Abstract
With the eventual goal of developing a tissue-engineered tear secretory system, we found that primary lacrimal gland acinar cells grown on solid poly(L-lactic acid) (PLLA) supports expressed the best histiotypic morphology. However, to be able to perform vectorial transport functions, epithelia must be supported by a permeable substratum. In the present study, we describe the use of a solvent-cast/particulate leaching technique to fabricate microporous PLLA membranes (mpPLLAm) from PLLA/polyethylene glycol blends. Scanning electron microscopy revealed pores on both the air-cured (∼4 μm) and glass-cured sides (<2 μm) of the mpPLLAm. Diffusion studies were performed with mpPLLAm fabricated from 57.1% PLLA/42.9% polyethylene glycol blends to confirm the presence of channelized pores. The data reveal that glucose, L-tryptophan, and dextran (a high molecular weight glucose polymer) readily permeate mpPLLAm. Diffusion of the immunoglobulin G through the mpPLLAm decreased with time, suggesting the possible adsorption and occlusion of the pores. Cells cultured on the mpPLLAm (57.1/42.9 wt%) grew to subconfluent monolayers but retained histiotypic morphological and physiological characteristics of lacrimal acinar cells in vivo. Our results suggest that mpPLLAm fabricated using this technique may be useful as a scaffold for a bioartificial lacrimal gland device.
Introduction
Poly(l-lactic acid) (PLLA) has been used extensively as a biodegradable and biocompatible scaffold for tissue development and also as a vehicle for drug and gene delivery.1–3 However, to promote cell ingrowth and thereby tissue development, scaffolds should possess appropriate pore size and porosity. Many techniques have been reported for fabricating porous polymeric scaffolds.4–6 Some of these include fiber bonding,7 gas foaming,8 solvent-cast/particulate leaching,9,10 phase separation,11 emulsion freeze drying,12 and melt molding.13 The solvent-cast/particulate leaching technique is one of the most commonly used. It involves dissolving a blend of the polymer and a water-soluble porogen, such as salt, in an organic solvent, casting in the desired shape mold,5 evaporation of the solvent, and removal of the porogen by leaching in water. This technique typically yields thin membranes or wafers (up to 3 mm thick) with very high porosity (>93%).10
Blending of PLLA with polyethylene glycol (PEG), an amphipathic polymer highly soluble in water as well as organic solvents such as chloroform and methyl chloride, is widely used to improve the physical and mechanical properties of PLLA.14–16 The miscibility, crystallization, structure, and thermal properties of these blends have been investigated in great detail.17–20 The resultant copolymer scaffold has been attractive for tissue engineering applications because of its hydrophilicity and mechanical characteristics, such as elongation and softness without much compromise of tensile strength.14,15 PLLA/PEG blends have also been used in the fabrication of microporous PLLA membranes (mpPLLAm).21–23 However, the resultant membranes were found to be only semiporous as they had surface pores on only one side of the membrane, and these pores were not channelized, in the sense that they were not interconnected and did not traverse from one side all the way to the other side of the membrane. Hence, these membranes could find limited use in tissue engineering applications such as drug transport and permeability, tissue remodeling, and electrophysiology, as these studies demand the bidirectional movement of fluid, chemical components, or biological components across the membrane. Further, the typical pore size range recommended for these studies should lie between 0.4 and 3 μm.
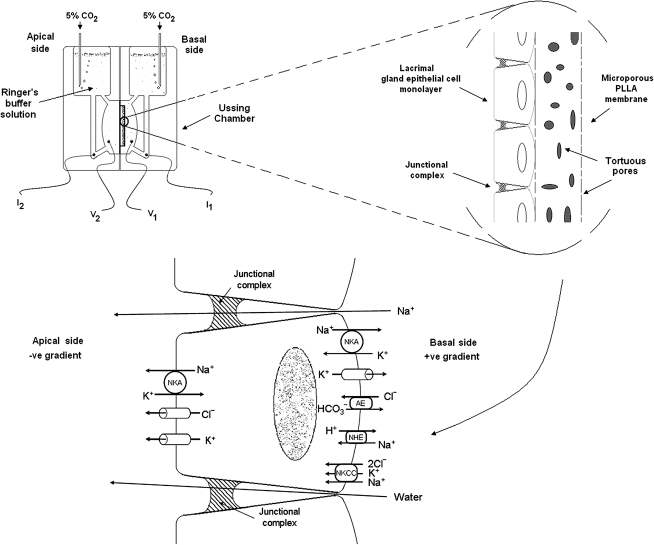
Our group has found PLLA superior to poly lactic-co-glycolic acid and collagen as a substratum for rabbit lacrimal gland acinar cells, which retained histiotypic morphology and certain cell functions but established only subconfluent monolayers.24 Although it is possible to establish continuous lacrimal epithelial cell monolayers on polyester membrane scaffolds, the monolayers maintain the high electrical resistances characteristic of transporting epithelia for only a few days,25 and the cells fail to exhibit certain distinctive morphological features.25 Hence, we anticipate that mpPLLAm might optimally support both the transepithelial electrophysiological properties and the cytophysiological apparatus that acinar cells normally use to deliver specific proteins into the ocular surface fluid. As lacrimal gland acinar cells employ an array of transmembrane ion transporters and aquaporins to secrete electrolytes and water across the acinar epithelium, such a membrane could be used to establish epithelial cell monolayers whose transepithelial electrophysiological behavior could be evaluated using the classic Ussing short-circuit method described previously (Fig. 1).25
FIG. 1.
Schematic illustration of how a microporous PLLA membrane (mpPLLAm) would find use for studying lacrimal epithelial transport physiology in an Ussing chamber. A part of the figure has been reprinted from Selvam et al.25 and used with permission of Am J Physiol Cell Physiol. PLLA, poly(L-lactic acid).
In the present study, we describe the use of the solvent-cast/particulate leaching technique to fabricate mpPLLAm with channelized pores with effective pore diameters within the range of 1–4 μm, and have demonstrated that such membranes are permeable to glucose, L-tryptophan, and dextran. Purified lacrimal gland acinar cells (pLGACs) were also cultured on the fabricated mpPLLAm (57.1/42.9 wt%), and the morphological and physiological characteristics of the cultured cells were evaluated.
Materials and Methods
Animals
Rabbits used throughout these studies were adult, female, New Zealand Whites (Irish Farms, Norco, CA), weighing ∼4 kg. Animals were used in accordance with the ARVO Resolution on Use of Animals in Ophthalmology Research and were maintained in a facility fully accredited by the American Association of Laboratory Animal Science. Animals were narcotized with a mixture of ketamine (20–40mg/mL) and xylazine (5–9 mg/mL), 1–1.5 mL, and euthanized with an overdose of Eutha-6CII (120 mg/mL).
