Abstract
As the only species that evolved to possess a language faculty, humans have been surprisingly generative in creating a diverse array of language systems. These systems vary in phonology, morphology, syntax, and written forms. Before the advent of modern brain-imaging techniques, little was known about how differences across languages are reflected in the brain. This chapter aims to provide an overview of an emerging area of research — cultural neurolinguistics — that examines systematic cross-cultural/crosslinguistic variations in the neural networks of languages. We first briefly describe general brain networks for written and spoken languages. We then discuss language-specific brain regions by highlighting differences in neural bases of different scripts (logographic vs. alphabetic scripts), orthographies (transparent vs. nontransparent orthographies), and tonality (tonal vs. atonal languages). We also discuss neural basis of second language and the role of native language experience in second-language acquisition. In the last section, we outline a general model that integrates culture and neural bases of language and discuss future directions of research in this area.
Keywords: cross-cultural, neurolinguistics, language, brain
Ex uno plures (from one, many). Among all the living species, humans are unique in having evolved to possess a universal language faculty and yet speaking more than 6000 different languages. The vast differences across these languages (i.e., phonology, morphology, syntax, semantics, and written forms) both reflect and contribute to historical–cultural differences in human mind (see Sapir, 1921, for a review; Vygotsky, 1934; Whorf, 1956). Because language is such an integral part of culture, anthropologists have relied heavily on language differences (but also on kinship relations, inheritance patterns) to establish cultural groups (Burton et al., 1996). Even the validity of population genetics initially relied on how well their conclusions corresponded to language families (Cavalli-Sforza et al., 1994).
Among the various aspects of culture, language may be the most extensively studied. Language sciences already encompass many disciplines, including the traditional linguistics, anthropology, psychology, information sciences, neuroscience, and their numerous subdisciplines. Researchers in these disciplines, especially in comparative linguistics, have documented differences in the smallest details of the world’s languages (see, e.g., Ethnologue: http://www.ethnologue.com/ and the World Atlas of Language Structures: http://wals.info/index). Few other aspects of culture have been examined to this extent either qualitatively or quantitatively.
However, these differences, though vast and well documented, have rarely entered the research on neural bases of language. This was perhaps due to two main reasons. First, earlier research on neural bases of language was typically based on patients with brain damages. Their small sample size and great individual variations in the extent and size of injuries or infarctions prevented meaningful comparisons across patients who speak different languages. Second, perhaps more importantly, researchers tended to believe that diversity in languages is purely cultural because infants can learn any language of the world.
Due to the advent of modern brain-imaging techniques, the first obstacle has been overcome. Researchers are able to use fMRI, PET, ERP, and other techniques to study samples of reasonable size from different cultures and to compare their findings with some precision. The second obstacle has been overcome by evidence from decades of research on brain plasticity. Even if the brain is universally the same initially, later experiences (including language experiences) can theoretically lead to diversity at the neural levels (both functionally and anatomically). Chiao and Ambady (2007) have already articulated that culture-specific early experience is one reason that brain structure and function may vary across cultures. After all, the vast differences across languages must be represented somehow at the neuronal level in the brain. The question is whether existing research tools are able to detect them. Indeed, research evidence is accumulating that different languages may have different neural bases. The time may have come for researchers to engage in a systematic exploration of cultural differences in the neural bases of language —cultural neurolinguistics. Specifically, cultural neurolinguistics aims to address questions such as the following. What are the similarities and variations in the brain networks, in terms of both functions and anatomy, that are used to process different languages? How did these variations come about developmentally? What are the implications of these variations for the learning of a new language?
This chapter aims to provide an overview of the emerging literature addressing the above questions. We will first briefly describe the general brain networks for written and spoken languages. Second, we will focus on research on cross-cultural differences in the language networks, emphasizing three distinctions — logographic versus alphabetic languages, transparent versus nontransparent orthographies, and tonal versus atonal languages. Third, we review the literature on the effects of prior language experience on second-language learning. Finally, we will provide an integrative discussion about culture and the neural basis of language and propose some future directions of research in this area.
