Abstract
Background and Purpose
To describe features of children with intracerebral hemorrhage (ICH) and to determine predictors of short-term outcome in a single-center prospective cohort study.
Methods
Single-center prospective consecutive cohort study of spontaneous ICH in children age 1-18 years from January 2006 to June 2008. Exclusion criteria were inciting trauma; intracranial tumor; isolated epidural, subdural, intraventricular, or subarachnoid hemorrhage; hemorrhagic transformation of ischemic stroke; and cerebral sinovenous thrombosis. Hospitalization records were abstracted. Follow-up assessments included outcome scores using the Pediatric Stroke Outcome Measure (PSOM) and King's Outcome Scale for Childhood Head Injury (KOSCHI). ICH volumes and total brain volumes (TBV) were measured by manual tracing.
Results
Twenty-two patients, median age of 10.3 years (range 4.2-16.6 years), had presenting symptoms of headache in 77%, focal deficits 50%, altered mental status 50%, and seizures 41%. Vascular malformations caused hemorrhage in 91%. Surgical treatment (hematoma evacuation, lesion embolization or excision) was performed during acute hospitalization in 50%. One patient died acutely. At median follow-up of 3.5 months (range 0.3-7.5 months), 71% of survivors had neurological deficits; 55% had clinically significant disability. Outcome based on PSOM and KOSCHI scores was worse in patients with ICH volume >2% of TBV (p=0.023) and altered mental status at presentation (p = 0.005).
Conclusions
Spontaneous childhood ICH was due mostly to vascular malformations. Acute surgical intervention was commonly performed. Although death was rare, 71% of survivors had persisting neurological deficits. Larger ICH volume and altered mental status predicted clinically significant disability.
Keywords: intracerebral hemorrhage, outcome, childhood, vascular malformation
Introduction
Stroke occurs in 2 to 13 children per 100,000 per year in developed countries 1, 2. Hemorrhagic stroke accounts for as much as half of pediatric stroke, whereas in adults, arterial ischemic stroke is more common1, 3. Intracerebral hemorrhage (ICH) devastates children with death reported in up to 33% and permanent deficits in up to 40%, including seizures, cognitive and motor impairment4-6. Although this disease is life-threatening, little research has been devoted to pediatric ICH. The few prospective cohort studies have small numbers2, 7 Case series and retrospective chart reviews comprise the remaining literature 7.
Much is known about the pathogenesis and outcome predictors of ICH in adults where hypertension and amyloid angiopathy are among the most common etiologies. Clinical and radiologic characteristics influencing adult ICH outcome cannot be applied to children because etiologies of ICH in children differ from those in adults. Additionally, published pediatric ICH outcome studies suggest that the course of recovery in children differs substantially from that in adults. One study reported that pediatric ICH caused by hematological abnormalities and infratentorial hemorrhage location is associated with worse prognosis8. A recent retrospective series demonstrated that ICH volume predicts poor outcome in children9. However, this association has not been evaluated in a prospective consecutive cohort. Also, other important factors portending poor recovery in adults, such as initial Glasgow Coma Score <9 and intraventricular extension10, have not been assessed prospectively in children. Our study's primary goals were to describe features of a prospective cohort of children with ICH and to examine whether hemorrhage volume and altered mental status predict outcome at short-term follow-up in children with intraparenchymal hemorrhage.
Materials and Methods
Study design
With institutional review board approval, a prospective consecutive cohort of patients with spontaneous ICH presenting between January 2006 and June 2008 was identified from a large tertiary care children's hospital stroke registry.
Case identification
During acute hospitalization, patients were identified for the study by neurosurgery and/or neurology providers participating in a multidisciplinary neurovascular care protocol targeting children admitted to the hospital with spontaneous ICH. Neurosurgery and hematology databases were cross referenced ensuring complete ascertainment. Inclusion criteria were children age 1 to 18 years with spontaneous intraparenchymal hemorrhage confirmed by neuroimaging. Exclusion criteria were head trauma; intracranial tumor; hemorrhage isolated to the epidural, subdural, intraventricular, or subarachnoid compartments; hemorrhagic transformation of arterial ischemic stroke; and hemorrhage from cerebral sinovenous thrombosis. Patients with brain tumor, hemorrhagic transformation of arterial ischemic stroke, and hemorrhagic venous infarction related to cerebral sinovenous thrombosis were excluded to avoid potentially confounding effects on outcome from the primary pathological process. Since our primary a priori hypothesis was that ICH volume would be a major predictor of outcome, we excluded isolated intraventricular hemorrhages as they have no measurable ICH volume.