Materials
PLLA (molecular weight (MW) 80,000–100,000; i.v. 1.3–1.6 dL/g) was purchased from Polysciences (Warrington, PA). PEG (average MW 400), reagent-grade chloroform, L-tryptophan, dextran (FITC conjugated; MW 4000), and immunoglobulin G (IgG) were purchased from Sigma-Aldrich (St. Louis, MO). Radiolabeled glucose, D-[3-3H]-, was purchased from New England Nuclear (Boston, MA). Standard 12-well tissue culture plates and 12-well Transwell polyester membrane cell culture inserts were obtained from Costar (Corning, NJ).
All cell culture procedures on the mpPLLAm were performed using Hepato-STIM® culture medium (HSM). HSM was purchased from BD Biosciences (Medford, MA). Fetal bovine serum (FBS) was purchased from Omega Scientific (Tarzana, CA).
Fabrication of mpPLLAm
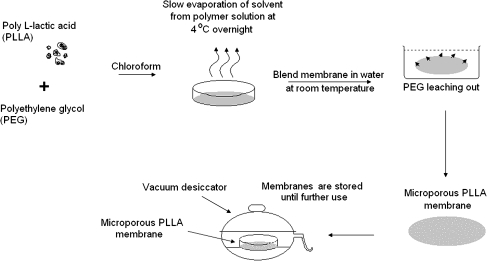
mpPLLAm were fabricated with the PEG solvent-cast technique described by Nakane et al.23 (Fig. 2). Different weights of PLLA (0.01, 0.02, 0.03, and 0.035 g) and 0.015 g PEG were dissolved in 5 mL of chloroform. The solutions were cast into glass Petri dishes (60 × 15 mm; VWR International, San Dimas, CA), placed in a perforated chamber (a 6″ × 6″ × 6″ aluminium basket covered on all sides with aluminium foil with perforations on the uppermost part of the foil on each side, leaving the top intact, was inverted over the Petri dish), and held overnight at 4°C to allow the chloroform to evaporate. The resulting homogenous membranes were then carefully removed and immersed in distilled water at room temperature for 4 h to leach out the PEG. The mpPLLAm were dried in open air at room temperature for 4 h and then held in a vacuum desiccator for at least 2 days before use. The thickness of the dried films was measured with a micrometer (Mitutoyo, Tokyo, Japan).
FIG. 2.
Schematic representation of the fabrication of mpPLLAm with polyethylene glycol (PEG) solvent-cast/particulate leaching technique.
Porosity determination
The porosity of the mpPLLAm was evaluated in methanol using an electronic densimeter (SD-200L; Mirage, Tokyo, Japan). The porosities of the membranes were calculated according to the following relation:
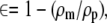 |
(1) |
where ∈ is the porosity of the mpPLLAm; ρm is the density of mpPLLAm, and ρp is the density of pure PLLA membranes.
Fabrication of mpPLLAm inserts
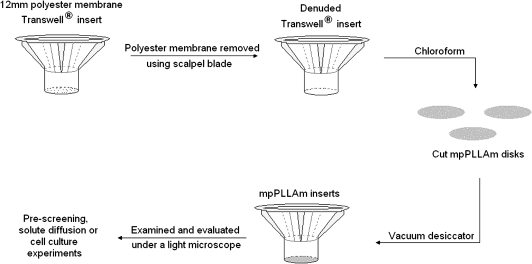
The mpPLLAm were cut into 15-mm-diameter disks and attached to denuded 12-mm Transwell® inserts with the use of chloroform (Fig. 3). Briefly, the polyester membrane from the Transwell inserts was scraped and peeled off with the help of a scalpel blade. The exposed edge of the inserts was then gently dipped in chloroform and was impressed onto the cut mpPLLAm disks. The mpPLLAm inserts were then placed in a vacuum desiccator overnight to remove residual chloroform. The inserts were examined and evaluated under a light microscope for structural integrity, washed thrice with distilled water, and placed into the wells of 12-well tissue culture plates.
FIG. 3.
Schematic illustration of the fabrication of mpPLLAm inserts with the use of chloroform.
For cell culture procedures, the air-cured side of the mpPLLAm was used as the cell culture side. The inserts were sterilized in 70% ethanol for 30 min and were placed into the wells of 12-well tissue culture plates (Costar) with sterile forceps. The inserts were rinsed twice in sterile phosphate-buffered saline (PBS) to remove residual alcohol. The plate was then used immediately for cell-seeding procedures.
Prescreening of the various mpPLLAm
To identify the best membrane for solute diffusion experiments, the mpPLLAm were prescreened by evaluating the diffusion of a dye, crystal violet (10 mg/mL in 20% ethanol; Sigma-Aldrich), across them (data not shown). Briefly, 0.5 mL of crystal violet solution was added to the apical chamber, and 1.5 mL of PBS was added to the basal chamber of the various mpPLLAm inserts. The diffusion of crystal violet in the basal chamber was observed for a period of 24 h using a bright white paper background placed under the tissue culture plate. Based on our findings, mpPLLAm 57.1/42.9 wt% demonstrated the best diffusion characteristics and hence was subsequently used for solute diffusion experiments.
Solute diffusion experiments
Solute diffusion experiments were performed with mpPLLAm (57.1/42.9 wt%) at 25°C. All diffusion experiments were performed with 1.5 mL of PBS in the basal chambers of the mpPLLAm inserts with the exception of the glucose diffusion experiments that contained 1.6 mL of basal medium. The apical chambers of the mpPLLAm inserts contained 0.5 mL of the solute in PBS unless otherwise stated. Glucose diffusion studies were initiated by adding glucose solution (2 mg/mL + 10 μCi radiolabeled glucose) to the apical chambers of the mpPLLAm inserts. Aliquots (10 μL) were withdrawn from the basal chamber at 15-min intervals for 4 h. Radioactivity was measured in a liquid beta scintillation counter (model LS 6000IC; Beckman Instruments, Fullerton, CA).
L-tryptophan (1 mg/mL in 0.1 N hydrochloric acid), dextran (0.5 mg/mL), and IgG (1 mg/mL) diffusion experiments were performed by adding the solute solution to the apical chambers of the mpPLLAm inserts. Aliquots (100 μL) were withdrawn from the basal chamber at 15 min intervals for a period of 4 h. The concentration of L-tryptophan or IgG from the aliquots was determined by measuring the optical density value at a wavelength of 280 nm using a UV spectrophotometer (model DU® 640; Beckman Instruments). The concentration of dextran was determined by measuring the fluorescence with a GENios multidetection microplate reader (Phenix, Hayward, CA) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. After optical density or fluorescence measurements, the aliquots were added back to basal chambers of the inserts.