The language brain
Language is a complex behavior that involves multiple senses and motor skills and the coordination among them. Consequently, the language brain is a network of brain regions that are semi-specialized for different functions. For example, comprehension of spoken language would involve Heschl’s gyrus in the primary auditory cortex (bilateral superior temporal gyrus) for initial spectrotemporal and phonological processing, the bilateral posterior inferior and middle temporal gyri for semantic processing, and the left posterior frontal lobe and the temporoparietal area for mapping sounds onto articulatory representations (Hickok and Poeppel, 2004, 2007).
Comprehension of a written language (i.e., reading), on the other hand, would involve the occipital cortex (primary visual analysis), the occipitotemporal regions (visual form processing), the posterior superior temporal gyrus (grapheme-to-phoneme conversion), the superior/middle temporal gyrus (semantic analysis), the inferior frontal gyrus (IFG) (phonological and semantic processing), and the precentral gyrus and cerebellum (motor skills for speech production) (for reviews, see Fiez and Petersen, 1998; Jobard et al., 2003; Paulesu et al., 2000; Price, 1998, 2000). Different types of reading would involve these brain regions to a different extent. For example, according to the dual-route cascade model of reading (Coltheart et al., 2001), there are two routes of phonological access: direct and indirect routes. The direct route (also called the lexical route or the addressed phonology) means that the meaning of a visual word is directly accessed. For the indirect route (also called the sub-lexical route or the assembled phonology), the different phonetic parts of visual words are first processed individually, then assembled (all at the sub-lexical level) to access the sound and the meaning of those words. The IFG appears to be important for both routes of phonological access, although the anterior portion (BA45/47) is more relevant to semantic processing, whereas the posterior portion (BA44/6) is more relevant to phonological processing (Poldrack et al., 1999). Accordingly, it has been proposed that the anterior portion of IFG is more involved in the lexical route, whereas the posterior portion of IFG is more involved in sub-lexical route (Jobard et al., 2003). Furthermore, addressed phonology tends to rely more on the fusiform gyrus in the occipitotemporal region, whereas assembled phonology appears to rely more on the superior temporal gyrus, angular gyrus, and supramarginal gyrus (Fiez and Petersen, 1998; Jobard et al., 2003; Price, 2000). This neural differentiation for different routes of phonological access is important because different languages tend to rely on different routes of phonological access (see next section).
Finally, Indefrey and Levelt (2004) have outlined brain regions involved in speech production: lemma retrieval and selection at the left middle temporal gyrus, phonological code retrieval at the left posterior middle temporal gyrus and posterior superior temporal gyrus, syllabification at the left posterior IFG, articulation at the bilateral sensorimotor and supplementary motor area, and self-monitoring at the bilateral superior temporal gyrus.
Major differences in language systems
Languages differ in many ways, including phonology, morphology, syntax, semantics, and written forms. Based on those differences, linguists have categorized the more than 6000 human languages into major language families (e.g., Niger-Congo, Austronesian, Sino-Tibetan family, Indo-European, and Afro-Asiatic families, with each containing hundreds of languages). Neurolinguistics is at a very early stage in exploring the differences in the neural bases of different languages. In this section, we will focus only on three major differences that have been examined cross-linguistically: scripts, orthography, and tonality.
Scripts
All written languages have their origin in pictographs. Out of those pictographs, some evolved to become logographs such as is the case for Chinese. There is apparent continuity between the visual configurations of the original pictographs and modern Chinese logographs. Modern Chinese logographs, as well as other logographic scripts such as Korean Hangul, typically consist of a number of strokes/units that are packed into a square. In contrast, alphabetic languages such as English use phonetic scripts that are a linear combination of letters (either from the left to the right or from the right to the left as in Hebrew and Arabic, which are also consonants-only scripts called abjads). Sometimes, researchers make a finer distinction between scripts and writing systems. For example, Perfetti et al. (2007) used scripts to describe visual appearance of the characters (logographic vs. alphabetic languages) and used writing system to describe the design principle (i.e., the basic unit size for mapping graphic units onto language units). According to this distinction, Chinese is a morph-syllabic system because Chinese characters map onto meaningful morphemes, whereas English and Korean use the letter-phoneme system, in which characters are mapped onto phonemes in the spoken language.