Clinical data
Acute hospital, neuroimaging, inpatient rehabilitation, pediatric stroke clinic and neurosurgery clinic follow-up records were abstracted. Patient details are summarized in an appendix table. Altered mental status (AMS) on admission was defined as present if medical records showed any of these terms/conditions describing the level of consciousness within the first 6 hours of hospitalization: 1) Glasgow Coma Score (GCS) ≤9; 2) description of the child as comatose, obtunded, or unresponsive; 3) intubation for deteriorating mental status. AMS was broadly defined because physicians did not uniformly record an initial GCS or because record of the first examination was not always available when a child was transferred from another hospital. Diagnostic testing was determined by clinical indications. Short-term outcome was assessed from findings on standard clinical care protocol follow-up examinations 2 to 6 months after initial symptom onset. For children with no follow-up visits during this period, the examination closest to target range midpoint (4 months) was used.
Hemorrhage analysis
ICH etiology was determined by medical history, neuroimaging, intraoperative observation, and available surgical pathology. Location of hemorrhage (supratentorial or infratentorial) and presence of IVH were determined via central review by a study neuroradiologist (RZ) blinded to clinical history and initial clinical radiographic interpretations. Hemorrhage volume and total brain volume (TBV) were measured on acute CT or MRI by manual segmentation tracing using online software, ITK-SNAP (www.itksnap.org), which has excellent intra- and inter-operator reliability for measuring regional brain volumes11. When MRI was the first imaging technique, T2 sequences were used for segmentation. Another pediatric ICH study used similar manual tracing techniques for ICH and TBV with excellent inter-rater reliability9. To account for varying brain volume among different aged children, hemorrhage volume was expressed as a percent of TBV. TBV included the cerebral hemispheres, cerebellum, and brainstem. Intraventricular hemorrhage was excluded from hemorrhage volume measurements; ventricular volume was excluded from TBV measurements. Supratentorial parenchymal hemorrhages ≤2% total brain volume were considered small, and those >2% total brain volume were considered large. Choosing 2% of TBV to define large ICH was based on the observation that hemorrhage greater than 30cc in adults is associated with functional impairment12. In an average adult with brain volume of 1400cc, an ICH volume of 30cc approximates 2% of TBV. The predictive significance of hemorrhage volume was evaluated in patients with supratentorial hemorrhage. Since the physiological effects of volume are likely different in the infratentorial compartment, and since only 2 children in our cohort had infratentorial hemorrhage, these patients were excluded from outcome analysis.
Outcome assessment
Outcome was evaluated and classified using two standardized instruments. The Pediatric Stroke Outcome Measure (PSOM) characterizes deficit type and severity on neurological examination and was validated in a cohort of children with arterial ischemic stroke13. The PSOM is based entirely on neurological examination with subscores in 5 domains: sensorimotor left, sensorimotor right, expressive language, receptive language, and cognition/behavior. PSOM subscores are graded 0 for no deficit, 0.5 for mild deficit that does not interfere with function, 1 for moderate deficit that interferes with function, and 2 for severe deficit with missing/absent function. The total PSOM score range is 0-10. Children who died were not assigned a PSOM score. The King's Outcome Scale for Childhood Head Injury (KOSCHI) is a pediatric modification of the Glasgow Outcome Scale, characterizing global functional status. The KOSCHI incorporates functional impairments plus periodic symptoms like headaches and seizures. It scores 1 for death, 2 for vegetative state, 3a and 3b for severe disability (3a worse), 4a and 4b for moderate disability (4a worse), and 5a and 5b for good recovery (5b full recovery, no residual symptoms)14. In our study, the KOSCHI was determined from history obtained via parental interview and neurological examination findings. Deficits were considered ‘clinically significant’ for a KOSCHI score ≤ 4b and/or the PSOM score ≥1 in any category. We chose these scores to ascertain fully all children with functional impairments.