The diffusion rate of each solute was calculated from the time-dependent increase in its concentration in the basal medium, expressed in units of (mg/mL)/min by measuring the slope of the release versus time profile. The concentration of the diffused component with respect to time was determined by tabulating and comparing the experimental data points with the standard curve of each component (i.e., quantification of radioactivity, transmittance, or fluorescence vs. component concentration).
Diffusion calculations
The diffusion of a component through the pores of the mpPLLAm from the high concentration (apical chamber) to the low concentration side (basal chamber) is determined by Fick's diffusion theory. The concentration of a component on the basal chamber according Fick's law is given by
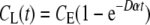 |
(2) |
where
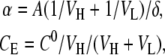 |
(3) |
CL is the concentration of the diffused component in the basal chamber (g/cm3), CE is the equilibrium concentration of the diffusing component, D is the diffusivity (cm2/s) of the component through the mpPLLAm, A is the total membrane flow area (1.12 cm2), δ is the thickness of the mpPLLAm (cm), VH and VL are the apical and basal volumes (cm3), and C0 is the initial concentration of the component in the apical chamber (g/cm3). From Equation (1) we derive
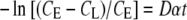 |
(4) |
Therefore, the slope of −ln [(CE − CL)/CE] plotted versus time (t) gives Dα. Equation (1) is a simplification of more general equations for diffusion in plane sheets and is expected to be valid when the amounts transported are small and boundary layer resistances are considered to be negligible.26
Purified lacrimal gland acinar cell preparation
The procedures for isolation of pLGACs were as previously described.21 Briefly, inferior lacrimal glands were removed aseptically, placed in a dish with Ham's complete medium, and finely minced with a pair of scalpel blades (No. 18). Ham's complete medium consists of Ham's F-12 supplemented with 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2 mM glutamine, 2 mM butyrate, 0.084 mg/L linoleic acid, 0.05 mg/mL trypsin inhibitor, 10 mM HEPES, and 5 mg/mL bovine serum albumin. The tissue fragments were then washed and digested with collagenase, DNase, and hyaluronidase in a shaking water bath for 35 min at 37°C. Gland digests were then centrifuged (700 rpm, 5 min), filtered through a 70-μm pore size cell strainer, and washed twice with Hanks' and Ham's complete media. The cell suspension was then further purified by centrifuging in a Ficoll gradient (190 rpm, 15 min) to reduce contamination by fibroblasts. The resultant cellular fraction was resuspended in HSM at a density of 1 × 106cells/mL. HSM is a serum-free, defined medium containing dexamethasone, ITS (insulin, transferrin, and selenium), and a proprietary formulation of hormones and metabolites. The medium was supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, 100 U/mL streptomycin, 0.25 μg/mL fungizone, and 5 ng/mL epidermal growth factor.
The upper chambers of the mpPLLAm inserts received 0.5 mL of the cell suspension, and the lower chambers received 1.5 mL of the culture medium. The plates were then left undisturbed in a 37°C incubator in 5% CO2 for 2 days to facilitate cell attachment.
Electron microscopy
mpPLLAm without cells were rinsed with 0.1 M cacodylate buffer, dehydrated in graded alcohols for 15 min each (50%, 70%, 85%, 95%, and twice in 100%), and left to dry overnight. They were then sputter coated with platinum and viewed under the scanning electron microscope (JEOL JSM-6390LV, Tokyo, Japan).
On day 4, cells cultured on the mpPLLAm were fixed with 1/2 strength Karnovosky's fixative solution and left at 4°C overnight for scanning electron microscopy (SEM) and transmission electron microscopy (TEM). After fixing, the samples were washed three times with PBS. Each sample was then cut into two equal halves. For SEM, one half of each sample was rinsed with 0.1 M cacodylate buffer and then osmicated in 2% OsO4 in the same buffer. The samples were rinsed again with 0.1 M cacodylate buffer, dehydrated in graded alcohols (50%, 70%, 85%, 95%, and twice in 100%), and infiltrated for 15 min each with a series of ethanol and hexamethyldisilazane (HMDS) (50:50, 25:75, 15:85, and 5:95). They were rinsed two more times in HMDS and left to dry overnight. The samples were then sputter coated with platinum and viewed under the scanning electron microscope.
For TEM, sections from the other half of each sample were rinsed thrice in 0.1 M cacodylate buffer, and then postfixed in 2% OsO4 for 1 h. The samples were then rinsed in 0.1 M cacodylate buffer and dehydrated through a graded series of ethanol rinses and infiltrated and embedded in Eponate 12 resin (Ted Pella, Redding, CA). Thin sections (∼70 nm) were cut with a diamond knife and placed on copper grids. The sections were later stained with uranyl acetate and lead citrate for viewing in a JEOL 1011 transmission electron microscope.
β-Hexosaminidase secretion
The secretion of β-hexosaminidase in response to stimulation with the cholinergic agonist carbachol (CCh) is an easily measured physiological function of lacrimal acinar cells. Secretion assays were carried out on day 4 with three sets of samples, each having three replicate samples. The first set received both apical and basal-lateral carbachol stimulation (A + B, 100 μM, 30 min), and the other two sets received basal-lateral CCh stimulation (100 μM) for 30 min (B30) and 2 h (B2h), respectively. Briefly, the culture medium in each well was aspirated, and 0.5 and 1.5 mL of fresh serum-free Dulbecco's modified Eagle's medium was added to the upper (apical) and lower (basal) chambers, respectively. The plate was then incubated at 37°C for 3 h; 350 and 800 μL of the apical and basal medium (unstimulated) were then removed, and freshly prepared 10 mM CCh solution was added to the three sets appropriately. The plate was then incubated for the prescribed time period at 37°C under 5% CO2. After incubation, the medium from the apical and basal chambers was collected and centrifuged at 700 rpm for 5 min at room temperature. The resulting supernatants were removed and stored frozen until they could be analyzed for β-hexosaminidase catalytic activity, using a GENios Plus microplate reader (Phenix) with 4-methylumbelliferyl-β-D-glucosaminide as substratum.