Orthography
Orthography literally means “correct writing” in Greek. In research literature, it is used to describe how a writing system is implemented in a particular language. Of most relevance to this chapter is the distinction between transparent and nontransparent orthography. Although both Italian and English use letter-phoneme mapping, Italian has a more regular mapping between letters and phonemes than English. Thus, Italian is a transparent or shallow orthography, whereas English is a quasi-transparent or deep orthography. For transparent orthography, phonological access can be achieved through assembled phonology, in which visual words are transformed into phonology through the grapheme-to-phoneme correspondences (GPC) rules. Korean is also a transparent orthography because of its near-perfect letter-phoneme mapping.
In contrast, Chinese is a nontransparent or the deepest orthography because there is no letter-phoneme mapping in Chinese. For nontransparent orthography, phonological access typically relies on addressed phonology, which directly maps the visual forms of words onto their sounds. For quasi-transparent orthography, such as English, assembled and addressed phonology are used to read regular and irregular words, respectively. It should be pointed out that, with increasing fluency in reading, there is a shift from assembled phonology to addressed phonology.
Due to the absence of GPC rules, Chinese logographic characters are to be learned by drill. Consequently, Chinese children rarely are able to read characters beyond their grade level, whereas many American children can do that because they can rely on the GPC rules (Lee et al., 1995). Consistent with this finding, McBride-Chang et al. (2005) recently found that phonological awareness was more important for reading English than for reading Chinese, whereas morphological structure awareness is more important for reading Chinese than for reading English. For the same reason, dyslexia in English can be of either surface and phonological subtypes (Marshall and Newcombe, 1973), but only of the surface subtype in Chinese (Meng et al., 2005).
Tonality
Pitch in human spoken language can convey several types of information, including speaker’s identity, affection, intonation, phonemic stress, and word meaning (Wong et al., 2004; see Wong, 2002, for a review). One typical language-specific pitch is the lexical tone. In tonal languages (e.g., Chinese and Thai), lexical tone is used to distinguish words. For example, in Chinese, the sound /ma/ spoken in a high pitch means “mother,” but the same sound spoken in a low falling–rising pitch means “horse.” In fact, there are four tones in Chinese Mandarin. The extent of tonality varies greatly across language systems. Languages such as English are atonal and they do not use tones to signal the meanings of words. However, most of atonal languages use stress, which on occasions provides some additional lexical information (e.g., CONtent vs. conTENT).
Different neural networks underlying different languages
The above-mentioned cross-cultural differences in scripts, orthography, and tonality can significantly affect the neural mechanisms of language processing. In a recent meta-analysis of neural bases of reading, Bolger et al. (2005) quantitatively compared the findings of 43 studies of different languages (25 with alphabetic languages, 9 with Chinese, 5 with Japanese Kana, and 4 with Japanese Kanji). They found that activations in the frontotemporal, occipitotemporal, and occipital regions were shared across languages. Important cross-language differences were found in the left middle frontal gyrus (MFG), temporoparietal region, and right fusiform cortex. Gandour (2005) has also provided a summary of the literature on the differential neural networks for tonal and atonal languages. In the following sections, we discuss in detail these relevant findings.
Neural bases of logographic and alphabetic scripts
As mentioned above, the visual configuration varies significantly across different writing systems or scripts. One of such distinctions is between alphabetic and logographic systems. For example, Chinese characters (a typical logographic system) possess a number of intricate strokes that are packed into a square shape, whereas the alphabetic systems have linear combination of letters. Given this visual characteristic of Chinese, the processing of Chinese characters might involve more visuospatial analysis than that of alphabetic writings. Visuospatial analysis (such as whole–part relations) is either bilateral or right-hemisphere dominant (Grill-Spector, 2001; Rossion et al., 2000). Consistent with this view, existing neuroimaging studies on Chinese processing have revealed bilateral or even right-dominated activation in the occipital and posterior occipitotemporal region. For instance, Tan et al. (2000) compared laterality between single Chinese characters and words, and found significant activation in the right occipital cortex for both types of materials. This is in clear contrast with the left-hemisphere dominance in the processing of alphabetic languages (e.g., Price et al., 1996; Vigneau et al., 2005). This finding of greater engagement of the right occipitotemporal region in Chinese processing than alphabetic processing was further confirmed by several other fMRI studies (Bolger et al., 2005; Chen et al., 2002; Fu et al., 2002; Kuo et al., 2001, 2003, 2004; Peng et al., 2004, 2003; Tan et al., 2005; Xue et al., 2005).