Statistical analysis
Fisher's exact test and relative risk (RR) with 95% exact confidence intervals (CI) were used to evaluate associations using STATA version 10.0 (Stata Corporation). Multivariate logistic regression analysis was limited due to small sample size and was therefore only attempted to adjust results from univariate analyses for age. A p-value of <0.05 was considered statistically significant.
Results
Demographics
Twenty-four patients with ICH were identified during the 2.5 year period. Parents of 22 patients (92%) consented to participation: 11 males, 11 females. Mean and median ages at presentation were 10.4 and 10.3 years, respectively (range 4.2 – 16.6 years). Racial distribution was 73% Caucasian and 27% African-American. All children were neurologically normal prior to ICH. Two children had medical conditions associated with ICH: 1 had sickle cell anemia (appendix, case 20), 1 had hereditary hemorrhagic telangiectasia (appendix, case 7).
Clinical presentations
Eighteen patients (82%) presented to local hospitals; 4 presented to our tertiary care center. Median time from symptom onset to presentation to the initial hospital was 70 minutes: 14 patients (64%) presenting within 3 hours, 5 patients (23%) presenting more than 24 hours from symptom onset. Symptoms included severe headache in 17 (77%), emesis in 13 (59%), altered mental status in 11 (50%), seizure in 9 (41%), and syncope in 1 (4.5%). Focal deficits were present on admission examination in 11 patients (50%).
Acute life-threatening intracranial hypertension or herniation syndromes requiring intervention occurred in 10 children (45%). Six (27%) received either hypertonic saline or mannitol. Three (14%) were hyperventilated. Moderate therapeutic hypothermia was instituted in 2 (9%). Six (27%) required ventriculostomy. Three (14%) underwent decompressive hemicraniectomy.
Hemorrhage Characteristics
Neuroimaging was performed in all children, with median time to the first study 2.4 hours (range 30 minutes to 10 days). Eleven children (50%) had their first diagnostic image performed within 3 hours of symptom onset. During their acute hospitalization, 20 children (91%) had head CT, 19 (86%) had MRI, 14 (64%) had MRA, 6 (27%) had CTA, and 17 (77%) had conventional angiogram. Four had no dedicated vascular imaging: 1 died prior to further evaluation, 3 had MRIs diagnostic for cavernoma. All children had normal platelet count, prothrombin time, international normalized ratio, and partial thromboplastin time. Vascular malformations were detected in 20 patients (91%): AVM in 12 (55%), cavernoma in 7 (32%), and aneurysm in 1 (4.5%). Hemorrhage etiology was not identified in 2 (9%).
Hemorrhage was supratentorial in 20 patients (91%). Two patients had infratentorial hemorrhages, one involving the pons and one the cerebellum. Intraventricular extension occurred in 10 patients (45%), 9 of whom had supratentorial ICH. Additional information on ICH locations is presented in the appendix.
Surgical management
Hematoma evacuation, hemicraniectomy, and/or surgical/endovascular management of vascular malformations were performed in 11 patients (50%) during the acute hospitalization. Children with more benign clinical courses or whose malformations were not diagnosed during the acute hospitalization were treated during subsequent hospitalizations. Indication for surgical interventions is presented in the appendix.
Outcome
Median hospitalization duration was 9 days (range 2–23 days). Median intensive care unit admission was 6.5 days (range 2–23 days). One death occurred on hospital day 2. Care was withdrawn from a patient with a cerebellar hematoma who had already herniated prior to transfer. Fourteen of 21 survivors (67%) were discharged with a neurological deficit. Eleven (52%) were discharged to inpatient rehabilitation.
Neurological follow-up was available in all patients who survived the acute hospitalization (n = 21) at a median of 3.5 months (range 0.3-7.5 months). Recurrent hemorrhage within the short-term follow-up interval occurred in one child with a cavernoma who developed a second ICH 11 days after the initial event. One child (4.5%) with AVM arising from the middle cerebral artery had a secondary ischemic stroke.
