Abstract
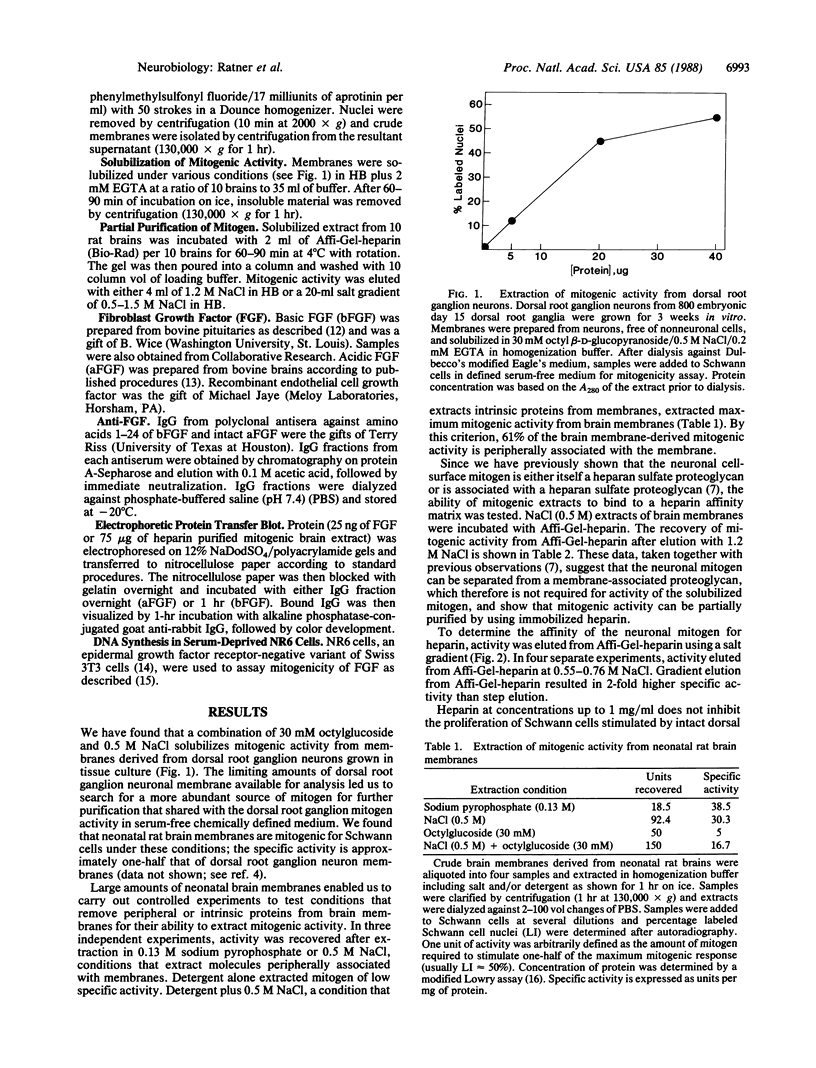
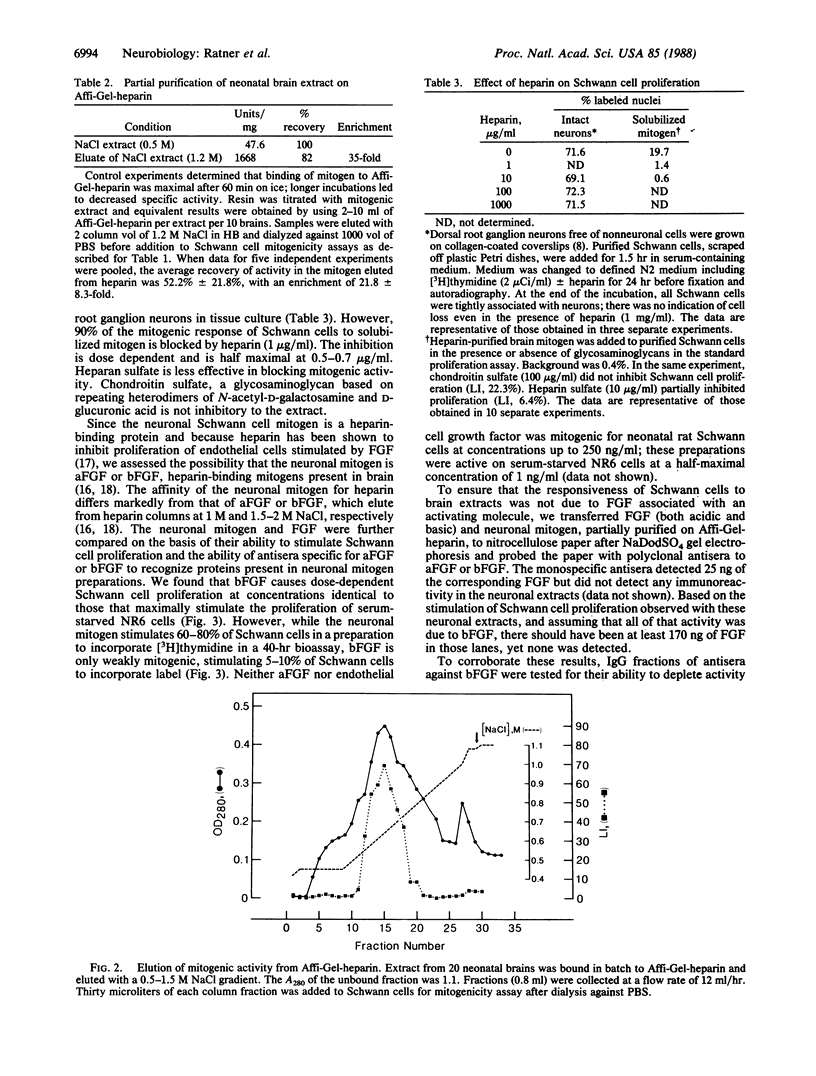
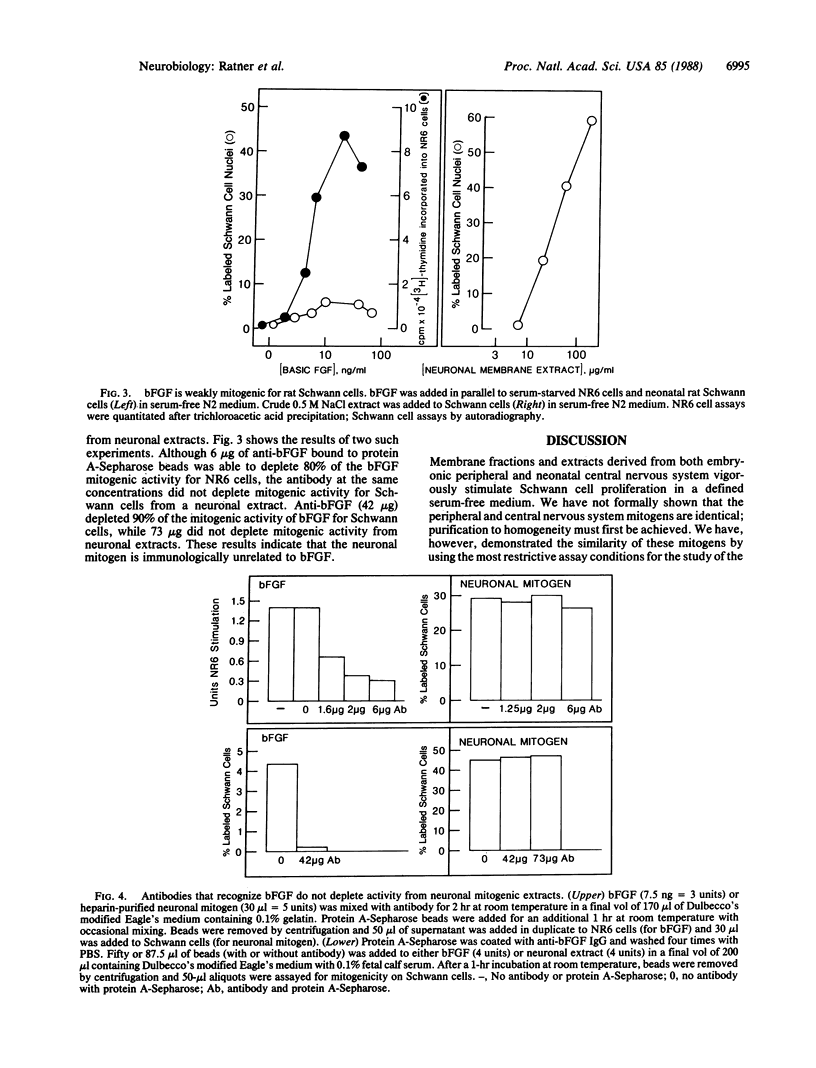
The cell surface of embryonic peripheral neurons provides a mitogenic stimulus for Schwann cells. We report (i) the solubilization of this mitogenic activity from rat dorsal root ganglion neurons grown in tissue culture and (ii) the solubilization and partial purification of mitogenic activity from neonatal rat brains. Extracted mitogenic activity is peripheral rather than intrinsic to the membrane, stable after extraction, and active as a mitogen in the absence of serum (the most stringent criterion defining the neuronal mitogen). We have previously provided evidence suggesting that a neuronal cell-surface heparan sulfate proteoglycan is required for expression of the neurons' mitogenic activity. We now show that mitogenic activity can be extracted from the membrane dissociated from proteoglycan as assayed by its ability to bind to immobilized heparin. After dissociation, low concentrations of heparin (1 micrograms/ml) inhibit the ability of the mitogen to stimulate Schwann cell division. Basic fibroblast growth factor (FGF) is weakly mitogenic for Schwann cells, but it is not present in mitogenic brain extracts (based on immunoblotting). Immunodepletion experiments with specific antibodies to FGF indicate that the mitogenic activity extracted from neurons is not a form of this heparin-binding mitogen. Acidic FGF is not mitogenic for Schwann cells and is not present in mitogenic brain extracts. We suggest that these and previous data indicate the neurite mitogen is a proteoglycan-growth factor complex that limits mitogenic activity to the axonal surface, protects mitogen against inactivation by other proteoglycans, and provides for effective presentation