Although there is a general consensus that Chinese logographic scripts resulted in the involvement of bilateral primary visual cortex, it is controversial whether the effect of scripts extends upstream to the middle portion of the occipitotemporal area, specifically the fusiform gyrus. Some researchers (Liu et al., 2007; Tan et al., 2005, 2000) have suggested that reading Chinese might be bilateral or even right lateralized in the fusiform gyrus. However, a direct quantitative comparison of the two hemispheres (Xue et al., 2005) revealed left-hemispheric dominance in the fusiform cortex when processing Chinese, a pattern similar to reading alphabetic writings. The latter finding seems to make sense because the fusiform gyrus is believed to play a fundamental role, although not necessarily an exclusive role (see Price and Devlin, 2003, for a review), in processing abstract visual word forms (Cohen and Dehaene, 2004; Cohen et al., 2002). Scripts should no longer matter when words are processed at the abstract level. Further complicating the role of the fusiform gyrus in visual word processing and possible cross-cultural differences, other studies have found that this region might also be involved in lexical, multimodal word processing (Buchel et al., 1998; Kronbichler et al., 2004), or in the integration of phonology and visual information during both word and picture processing (McCrory et al., 2005; Price and Friston, 2005; Xue et al., 2006b). Further research can help clarify the role of the fusiform in visual word processing and determine how far upstream scripts can affect the neural basis of language.
Neural bases of transparent and nontransparent orthographies
Depending on orthographic transparency, different languages rely on different routes of phonological access: addressed phonology for nontransparent orthography and assembled phonology for transparent orthography. These different routes of phonological access involve distinct neural mechanisms. Specifically, the left temporoparietal area has been implicated for assembled phonology. In a PET study, Paulesu et al. (2000) found that reading Italian (a transparent orthography) induced more activation in the left posterior superior temporal gyrus than reading English (a quasi-transparent orthography), whereas reading English elicited more activation in the left posterior inferior temporal region and the left IFG. When comparing English with Chinese (a nontransparent orthography), researchers have found that reading English activated the posterior superior temporal gyrus and adjacent supermarginal cortex, whereas reading Chinese activated the dorsal extent of the inferior parietal lobule (perhaps because this area is also involved in visuospatial analysis of Chinese characters) (see Tan et al., 2005, for a review).
There is also evidence that different orthographies might result in differences in other brain regions such as the frontal lobe. For example, Tan and colleagues have found that the left MFG (BA9) is more activated when reading Chinese than when reading English (Tan et al., 2005, 2001, 2000). These researchers believe that this region might play a role in addressed phonology when reading Chinese. They even reported anatomical differences in this region favoring Chinese subjects (Kochunov et al., 2003). Furthermore, they found decreased activation and reduced gray matter in left MFG in Chinese dyslexics (Siok et al., 2008, 2004). However, several other studies have failed to find MFG activation when subjects were reading Chinese (Chee et al., 1999a, 2003, 2000; Kuo et al., 2003; Lee et al., 2004; Xue et al., 2005, 2004a, b; Zhang et al., 2004). It remains to be seen what specific roles this region might play in processing Chinese.
Neural bases of tonal and atonal languages
Previous neuroimaging studies have observed double dissociation in the neural networks of lexical tone and nonlinguistic pitch processing. For lexical tone perception, activations have been mainly reported in the left inferior frontal regions (Gandour et al., 1998, 2000, 2002, 2003; Klein et al., 2001; Wang et al., 2003, 2004) and the temporal regions (Wang et al., 2003, 2007; Xu et al., 2006). In contrast, nonlinguistic pitch processing typically elicit activations in homologous areas in the right hemisphere (e.g., Zatorre et al., 1994, 1992). In speech production, however, Liu et al. (2006) found that tones (suprasegmental elements) activated the right frontal gyrus more than did consonants (segmental elements).