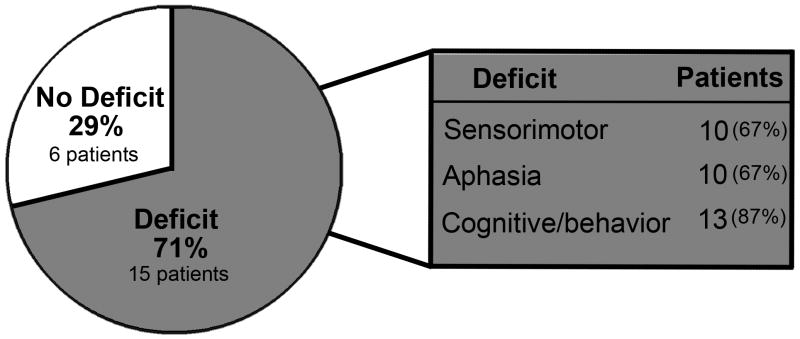
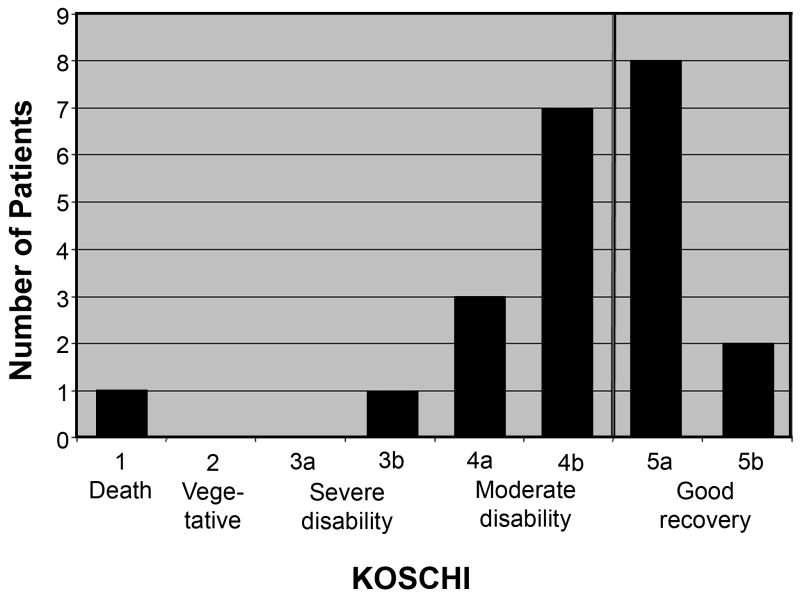
Of the 21 living children, parents of 13 (62%) reported their child had not fully recovered from the hemorrhage. Six did not attend school due to stroke; 3 others had modified school programs. Five children (24%) suffered from mood alterations, 2 of whom had overt signs of depression. Ten patients (48%) had chronic headaches. Of 3 patients who developed epilepsy, only 1 had seizures at initial presentation. Five children were readmitted to the hospital due to hemorrhage-related problems including seizures, re-bleed, or definitive treatment of vascular malformations. Fifteen children (71%) had abnormal neurological examinations (Figure 1). However, all were ambulatory and able to communicate. Twelve (55%) had clinically significant disability, all of whom had a PSOM score ≥ 1 in at least one category (appendix) and a KOSCHI score ≤ 4b (Figure 2 and appendix).
Figure 1.
Neurological Deficits at Follow-up, N = 21
Figure 2.
King's Outcome Scale for Childhood Head Injury (KOSCHI), dark vertical line represents cut-off between good and poor outcomes
Outcome Predictors
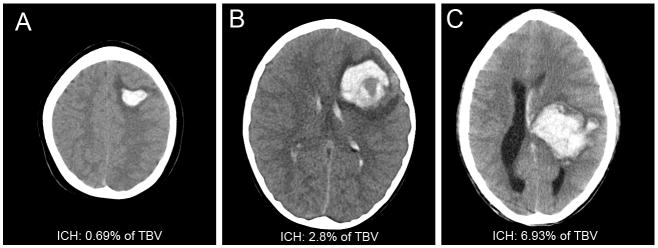
Among children with supratentorial ICH, large ICH volume was associated with clinically significant disability in univariate analysis. A diagnostic image performed within the first day of hospitalization was available in 19 of 20 children with supratentorial ICH (16 HCT, 3 MRI). Median ICH volume expressed as a percent of TBV was 1.68% (range 0.005-6.93%). Median absolute hemorrhage volume was 22.8cc (range 0.07-92cc). Children with ICH volumes >2% TBV had worse outcome compared to children with ICH volumes ≤2% (p = 0.023, RR 3.9, 95% CI 1.1–14.1). Figure 3 shows examples of small and large hemorrhages.