of mitogen to the Schwann cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A. J., Peyronnard J. M., Terry L. C., Romine J. S., Bray G. M. Neonatal neuronal loss in rat superior cervical ganglia: retrograde effects on developing preganglionic axons and Schwann cells. J Neurocytol. 1976 Apr;5(2):137–155. doi: 10.1007/BF01181653. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Fields K. L., Raff M. C. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979 Apr 6;165(1):105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Cassel D., Wood P. M., Bunge R. P., Glaser L. Mitogenicity of brain axolemma membranes and soluble factors for dorsal root ganglion Schwann cells. J Cell Biochem. 1982;18(4):433–445. doi: 10.1002/jcb.1982.240180405. [DOI] [PubMed] [Google Scholar]
- DeVries G. H., Minier L. N., Lewis B. L. Further studies on the mitogenic response of cultured Schwann cells to rat CNS axolemma-enriched fractions. Brain Res. 1983 Jul;285(1):87–93. doi: 10.1016/0165-3806(83)90112-8. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Massoglia S., Cheng J., Fujii D. K. Effect of fibroblast growth factor and lipoproteins on the proliferation of endothelial cells derived from bovine adrenal cortex, brain cortex, and corpus luteum capillaries. J Cell Physiol. 1986 Apr;127(1):121–136. doi: 10.1002/jcp.1041270116. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Molecular and biological characterization of fibroblast growth factor, an angiogenic factor which also controls the proliferation and differentiation of mesoderm and neuroectoderm derived cells. Cell Differ. 1986 Jul;19(1):1–17. doi: 10.1016/0045-6039(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Hinds J. W. Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. J Comp Neurol. 1968 Nov;134(3):287–304. doi: 10.1002/cne.901340304. [DOI] [PubMed] [Google Scholar]