Statistical analysis
Four mpPLLAm specimens of each type were used for density and porosity measurements. Solute diffusion studies were performed with mpPLLAm inserts prepared from at least three specimens of mpPLLAm (57.1/42.9 wt%). Results are means ± SE of six separate measurements for glucose, L-tryptophan, and IgG, and four separate experiments for dextran. For β-hexosaminidase assays, data are expressed as the relative increase above basal value. Data were analyzed using a Student's t-test for unpaired samples assuming equal variance when comparing group means. Data are means ±SE of four separate determinations.
Results
Properties of mpPLLAm
Figure 2 is a schematic representation of the fabrication of mpPLLAm using the PEG solvent-cast/particulate leaching technique. The films were translucent with thicknesses ranging between 5 and 40 μm and averaging 19.92 ± 9.51 μm. Table 1 summarizes the porosity values of the various mpPLLAm fabricated with varying PLLA weight ratios. The values ranged from 2% to 13%, with mpPLLAm (66.7/33.3 wt%) the least porous and mpPLLAm (57.1/42.9 wt%) the most porous.
Table 1.
Porosity of the Various Poly(L-Lactic Acid) Membranes
| Membranes | PLLA (g) | PEG (g) | Wt% | Density (g/cm3) | 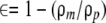 |
|---|---|---|---|---|---|
| PLLA | 0.01 | — | 1.2624 ± 0.0367 (ρp) | 0.0444 | |
| PLLA/PEG | 0.01 | 0.015 | 40/60 | 1.2063 ± 0.0325 (ρm) | |
| PLLA | 0.02 | — | 1.2762 ± 0.0243 (ρp) | 0.1247 | |
| PLLA/PEG | 0.02 | 0.015 | 57.1/42.9 | 1.1170 ± 0.0456 (ρm) | |
| PLLA | 0.03 | — | 1.2623 ± 0.0156 (ρp) | 0.0239 | |
| PLLA/PEG | 0.03 | 0.015 | 66.7/33.3 | 1.2321 ± 0.0106 (ρm) | |
| PLLA | 0.035 | — | 1.2681 ± 0.0044 (ρp) | 0.0242 | |
| PLLA/PEG | 0.035 | 0.015 | 70/30 | 1.2374 ± 0.009 (ρm) |
The underlined digits observed on the densimeter represent the unreliability of the densimeter under measuring conditions.
Electron microscopy of mpPLLAm
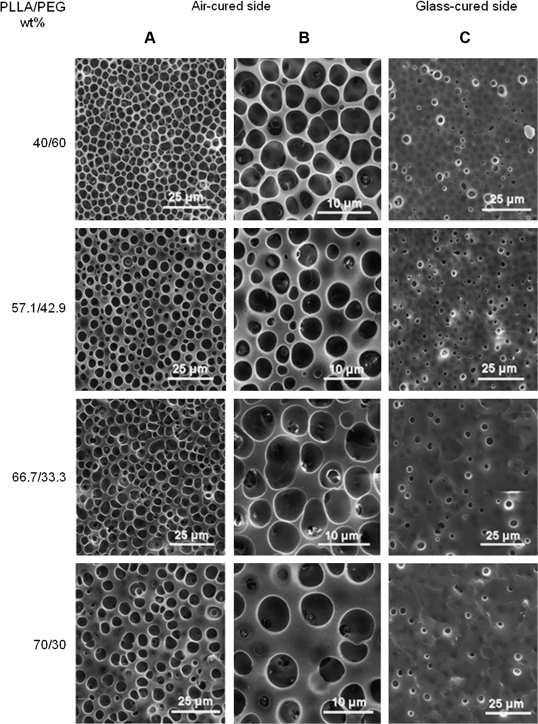
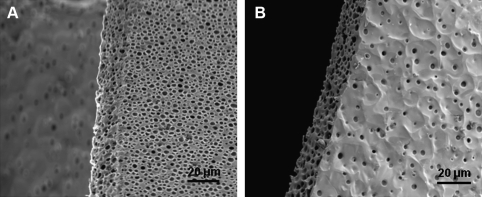
SEM images demonstrated the presence of pores on both sides of the mpPLLAm (Fig. 4). The pores were found to be homogenous on each side of the membrane. However, it was observed that the pore size and pore density on the air-cured and glass-cured side of the membranes were observed to be significantly different. The average pore size on the air-cured side of the membrane was ∼4 μm, whereas the glass-cured side had a pore size <2 μm. Further, a large fraction of the open pores present on the air-cured side seemed to demonstrate channelization. In accord with the relationship between porosity and composition, the pore densities on both sides of the fabricated mpPLLAm decreased gradually with increase in weight ratios of PLLA with the exception of the glass-cured surface of mpPLLAm (40/60 wt%).
FIG. 4.
SEM images of mpPLLAm. (A) Pores on the air-cured side of the fabricated membrane. (B) Magnified image of pores on the air-cured side of the membrane. (C) Pores on the glass-cured side of the fabricated membrane.
The microstructure of an angled cross section of mpPLLAm (57.1/42.9 wt%) revealed a sponge-like appearance (Fig. 5A, B). It was observed that the membrane exhibited a uniformly distributed microporous structure with interconnected open pores. Edge effects introduced by the compressional pressure applied during the sectioning process were also observed (Fig. 5C).
FIG. 5.
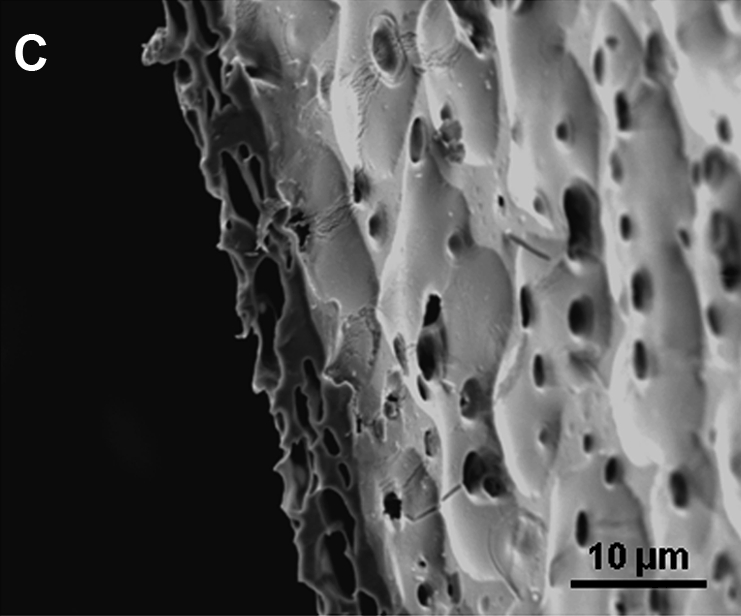
Scanning electron microscopy (SEM) image of the cross section of mpPLLAm (57.1/42.9 wt%). (A) An angled cross section of the air-cured side of the membrane. (B) An angled cross section of the glass-cured side of the membrane. (C) The crumpled edge effects introduced by the compressional pressure applied during sectioning of the specimen.