Figure 3.
Example Head Computed Tomography
A, Intracerebral hemorrhage (ICH) 0.69% of total brain volume (TBV); B, ICH 2.8%TBV; C, ICH 6.93%TBV
AMS was associated with clinically significant disability in univariate analysis (p=0.005, RR 7.2, 95% CI 1.1-46.9). Documentation of mental status within 6 hours of hospital arrival was available in 19 of 20 children with supratentorial ICH; 10 of these 19 had AMS. AMS was associated with ICH volume >2% TBV (p=0.005, RR 7.2, 95% CI 1.1-46. 9). Only 2/10 children with AMS had ICH volume ≤2% TBV compared to 8/9 children without AMS. Given the modest sample size, we were unable to test formally for interaction or to adjust for other potential confounders.
Patients with clinically significant disability were slightly older than those without clinically significant disability in univariate analysis (11.9 ± 3.5 years vs.8.9 ± 3.1 years, p=0.06 by t-test). All other univariate analyses performed were post hoc (Table 1). Although clinically significant disability was numerically more common in patients with IVH, seizures, or focal deficit at presentation, these findings were not statistically significant. The 2 children with infratentorial hemorrhage had the worst outcomes: 1 died, 1 had severe disability.
Table 1.
Univariate Analysis of Outcome Predictors in Supratentorial Hemorrhage
| Variable | # with clinically significant disability/# with variable | # with clinically significant disability/# without variable | Relative Risk | 95% Confidence Interval | p-value |
|---|---|---|---|---|---|
| Hemorrhage volume >2% TBV* | 7/9 | 2/10 | 3.9 | 1.1-14.1 | 0.02 |
| Altered mental status* | 8/10 | 1/9 | 7.2 | 1.1-46.9 | 0.005 |
| Intraventricular hemorrhage | 6/9 | 4/11 | 1.8 | 0.7-4.5 | 0.37 |
| Seizure | 6/9 | 4/11 | 1.8 | 0.7-4.5 | 0.37 |
| Focal deficit | 7/10 | 3/10 | 2.3 | 0.8-6.5 | 0.18 |
| Surgery during acute hospitalization | 7/11 | 3/9 | 1.9 | 0.7-5.3 | 0.37 |
| Male gender | 5/10 | 5/10 | 1.0 | 0.4-2.4 | 1.00 |
| African-American race | 3/6 | 7/14 | 1.0 | 0.4-2.6 | 1.00 |
One patient did not have imaging available for hemorrhage volume measurement or documentation of mental status and was not included in these analyses.
Multivariate analysis was performed to determine if age confounded the associations between outcome and either ICH volume or early AMS. Age was not independently associated with outcome in either analysis. However, age modestly impacted the odds ratios of the covariates above but did not alter their significant outcome associations.
Discussion
In this study, we described presentations, etiologies, and short-term outcomes of a prospective consecutive cohort of children with spontaneous ICH. More than half of the children had clinically significant disability on the PSOM and/or KOSCHI at follow-up. We considered clinically significant disability to be present in any children with moderate disability or worse since these patients are unable to function normally and require additional care. Children with clinically significant disability face critical difficulties in educational and social arenas. Fourteen percent had epilepsy, 19% were re-hospitalized, 43% could not attend regular school programs, over 60% had cognitive deficits, and nearly half suffered from chronic headaches. These percentages are similar to retrospective study findings that describe long-term outcomes. One found that nearly 11% of children developed epilepsy and that evidence of cognitive deficits were observed in nearly half of surviving patients at 10-year follow-up5. Another reported that patients with good early outcome scores had neuropsychological impairment at 3-year follow-up8.
