The estimated statistics were very similar across sources. The geometric mean BO-Hb levels ranged from 27.7 to 33.1 pmol/g globin. Neither of the comparisons of mean (logged) BO-Hb levels between sources (adult conventional vs. adult DBS and adult DBS vs. newborn DBS) was significant at a P-value of 0.05.
Table 1.
Summary statistics of BO-Hb levels in samples of globin isolated from different sources (n = 9 specimens per source).
| Source of globin | Estimated mean of logged BO-Hb levels | Estimated SD of logged BO-Hb levels | Range of logged BO-Hb levels | Estimated geometric mean BO-Hb level (pmol/g globin) |
|---|---|---|---|---|
| Adult DBS | 3.32 | 0.326 | 2.98 – 3.83 | 27.7 |
| Newborn DBS | 3.47 | 0.449 | 3.02 – 4.48 | 32.1 |
| Adult conventional | 3.50 | 0.351 | 2.99 – 3.94 | 33.1 |
Legend: DBS, dried blood spot; BO-Hb, hemoglobin adduct of benzene oxide; SD, standard deviation.
Figure 2 shows a scatter plot of the (logged) BO-Hb levels in globin derived from adult conventional red blood cells and DBS in single specimens of blood obtained from the same subjects. The Pearson correlation coefficient for the 9 data pairs was r = 0.732.
Figure 2.
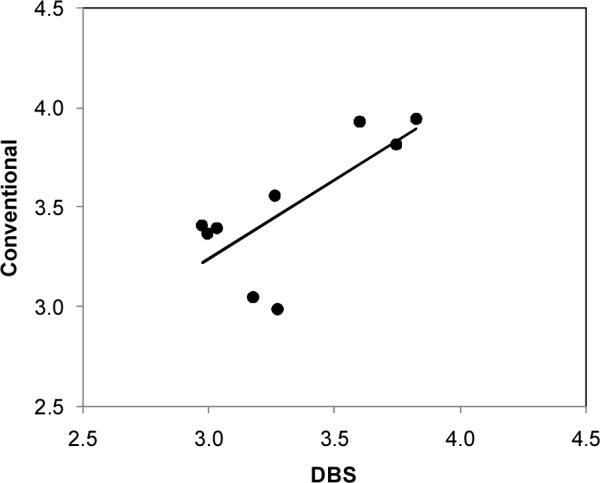
Scatterplot of logged levels of benzene oxide-hemoglobin adducts measured in globin isolated from dried blood spots (DBS) and from conventional red blood cells from the same 9 adult blood specimens (r = 0.732).
Discussion
Although blood protein adducts of targeted electrophiles have been measured in humans exposed to prominent genotoxins [see review by Tornqvist et al. (5)], all prior investigations employed venous blood samples. The purpose of this investigation was to prove the concept that protein adducts can be measured in DBS-derived globin from both newborn and adult subjects. We focused upon one adduct, namely BO-Hb, that we had previously measured in globin isolated from benzene-exposed workers and control subjects from Shanghai, China (18).
An important innovation of our assay involves the isolation of relatively pure Hb from a DBS based upon precipitation in 43% ethanol. Conventional Hb adduct assays have relied on first separating red blood cells (which are comprised almost exclusively of Hb) from the plasma by centrifugation. This is not possible with DBS because red blood cells are lysed during the blood drying process, giving rise to a more complex whole blood matrix. Precipitating Hb from DBS via addition of ethanol provides a relatively simple and inexpensive means of obtaining globin for use in molecular epidemiology studies. While our focus here concerned isolation of Hb, it should be noted that, following precipitation of Hb, the supernatant fraction could also be used to provide additional proteins, notably HSA.
We successfully measured BO-Hb in all DBS specimens from 9 newborns and 9 adults in North Carolina and also in globin isolated from conventional red blood cells for the same adult subjects. No significant differences in mean (logged) BO-Hb concentrations were detected between samples of globin isolated from the different sources (adult DBS vs. newborn DBS and adult DBS vs. adult conventional). Since these t-tests were conducted with the logged adduct levels, in natural scale they tested for differences between geometric mean BO-Hb concentrations in the different sources of Hb. The lack of significant differences between geometric mean levels indicates that the typical blood concentrations of BO-Hb were comparable across sources. This indicates that the typical BO-Hb level in adult DBS was similar to that measured in conventionally-isolated adult globin, and that the typical BO-Hb level in a North Carolina newborn was similar to that measured in a North Carolina adult. Given a pooled estimate of the variance of 0.144 for logged BO-Hb concentrations, our tests (with 9 specimens per group) had a power to detect a 1.7-fold difference in geometric mean values.