- Lemke G. E., Brockes J. P. Identification and purification of glial growth factor. J Neurosci. 1984 Jan;4(1):75–83. doi: 10.1523/JNEUROSCI.04-01-00075.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. R., Harper J. W., Fett J. W. Purification of heparin-binding growth factors. Anal Biochem. 1986 Apr;154(1):1–14. doi: 10.1016/0003-2697(86)90487-2. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Nagasaki T., Lieberman M. A. Heparin potentiates the action of plasma membrane-associated growth stimulatory activity. J Cell Physiol. 1987 Nov;133(2):365–371. doi: 10.1002/jcp.1041330222. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3918–3921. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner N., Bunge R. P., Glaser L. A neuronal cell surface heparan sulfate proteoglycan is required for dorsal root ganglion neuron stimulation of Schwann cell proliferation. J Cell Biol. 1985 Sep;101(3):744–754. doi: 10.1083/jcb.101.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner N., Elbein A., Bunge M. B., Porter S., Bunge R. P., Glaser L. Specific asparagine-linked oligosaccharides are not required for certain neuron-neuron and neuron-Schwann cell interactions. J Cell Biol. 1986 Jul;103(1):159–170. doi: 10.1083/jcb.103.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner N., Glaser L., Bunge R. P. PC12 cells as a source of neurite-derived cell surface mitogen, which stimulates Schwann cell division. J Cell Biol. 1984 Mar;98(3):1150–1155. doi: 10.1083/jcb.98.3.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J. L., Bunge R. P., Glaser L. Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J Cell Biol. 1980 Mar;84(3):767–778. doi: 10.1083/jcb.84.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J. L., Bunge R. P. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol. 1980 Mar;84(3):739–752. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J. L., Williams A. K., Glaser L., Bunge R. P. Studies of Schwann cell proliferation. II. Characterization of the stimulation and specificity of the response to a neurite membrane fraction. J Cell Biol. 1980 Mar;84(3):753–766. doi: 10.1083/jcb.84.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoff R. P., Price D. L., Stocks A. Electron microscopic autoradiographic studies of gliogenesis in rat optic nerve. II. Time of origin. J Comp Neurol. 1976 Oct 1;169(3):313–334. doi: 10.1002/cne.901690304. [DOI] [PubMed] [Google Scholar]
- Sobue G., Kreider B., Asbury A., Pleasure D. Specific and potent mitogenic effect of axolemmal fraction on Schwann cells from rat sciatic nerves in serum-containing and defined media. Brain Res. 1983 Dec 5;280(2):263–275. doi: 10.1016/0006-8993(83)90056-2. [DOI] [PubMed] [Google Scholar]
- Wice B., Milbrandt J., Glaser L. Control of muscle differentiation in BC3H1 cells by fibroblast growth factor and vanadate. J Biol Chem. 1987 Feb 5;262(4):1810–1817. [PubMed] [Google Scholar]
- Wood P. M., Bunge R. P. Evidence that axons are mitogenic for oligodendrocytes isolated from adult animals. Nature. 1986 Apr 24;320(6064):756–758. doi: 10.1038/320756a0. [DOI] [PubMed] [Google Scholar]
- Wood P. M., Bunge R. P. Evidence that sensory axons are mitogenic for Schwann cells. Nature. 1975 Aug 21;256(5519):662–664. doi: 10.1038/256662a0. [DOI] [PubMed] [Google Scholar]
- Wood P. M., Williams A. K. Oligodendrocyte proliferation and CNS myelination in cultures containing dissociated embryonic neuroglia and dorsal root ganglion neurons. Brain Res. 1984 Feb;314(2):225–241. doi: 10.1016/0165-3806(84)90045-2. [DOI] [PubMed] [Google Scholar]