Solute diffusion
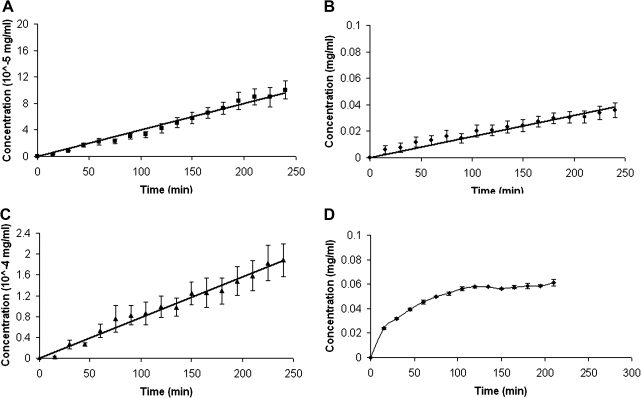
The concentrations of glucose and L-tryptophan and dextran in the basal compartment increased linearly with time throughout the 4-h period of the measurements (Fig. 6). In contrast, the rate of IgG movement decreased progressively over time (Fig. 6). The diffusivities of glucose, L-tryptophan, dextran, and IgG were calculated to be 6.38 × 10−12, 6.66 ×10−9, 6.46 × 10−11, and 2.09 × 10−8 cm2/s, respectively. Table 2 shows the summary of the results of the diffusion experiments.
FIG. 6.
Concentration versus time plots of (A) glucose, (B) L-tryptophan, (C) dextran, and (D) IgG in phosphate-buffered saline (PBS) over a period of 4 h. Data are expressed as the means ± SE of six separate measurements for glucose, L-tryptophan, and IgG, and four separate experiments for dextran.
Table 2.
Results of the Solute Diffusion Experiments
| Solute | MW | d (Å) | C0(mg/mL) | CLa(mg/mL) | CEb(mg/mL) | D (cm2/s) |
|---|---|---|---|---|---|---|
| Glucose | 180 Da | 3.844 | 2 | 0.0001 | 0.5 | (6.38 ± 1.33) × 10−12 |
| L-Tryptophan | 204 Da | <40 | 1 | 0.036 | 0.25 | (6.66 ± 0.99) × 10−9 |
| Dextran | 4000 Da | 2645 | 0.5 | 0.0001 | 0.125 | (6.46 ± 1.07) × 10−11 |
| IgG | 150 k Da | 11346 | 1 | 0.061 | 0.25 | (2.09 ± 0.06) × 10−8 |
Concentration of the components at the end of 4 h.
Equilibrium concentrations in the PBS when the substances could pass through the membrane freely.
MW, molecular weight; d, effective hydrodynamic diameter.
Electron microscopy of pLGACs on mpPLLAm
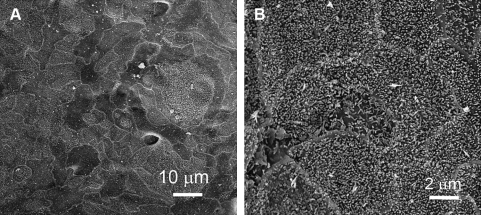
SEM images showed that cells cultured on mpPLLAm grew to subconfluent monolayers with acinus-like cell clusters of varying sizes occasionally seen in the cell monolayers. The cell monolayers demonstrated contiguous arrangement of cells with well-defined cell boundaries and the presence of numerous short interdigitating microvilli on the apical surface (Fig. 7).
FIG. 7.
SEM images of purified lacrimal gland acinar cells (pLGACs) cultured on mpPLLAm (57.1/42.9 wt%). (A) A distinct patch of a cell monolayer with contiguous arrangement of cells and well-defined cell boundaries. (B) The presence of numerous short interdigitating microvilli on the apical surface.
TEM micrographs demonstrated a flattened, polarized monolayer with a histiotypic apical–basal orientation, that is, basal side facing the mpPLLAm and apical side facing the culture medium (Fig. 8). It was observed that the cells had strongly adhered to the mpPLLAm by extending their pseudopods into the surface pores present along the surface of the membrane and gradually grew into them confining to the shape of the pits. However, cells were not seen to grow into the inside pores of the membrane. The apical surface of the cells was intermittently studded with short microvilli, and the apical cytoplasm contained a vast number of secretory granules characteristic of lacrimal acinar cells, clear vesicles, and frequent profiles of exocytosis. The cells were well connected by junctional complexes, including tight junctions, seen beneath the plane of the apical surface.
FIG. 8.
Transmission electron micrographs of pLGACs cultured on the mpPLLAm (57.1/42.9 wt%) (m). (A) The cells demonstrated a polarized monolayer with features typical of a glandular epithelium with apical microvilli (mv), secretory granules (g), and a basally positioned nucleus (n). (B) Magnification of the boxed area depicting cells joined by junctional complexes (jc) that included a tight junction zone (tj) near the apical surface.
β-Hexosaminidase assay
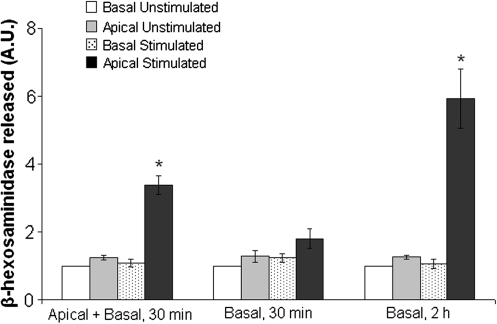
Analysis of β-hexosaminidase revealed that stimulation of 100 μM carbachol significantly increased the secretion of β-hexosaminidase to the apical bathing medium (p ≤ 0.05) in A + B and B2h groups but had no significant effect on secretion to the basal medium in all three groups (Fig. 9). The increase in β-hexosaminidase secretion to the apical medium was nearly threefold and fivefold greater than secretion to the apical medium in the unstimulated state for A + B and B2h groups, respectively, but had no significant effect on secretion in B30 group. Further, when compared between the stimulated groups, the apical secretion of β-hexosaminidase in the A + B and B2h groups, respectively, was nearly twofold and threefold greater than apical secretion in the B30 group.
FIG. 9.