Our ability to measure BO-Hb in DBS proves the concept that a single DBS provides suitable Hb for determinations of a prominent adduct in human populations. This conclusion is reinforced by the high pairwise correlation of BO-Hb levels measured in globin from DBS and conventional red blood cells from the same 9 adult subjects (Figure 2). Since DBS can be collected much more simply and with greater subject acceptance than venous blood samples, this opens the door to the use of protein adducts as biomarkers of human exposure to benzene, and, ultimately, to a host of genotoxic and carcinogenic substances in either adults or newborns. With the relentless improvements in analytical sensitivity that we are witnessing, it is reasonable to expect that it will soon be possible to conduct assays of numerous protein adducts with a small portion of a DBS rather than an entire DBS as reported here.
Given the relatively long residence time of 60 d for chemically stable Hb adducts in human blood (5), Hb adducts provide rather steady measures of human exposure, either in utero or in adult populations. Such steady biomarkers provide less biasing surrogates of the true long-term exposure levels which give rise to human diseases and are, therefore, preferable to short-term biomarkers, such as urinary metabolites, whose levels vary greatly from day to day (23).
It is also interesting to note that the geometric mean concentration of BO-Hb, which in our adult subjects (DBS and conventional globin, n = 18) was e(3.32+3.50)/2 = e3.41 = 30.3 pmol/g globin, is quite similar to the median value of 37.1 pmol/g globin reported in 44 control subjects from Shanghai, China (18). This suggests that the magnitudes of environmental, dietary, and endogenous sources of the precursors of BO-Hb, which include environmental benzene as well as other (unknown) contributors, are similar in different parts of the world.
Acknowledgments
Financial support for this work was provided through the trans-NIH Genes, Environment and Health Initiative, grant U54ES016115, and by the National Institute for Environmental Health Sciences through a pilot project under center grant P30ES10126,, by training grant T32ES07018, and by the Chemistry Core of P42ES005948. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institute of Environmental Health Sciences or the National Institutes of Health.
References
- 1.Lin YS, Kupper LL, Rappaport SM. Air samples versus biomarkers for epidemiology. Occup Environ Med. 2005;62:750–60. doi: 10.1136/oem.2004.013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller EC, Miller JA. Mechanisms of chemical carcinogenesis: nature of proximate carcinogens and interactions with macromolecules. Pharmacol Rev. 1966;18:805–38. [PubMed] [Google Scholar]
- 3.Ehrenberg L, Hiesche KD, Osterman-Golkar S, Wennberg I. Evaluation of genetic risks of alkylating agents: tissue doses in the mouse from air contaminated with ethylene oxide. Mutation Research. 1974;24:83–103. doi: 10.1016/0027-5107(74)90123-7. [DOI] [PubMed] [Google Scholar]
- 4.Groth U, Neumann HG. The relevance of chemico-biological interactions for the toxic and carcinogenic effects of aromatic amines. V. The pharmacokinetics of related aromatic amines in blood. Chem Biol Interact. 1972;4:409–19. doi: 10.1016/0009-2797(72)90061-0. [DOI] [PubMed] [Google Scholar]
- 5.Tornqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:279–308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 6.Ehrenberg L, Moustacchi E, Osterman Golkar S. Dosimetry of genotoxic agents and dose-response relationships of their effects. Mut. Res. 1983;123:121–182. doi: 10.1016/0165-1110(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 7.Granath F, Ehrenberg L, Tornqvist M. Degree of alkylation of macromolecules in vivo from variable exposure. Mutat. Res. 1992;284:297–306. doi: 10.1016/0027-5107(92)90014-s. [DOI] [PubMed] [Google Scholar]