Our finding that hemorrhage volume >2% of TBV was associated with worse neurologic outcome in childhood ICH confirms the findings of a prior retrospective report9. Furthermore, our study demonstrates that AMS within 6 hours of hospitalization was associated with worse outcome. Additionally, AMS within 6 hours of hospitalization was associated with supratentorial ICH volume >2% of TBV and therefore may serve as an early clinical indicator of larger, more severe ICH. Larger studies are required to determine the independence of these two factors.
Mortality in our cohort, 4.5% at 30-days, was low compared to rates of nearly 25% reported in other studies4-6, 8. Furthermore, mortality in our cohort was far lower than that in adult studies in which 6-month mortality ranges from 23% to 58%15. Several possible explanations exist for our low mortality. First, our cohort included only 1 hemorrhage ≥4% TBV, while a previously published report with more deaths included 8 children with hemorrhage volume ≥4% TBV9. Further, we excluded patients with brain tumor and with hemorrhage associated with cerebral venous sinus thrombosis, primary conditions that may portend poor prognosis. No child in our cohort had ICH resulting from coagulopathy, an etiology associated with poor outcome in a retrospective study8. Referral bias may exist such that some patients may have died prior to transfer to our tertiary care center. Finally, children in our study who rapidly deteriorated were treated aggressively with hemorrhage evacuation and/or hemicraniectomy and maximal medical therapy for intracranial hypertension.
The association of poor outcome and IVH reported in adult ICH was not definitively demonstrated in our study. In this cohort, intraventricular hemorrhage was not statistically associated with clinically significant disability in children with supratentorial ICH. Due to the small numbers, it is possible that the added negative effect of IVH was too small to detect. Infratentorial ICH is also associated with poor outcome in adults. Only 2 patients presented with infratentorial ICH, so this study could not adequately evaluate infratentorial location as an outcome predictor. Note that these two patients suffered severe outcomes: death in 1, severe disability in the other.
Another study limitation is that no validated standard outcome measure exists in pediatric ICH. The two measures used here have been used in other related disease processes: the PSOM for pediatric ischemic stroke, the KOSCHI for childhood head injury. Other studies have employed non-validated tools such as the Glasgow Outcome Scale8, 9 and the modified Rankin scale5. In a head trauma study, the KOSCHI had limited utility predicting long-term outcome, so the implications for long-term outcome in our study should be interpreted cautiously16. Some children in this cohort with clinically significant disability on short-term KOSCHIs and PSOMs may continue to improve, so early outcomes may over-estimate long-term deficits. However, some children may manifest neurocognitive problems as they mature. The benefit of using the KOSCHI plus the PSOM, is that the former accounts for seizures and headaches, thereby separating children with no sequelae from the injury from those with no deficit but with other repercussions. Larger studies with greater follow-up duration should be performed to elucidate the relationships between hemorrhage volume and long-term outcomes and that between early altered mental status and long-term outcomes.
The strength of our findings is enhanced by the prospective design, by the high rate of inclusion and ascertainment, and by our use of objective standardized outcome measures. The prospective design particularly eliminates the selection and ascertainment bias that often plagues retrospective studies based on ICD-9 code searches which have been inadequate in identifying children with stroke diagnoses6, 17, 18. The prospective design and use of objective standardized outcome measures further strengthens our conclusions by minimizing recall bias for patient presentations and for interpreting clinical examinations and outcomes.
Summary and Future Directions
In this single-center prospective cohort of children with ICH, hemorrhage volume >2% of total brain volume and altered mental status within 6 hours of hospital presentation were associated with clinically significant disability at short-term follow-up. Seventy percent of children had neurological deficits and 55% had clinically significant disability. Enhanced understanding of factors affecting outcome for children with ICH is needed to provide better prognostic counseling for families and to design clinical trials for management strategies. Validating our results in a larger prospective cohort and identifying other potentially modifiable factors associated with outcome may lead to significant improvements in the care of children with ICH.