β-Hexosaminidase protein released on the basal and apical side of the culture medium from pLGACs cultured on 12-well mpPLLAm inserts when exposed with or without carbachol (100 μM). Results are means ± SE expressed in arbitrary units (A.U.) of four separate determinations (n = 4). *p ≤ 0.05, significant increase from resting values and stimulated secretion on the apical side of the cultured cells.
Discussion
PLLA-PEG copolymers have found tremendous use in various biomedical applications. These include nanoparticles for intranasal and transdermal drug delivery,27,28 as vehicles for controlled drug release29 and as scaffolds for bone and cartilage tissue engineering.30,31
The approach of blending PLLA with PEG in a common solvent followed by water extraction of PEG to form porous PLLA scaffolds has been previously reported in literature for possible use in biomedical applications.19,21–23 However, the fabricated membranes showed only the presence of surface pores on one side of the membrane with no evidence of channelization. Further, neither diffusion nor cell culture studies were performed to demonstrate the permeability and biocompatibility of such membranes. In this study, we first report the fabrication of mpPLLAm with channelized pores using PEG solvent-cast/particulate leaching technique.
Porous PLLA membranes with different pore sizes and porosities have been obtained using this method by blending the two polymers with different molecular weights and weight ratios.19,21,23 It has been suggested that the characteristic pore morphology in the membranes results from the phase separation of PLLA and PEG into PLLA-rich and PEG-rich phases.19 The phase separation could be induced by the slow vaporization rate of the cosolvent and also in part due to the mobility of the PEG molecular chain during the process of membrane formation.23 In addition, a low casting temperature has also been shown to yield homogenous, flexible membranes close to an amorphous state.23
The differences in density between pure PLLA membranes and the mpPLLAm were very small, indicating that the mpPLLAm were not highly porous. This could be due to the loss in the physical integrity of the mpPLLAm in methanol during density measurement and also to the relatively low sensitivity of the densimeter to very light membranes. However, aside from porosity, the uniformity of pore size with a nominal pore density is a critical property influencing transport phenomena and cellular metabolic activities. Membranes with large pores (3 μm and above) have been used to study cell invasion,32 chemotaxis,33,34 and cell motility.34 Smaller pore sizes, that is, between 0.4 and 3 μm, have been primarily used as scaffolds for cells in in vitro studies of various phenomena, including epithelial drug transport and permeability studies,35,36 electrophysiology,25 cell polarity,37 endocytosis,38,39 coculture,40 and tissue remodeling.41
It seems possible that mpPLLAm with pore sizes between 0.4 and 3 μm could be used to improve upon current models for studying the transepithelial bioelectrical properties of cultured lacrimal gland acinar cells. As demonstrated by SEM, one of the mpPLLAm, 57.1/42.9 wt%, demonstrated maximum porosity and exhibited a uniformly distributed microporous surface structure. The cross section of the membrane also revealed a sponge-like appearance, which is an indirect evidence for the presence of interconnected open pores.42 Although the proportion of pores that completely traversed the membrane could not be defined, due to the pores' tortuosity, the observation that such membranes permitted diffusion of aqueous solutes suggests that they are sufficiently permeable to serve as tissue-engineered scaffolds. Further, the effective pore diameters across the membrane could be well within the 0.4 and 3 μm range as the pore sizes ranged from 2 μm (glass-cured side) to 4 μm (air-cured side) and hence could be used for in vitro cell culture studies.
A tissue-engineered scaffold should possess an adequate pore structure for the effective diffusion of oxygen and cell nutrients and the removal of waste products.43 Essential cell nutrients for cell growth and proliferation include carbohydrates, amino acids, vitamins, and growth factors. In addition, various inorganic ions such as Na+, K+, Cl−, and Ca2+ are also important for the culture of fluid-secreting lacrimal epithelial cells.25 Further, the scaffold should be designed to selectively inhibit the diffusion of immunocytes and immunoglobulins for successful use for clinical applications. Our diffusion experiments demonstrate that the mpPLLAm (57.1/42.9 wt%) was permeable to glucose, L-tryptophan, and dextran and semipermeable to IgG. The diffusion rates of the components were different because different initial concentrations were used in the diffusion experiments. Although the fabricated mpPLLAm was permeable to the various solute components, the membrane could not selectively inhibit the diffusion of solutes based on their molecular size, as pore sizes were too large, allowing even high molecular weight IgG to pass through. However, the diffusive rate of IgG plateaued off quickly as the molecules could have agglomerated overtime, thereby occluding the pores of the membrane. Moreover, the diffusion rate of a solute could well depend on its physical interaction with the surface of the membrane. Further, the diffusion data show only average diffusivities of the solutes, suggesting the presence of too few open channels. To overcome these issues, studies currently aim at controlling the pore size and pore density of the fabricated mpPLLAm by modifying the surface polarities of the glass surface or by using different casting substrates.
Results from our cell culture studies show that the fabricated mpPLLAm provide good microenvironments for the growth and maintenance of acinar cells. SEM images showed that pLGACs grew to subconfluent monolayers on the mpPLLAm. TEM micrographs revealed a strong, adherent, polarized monolayer of cells demonstrating a histiotypic apical–basal orientation with the basal side facing the polymeric substratum and the apical side facing the culture media. The ultrastructural view of the cells closely resembled lacrimal acinar cells in vivo with histiotypic characteristics such as apical microvilli, typical secretory granules and profiles of exocytosis, apical clear vesicles, junctional complexes, and basally positioned nuclei.
In addition to forming subconfluent monolayers that express histiotypic ultrastructural features, the cells also retained the ability to secrete proteins in response to appropriate stimuli. β-Hexosaminidase secretion studies suggest that the cultured acinar cells maintain a constitutive secretory process directed toward the apical plasma membrane in response to carbachol-induced stimulation. It was also observed that mpPLLAm poses a substantial diffusion barrier for carbachol as it takes nearly 2 h of incubation in carbachol (B2h group) to observe a significant β-hexosaminidase release response when compared to the release response in the B30 group. However, the observation that these cells can be stimulated basal-laterally to secrete proteins apically confirms the fact that carbachol can sufficiently permeate through the pores of the membrane. Hence, the fabricated mpPLLAm (57.1/42.9 wt%) appear to have good potential for use in ex vivo reconstitution of an electrophysiologically functional lacrimal gland epithelial tissue. However, a confluent lacrimal acinar cell monolayer needs to be established on these membranes before the epithelium will be capable of secreting electrolytes, and our current efforts are focused on achieving this goal.