- 8.Rappaport SM, Waidyanatha S, Qu Q, Shore R, Jin X, Cohen B, Chen LC, Melikian AA, Li G, Yin S, Yan H, Xu B, Mu R, Li Y, Zhang X, Li K. Albumin adducts of benzene oxide and 1,4-benzoquinone as measures of human benzene metabolism. Cancer Res. 2002;62:1330–7. [PubMed] [Google Scholar]
- 9.Guthrie R, Susi A. A Simple Phenylalanine Method For Detecting Phenylketonuria In Large Populations Of Newborn Infants. Pediatrics. 1963;32:338–43. [PubMed] [Google Scholar]
- 10.O'Broin S, Gunter EW. Screening of folate status with use of dried blood spots on filter paper. Am J Clin Nutr. 1999;70:359–367. doi: 10.1093/ajcn/70.3.359. [DOI] [PubMed] [Google Scholar]
- 11.McDade TW, Shell-Duncan B. Whole blood collected on filter paper provides a minimally invasive method for assessing human transferrin receptor level. J Nutr. 2002;132:3760–3. doi: 10.1093/jn/132.12.3760. [DOI] [PubMed] [Google Scholar]
- 12.Tanner S, Shell-Duncan B, McDade T. Use of combined measures from capillary blood to assess iron deficiency in rural Kenyan children. J Nutr. 2004;134:384–7. doi: 10.1093/jn/134.2.384. [DOI] [PubMed] [Google Scholar]
- 13.Tanner S, McDade TW. Enzyme immunoassay for total immunoglobulin E in dried blood spots. Am J Hum Biol. 2007;19:440–2. doi: 10.1002/ajhb.20635. [DOI] [PubMed] [Google Scholar]
- 14.McDade TW, Stallings JF, Angold A, Costello EJ, Burleson M, Cacioppo JT, Glaser R, Worthman CM. Epstein-Barr virus antibodies in whole blood spots: a minimally invasive method for assessing an aspect of cell-mediated immunity. Psychosom Med. 2000;62:560–7. doi: 10.1097/00006842-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Miller AA, Sharrock KC, McDade TW. Measurement of leptin in dried blood spot samples. Am J Hum Biol. 2006;18:857–60. doi: 10.1002/ajhb.20566. [DOI] [PubMed] [Google Scholar]
- 16.McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50:652–4. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- 17.Longe JL, Blanchfield DS. The Gale encyclopedia of medicine. Gale Group; Detroit: 2002. [Google Scholar]
- 18.Yeowell-O'Connell K, Rothman N, Waidyanatha S, Smith MT, Hayes RB, Li G, Bechtold WE, Dosemeci M, Zhang L, Yin S, Rappaport SM. Protein Adducts of 1,4-Benzoquinone and Benzene Oxide among Smokers and Nonsmokers Exposed to Benzene in China. Cancer Epidemiol Biomarkers Prev. 2001;10:831–8. [PubMed] [Google Scholar]
- 19.Yeowell-O'Connell K, Rothman N, Smith MT, Hayes RB, Li G, Waidyanatha S, Dosemeci M, Zhang L, Yin S, Titenko-Holland N, Rappaport SM. Hemoglobin and albumin adducts of benzene oxide among workers exposed to high levels of benzene. Carcinogenesis. 1998;19:1565–71. doi: 10.1093/carcin/19.9.1565. [DOI] [PubMed] [Google Scholar]
- 20.Lin YS, McKelvey W, Waidyanatha S, Rappaport SM. Variability of albumin adducts of 1,4-benzoquinone, a toxic metabolite of benzene, in human volunteers. Biomarkers. 2006;11:14–27. doi: 10.1080/13547500500382975. [DOI] [PubMed] [Google Scholar]
- 21.Yeowell-O'Connell K, McDonald TA, Rappaport SM. Analysis of hemoglobin adducts of benzene oxide by gas chromatography- mass spectrometry. Anal Biochem. 1996;237:49–55. doi: 10.1006/abio.1996.0199. [DOI] [PubMed] [Google Scholar]
- 22.Kates EH, Kates JS. Anemia and Polycythemia in the Newborn. Pediatr Rev. 2007;28(1):33–34. doi: 10.1542/pir.28-1-33. [DOI] [PubMed] [Google Scholar]
- 23.Rappaport SM, Kupper LL. Quantitative Exposure Assessment. Stephen Rappaport; El Cerrito, CA: 2008. http://www.lulu.com/content/1341905. [Google Scholar]