Supplementary Material
Acknowledgments
We acknowledge The Children's Hospital of Philadelphia Research Institute. It has generously supported Dr. Ichord's development of the Pediatric Stroke Program at The Children's Hospital of Philadelphia as well as the development and maintenance of The Children's Hospital of Philadelphia Stroke Registry.
We also acknowledge the contributions of our research assistants, Stefanie Mason BA, Erica D Kane BA, Charlene I Jones BA, Aviva R Halperin BA, Danielle M Gordon BA, Leila Glass, and Stephen J Tang BS, for their help with data collection and data entry.
Dr. Licht is supported by grants from the NINDS (K23-NS52380), Dana Foundation, and the Wolfson Family Foundation.
Dr. Smith is supported by a grant from the NINDS (K12-NS049453).
Dr. Ichord is supported by grants from the NINDS (RO1-207320801, K23 NS062110).
Dr. Jordan is supported by a grant from the NINDS (K23-NS062110).
Footnotes
None of the authors has any conflict of interest or disclosures.
References
- 1.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: Ethnic and gender disparities. Neurology. 2003;61:189–194. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 2.Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of dijon, france: A study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48:1343–1348. doi: 10.1016/0895-4356(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 3.Broderick J, Talbot GT, Prenger E, Leach A, Brott T. Stroke in children within a major metropolitan area: The surprising importance of intracerebral hemorrhage. J Child Neurol. 1993;8:250–255. doi: 10.1177/088307389300800308. [DOI] [PubMed] [Google Scholar]
- 4.Al-Jarallah A, Al-Rifai MT, Riela AR, Roach ES. Nontraumatic brain hemorrhage in children: Etiology and presentation. J Child Neurol. 2000;15:284–289. doi: 10.1177/088307380001500503. [DOI] [PubMed] [Google Scholar]
- 5.Blom I, De Schryver EL, Kappelle LJ, Rinkel GJ, Jennekens-Schinkel A, Peters AC. Prognosis of haemorrhagic stroke in childhood: A long-term follow-up study. Dev Med Child Neurol. 2003;45:233–239. doi: 10.1017/s001216220300046x. [DOI] [PubMed] [Google Scholar]
- 6.Lo WD, Lee J, Rusin J, Perkins E, Roach ES. Intracranial hemorrhage in children: An evolving spectrum. Arch Neurol. 2008;65:1629–1633. doi: 10.1001/archneurol.2008.502. [DOI] [PubMed] [Google Scholar]
- 7.Jordan LC, Hillis AE. Hemorrhagic stroke in children. Pediatr Neurol. 2007;36:73–80. doi: 10.1016/j.pediatrneurol.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: Aetiology, presentation and outcome. Brain Dev. 2003;25:416–421. doi: 10.1016/s0387-7604(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 9.Jordan LC, Kleinman JT, Hillis AE. Intracerebral hemorrhage volume predicts poor neurologic outcome in children. Stroke. 2009;40:1666–1671. doi: 10.1161/STROKEAHA.108.541383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ich score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 11.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3d active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 13.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15:316–324. doi: 10.1177/088307380001500508. [DOI] [PubMed] [Google Scholar]
- 14.Crouchman M, Rossiter L, Colaco T, Forsyth R. A practical outcome scale for paediatric head injury. Arch Dis Child. 2001;84:120–124. doi: 10.1136/adc.84.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 16.Calvert S, Miller HE, Curran A, Hameed B, McCarter R, Edwards RJ, Hunt L, Sharples PM. The king's outcome scale for childhood head injury and injury severity and outcome measures in children with traumatic brain injury. Dev Med Child Neurol. 2008;50:426–431. doi: 10.1111/j.1469-8749.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 17.Golomb MR, Garg BP, Saha C, Williams LS. Accuracy and yield of icd-9 codes for identifying children with ischemic stroke. Neurology. 2006;67:2053–2055. doi: 10.1212/01.wnl.0000247281.98094.e2. [DOI] [PubMed] [Google Scholar]
- 18.Golomb MR, Garg BP, Williams LS. Accuracy of icd-9 codes for identifying children with cerebral sinovenous thrombosis. J Child Neurol. 2007;22:45–48. doi: 10.1177/0883073807299959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.