In summary, our results demonstrate that mpPLLAm with channelized pores could be prepared by water extraction of PEG from solution cast blend membranes using the solvent-cast/particulate leaching technique. Membrane pore density could be controlled by varying the blend ratio of the two polymers. Solute diffusion measurements showed that mpPLLAm (57.1/42.9 wt%) was permeable to glucose, L-tryptophan, and dextran, but semipermeable to IgG. Further, pLGACs cultured on the mpPLLAm (57.1/42.9 wt%) grew to subconfluent monolayers and retained histiotypic morphological and physiological characteristics of lacrimal acinar cells in vivo. These results indicate that the fabricated mpPLLAm show great promise for use in lacrimal epithelial transport studies and also in the tissue engineering of biomaterial grafts for clinical applications.
Footnotes
This work was performed at the Doheny Eye Institute.
Acknowledgments
We are grateful to Ernesto Barron and Michael Pidgeon for expert technical assistance with electron microscopy, and to Susan Clarke for editorial assistance. This study was supported by NEI Grants EY03040, EY015457, EY012689, and EY005801 and Baxter Foundation Junior Faculty Award to Dr. Yiu. The study was also supported in part by an unrestricted grant from Research to Prevent Blindness.
Disclosure Statement
No competing financial interests exist.
References
- 1.Chandy T. Rao G.H. Wilson R.F. Das G.S. Development of poly(Lactic acid)/chitosan co-matrix microspheres: controlled release of taxol-heparin for preventing restenosis. Drug Deliv. 2001;8:77. doi: 10.1080/107175401750177025. [DOI] [PubMed] [Google Scholar]
- 2.Chen J. Tian B. Yin X. Zhang Y. Hu D. Hu Z. Liu M. Pan Y. Zhao J. Li H. Hou C. Wang J. Zhang Y. Preparation, characterization and transfection efficiency of cationic PEGylated PLA nanoparticles as gene delivery systems. J Biotechnol. 2007;130:107. doi: 10.1016/j.jbiotec.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Wang C.H. Hsiue G.H. Polymer-DNA hybrid nanoparticles based on folate-polyethylenimine-block-poly(L-lactide) Bioconjug Chem. 2005;16:391. doi: 10.1021/bc049754j. [DOI] [PubMed] [Google Scholar]
- 4.Yang S. Leong K.F. Du Z. Chua C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7:679. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 5.Mikos A.G. Temenoff J.S. Formation of highly porous biodegradable scaffolds for tissue engineering. Electron J Biotechnol. 2000;3:114. [Google Scholar]
- 6.Sachlos E. Czernuszka J.T. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater. 2003;5:29. doi: 10.22203/ecm.v005a03. [DOI] [PubMed] [Google Scholar]
- 7.Cima L.G. Vacanti J.P. Vacanti C. Ingber D. Mooney D. Langer R. Tissue engineering by cell transplantation using degradable polymer substrates. J Biomech Eng. 1991;113:143. doi: 10.1115/1.2891228. [DOI] [PubMed] [Google Scholar]
- 8.Mooney D.J. Baldwin D.F. Suh N.P. Vacanti J.P. Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- 9.Mikos A.G. Sarakinos G. Leite S.M. Vacanti J.P. Langer R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14:323. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- 10.Mikos A.G. Thorsen A.J. Czerwonka L.A. Bao Y. Langer R. Winslow D.N. Vacanti J.P. Preparation and characterization of poly(L-lactic acid) foams. Polymer. 1994;35:1068. [Google Scholar]
- 11.Schugens C. Maquet V. Grandfils C. Jerome R. Teyssie P. Polylactide macroporous biodegradable implants for cell transplantation. II. Preparation of polylactide foams by liquid-liquid phase separation. J Biomed Mater Res. 1996;30:449. doi: 10.1002/(SICI)1097-4636(199604)30:4<449::AID-JBM3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Whang K. Thomas C.H. Healy K.E. Nuber G. A novel method to fabricate bioabsorbable scaffolds. Polymer. 1995;36:837. [Google Scholar]
- 13.Thomson R.C. Yaszemski M.J. Powers J.M. Mikos A.G. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J Biomater Sci Polym Ed. 1995;7:23. doi: 10.1163/156856295x00805. [DOI] [PubMed] [Google Scholar]
- 14.Zoppi R.A. Duek E.A.R. Coraca D.C. Barros P.P. Preparation and characterization of poly (L-lactic acid) and poly(ethylene oxide) blends. Mater Res. 2001;4:117. [Google Scholar]
- 15.Jonnalagadda S. Robinson D.H. Effect of thickness and PEG addition on the hydrolytic degradation of PLLA. J Biomater Sci Polym Ed. 2004;15:1317. doi: 10.1163/1568562041959982. [DOI] [PubMed] [Google Scholar]
- 16.Santovena A. Alvarez-Lorenzo C. Concheiro A. Llabres M. Farina J.B. Structural properties of biodegradable polyesters and rheological behaviour of their dispersions and films. J Biomater Sci Polym Ed. 2005;16:629. doi: 10.1163/1568562053783768. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y. Hu Y.S. Topolkaraev V. Hiltner A. Baer E. Crystallization and phase separation in blends of high stereoregular poly(lactide) with poly(ethylene glycol) Polymer. 2003;44:5681. [Google Scholar]
- 18.Huh K.M. Bae Y.H. Synthesis and characterization of poly(ethylene glycol)/poly(l-lactic acid) alternating multiblock copolymers. Polymer. 1999;40:6147. [Google Scholar]
- 19.Younes H. Cohn D. Phase-separation in poly(ethyleneglycol) poly(lactic acid) blends. Eur Polym J. 1988;24:765. [Google Scholar]
- 20.Nakafuku C. Sakoda M. Melting and crystallization of poly(L-lactic acid) and poly(ethylene oxide) binary mixture. Polym J. 1993;25:909. [Google Scholar]
- 21.Tsuji H. Smith R. Bonfield W. Ikada Y. Porous biodegradable polyesters. I. Preparation of porous poly(L-lactide) films by extraction of poly(ethylene oxide) from their blends. J Appl Polym Sci. 2000;75:629. [Google Scholar]
- 22.Swaminathan V. Tchao R. Jonnalagadda S. Physical characterization of thin semi-porous poly(L-lactic acid)/poly(ethylene glycol) membranes for tissue engineering. J Biomater Sci Polym Ed. 2007;18:1321. doi: 10.1163/156856207782177864. [DOI] [PubMed] [Google Scholar]
- 23.Nakane K. Hata Y. Morita K. Ogihara T. Ogata N. Porous poly(L-lactic acid)/poly(ethylene glycol) blend films. J Appl Polym Sci. 2004;94:965. [Google Scholar]
- 24.Selvam S. Thomas P.B. Trousdale M.D. Stevenson D. Schechter J.E. Mircheff A.K. Jacob J.T. Smith R.E. Yiu S.C. Tissue-engineered tear secretory system: functional lacrimal gland acinar cells cultured on matrix protein-coated substrata. J Biomed Mater Res B Appl Biomater. 2007;80:192. doi: 10.1002/jbm.b.30584. [DOI] [PubMed] [Google Scholar]
- 25.Selvam S. Thomas P.B. Gukasyan H.J. Yu A.S. Stevenson D. Trousdale M.D. Mircheff A.K. Schechter J.E. Smith R.E. Yiu S.C. Transepithelial bioelectrical properties of rabbit acinar cell monolayers on polyester membrane scaffolds. Am J Physiol Cell Physiol. 2007;293:C1412. doi: 10.1152/ajpcell.00200.2007. [DOI] [PubMed] [Google Scholar]
- 26.Cussler E.L. Diffusion: Mass Transfer in Fluid Systems. Cambridge: Cambridge University Press; 1984. [Google Scholar]
- 27.Vila A. Gill H. McCallion O. Alonso M.J. Transport of PLA-PEG particles across the nasal mucosa: effect of particle size and PEG coating density. J Control Release. 2004;98:231. doi: 10.1016/j.jconrel.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Shim J. Seok Kang H. Park W.S. Han S.H. Kim J. Chang I.S. Transdermal delivery of mixnoxidil with block copolymer nanoparticles. J Control Release. 2004;97:477. doi: 10.1016/j.jconrel.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Yu J.J. Lee H.A. Kim J.H. Kong W.H. Kim Y. Cui Z.Y. Park K.G. Kim W.S. Lee H.G. Seo S.W. Bio-distribution and anti-tumor efficacy of PEG/PLA nano particles loaded doxorubicin. J Drug Target. 2007;15:279. doi: 10.1080/10611860701357235. [DOI] [PubMed] [Google Scholar]
- 30.Yoneda M. Terai H. Imai Y. Okada T. Nozaki K. Inoue H. Miyamoto S. Takaoka K. Repair of an intercalated long bone defect with a synthetic biodegradable bone-inducing implant. Biomaterials. 2005;26:5145. doi: 10.1016/j.biomaterials.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 31.Hiemstra C. Zhong Z. Dijkstra P.J. Feijen J. PEG-PLA hydrogels by stereocomplexation for tissue engineering of cartilage. J Control Release. 2005;101:332. [PubMed] [Google Scholar]
- 32.Dias S. Hattori K. Zhu Z. Heissig B. Choy M. Lane W. Wu Y. Chadburn A. Hyjek E. Gill M. Hicklin D.J. Witte L. Moore M.A. Rafii S. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest. 2000;106:511. doi: 10.1172/JCI8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiaradonna F. Fontana L. Iavarone C. Carriero M.V. Scholz G. Barone M.V. Stoppelli M.P. Urokinase receptor-dependent and -independent p56/59(hck) activation state is a molecular switch between myelomonocytic cell motility and adherence. EMBO J. 1999;18:3013. doi: 10.1093/emboj/18.11.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashido Y. Lucas A. Rougeot C. Godyna S. Argraves W.S. Rochefort H. Estradiol and fibulin-1 inhibit motility of human ovarian- and breast-cancer cells induced by fibronectin. Int J Cancer. 1998;75:654. doi: 10.1002/(sici)1097-0215(19980209)75:4<654::aid-ijc26>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Franke H. Galla H. Beuckmann C.T. Primary cultures of brain microvessel endothelial cells: a valid and flexible model to study drug transport through the blood-brain barrier in vitro. Brain Res Brain Res Protoc. 2000;5:248. doi: 10.1016/s1385-299x(00)00020-9. [DOI] [PubMed] [Google Scholar]
- 36.Liu F. Soares M.J. Audus K.L. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am J Physiol. 1997;273:C1596. doi: 10.1152/ajpcell.1997.273.5.C1596. [DOI] [PubMed] [Google Scholar]
- 37.Matlin K.S. Haus B. Zuk A. Integrins in epithelial cell polarity: using antibodies to analyze adhesive function and morphogenesis. Methods. 2003;30:235. doi: 10.1016/s1046-2023(03)00030-6. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y. Cai H. Cebotaru L. Hryciw D.H. Weinman E.J. Donowitz M. Guggino S.E. Guggino W.B. ClC-5: role in endocytosis in the proximal tubule. Am J Physiol Renal Physiol. 2005;289:F850. doi: 10.1152/ajprenal.00011.2005. [DOI] [PubMed] [Google Scholar]
- 39.Stins M.F. Badger J. Sik Kim K. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb Pathog. 2001;30:19. doi: 10.1006/mpat.2000.0406. [DOI] [PubMed] [Google Scholar]
- 40.Wong C.K. Wang C.B. Ip W.K. Tian Y.P. Lam C.W. Role of p38 MAPK and NF-kB for chemokine release in coculture of human eosinophils and bronchial epithelial cells. Clin Exp Immunol. 2005;139:90. doi: 10.1111/j.1365-2249.2005.02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swartz M.A. Tschumperlin D.J. Kamm R.D. Drazen J.M. Mechanical stress is communicated between different cell types to elicit matrix remodeling. Proc Natl Acad Sci USA. 2001;98:6180. doi: 10.1073/pnas.111133298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nam Y.S. Park T.G. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J Biomed Mater Res. 1999;47:8. doi: 10.1002/(sici)1097-4636(199910)47:1<8::aid-jbm2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 43.Karande T.S. Ong J.L. Agrawal C.M. Diffusion in musculoskeletal tissue engineering scaffolds: design issues related to porosity, permeability, architecture, and nutrient mixing. Ann Biomed Eng. 2004;32:1728. doi: 10.1007/s10439-004-7825-2. [DOI] [PubMed] [Google Scholar]
- 44.Pappenheimer J.R. Renkin E.M. Borrero L.M. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol. 1951;167:13. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- 45.Thorne R.G. Hrabetova S. Nicholson C. Diffusion of epidermal growth factor in rat brain extracellular space measured by integrative optical imaging. J Neurophysiol. 2004;92:3471. doi: 10.1152/jn.00352.2004. [DOI] [PubMed] [Google Scholar]
- 46.Hennink W.E. Talsma H. Borchert J.C.H. De Smedt S.C. Demeester J. Controlled release of proteins from dextran hydrogels. J Control Rel. 1996;39:47. [Google Scholar]