More direct evidence for the effect of linguistic factors or language experience on lexical tone processing comes from crosslinguistic/cross-cultural studies. Recently, several studies have compared neural mechanisms of tone processing in speakers of a tonal language (e.g., Chinese and Thai) with those of an atonal language (e.g., English), and found that speakers of a tonal language showed more left-lateralized activations in the frontotemporal regions in contrast with atonal language speakers (Gandour et al., 2003, 1998; Klein et al., 2001; Wong et al., 2004; Xu et al., 2006). There is evidence that the left hemisphere is more effective in learning lexical tones than the right hemisphere (Wong et al., 2007).
Furthermore, tone processing appears to be language specific. Neural patterns of tone processing do not seem to transfer from one tonal language to another tonal language. For example, when processing Thai tones, native Chinese speakers, although having years of experience in Chinese tones, showed different neural patterns from those of native Thai speakers (Gandour et al., 2002, 2003).
Other cross-cultural differences in the neural basis of language processing
Although cross-cultural differences in neural bases of speech processing and reading have been most often studied, researchers have also begun to document cross-cultural differences in neural bases of other aspects of language processing. For example, studies of speakers of English and other Indo-European languages have typically found that verbs are represented in the frontal region (e.g., the left prefrontal cortex), whereas nouns are represented in the posterior regions (the temporal–occipital regions) (Petersen et al., 1989). Nouns and verbs in Chinese, however, activate a wide range of overlapping brain areas in distributed networks, in both the left and the right hemispheres (Li et al., 2004). The reason for this cross-cultural difference is probably that categorization of words into different grammatical classes is less clear-cut in Chinese than in English. Many individual words in Chinese cannot be easily distinguished into nouns or verbs, mainly due to a lack of inflectional morphology in Chinese. Most words play multiple grammatical roles, resulting in an abundance of class-ambiguous words that can be used as either nouns or verbs. Much more research is needed to understand crosslinguistic variations in the neural bases of semantic processing.
Second-language learning
Thus far, we have focused on comparisons of neural bases of different languages. Cultures are not isolated from one another. Cultural encounters lead to exposure to and acquisition of second languages. Neural bases of second language and especially the role of native language in second-language acquisition are an important topic of research in cultural neurolinguistics.
Earlier studies of bilinguals (Dehaene et al., 1997; Kim et al., 1997; Perani et al., 1998) reported neural dissociations between native and second language. Later studies typically found a largely shared neural network in both native and second-language processing, even for two drastically different languages such as Chinese and English (Chee et al., 1999a, b, 2000; Klein, 2003; Klein et al., 1995, 1999; Xue et al., 2004a, b). Given the differences in neural networks for different native languages (see the previous section), it is puzzling why neural patterns for first and second language (especially for Chinese and English) are not more distinct. One explanation of this overlap between native and second language’s neural networks is that the neural mechanisms for second-language processing are shaped by native language experience. In fact, some recent studies showed that the brain network shaped by native language experience is optimal for learning a new language (Chen et al., 2007; Dong et al., 2008; Mei et al., 2008; Xue et al., 2006a).
Perfetti et al. (2007) have proposed an intriguing model that consists of two processes, namely assimilation and accommodation in Piagetian sense, to account for the effects of the native language on neural mechanisms involved in learning a second language. The assimilation hypothesis assumes that the brain will read a second language as if it is the native language and use the native language network to support the second language. In contrast, the accommodation hypothesis assumes that the brain’s reading network must adapt to the features of a new writing system to the extent that those features require different reading procedures. Supporting evidence for this model comes from several studies. For example, a study by Tan et al. (2003) provided evidence for neural assimilation by showing that for Chinese subjects who were learning English as their second language, the superior temporal gyrus was not activated when reading English although this region was activated for native English readers. Instead, the left MFG, usually involved in processing Chinese, was activated. On the other hand, for English speakers who are learning Chinese, the bilateral visual form and left MFG were activated when processing Chinese, which is consistent with the accommodation hypothesis (Liu et al., 2007; Nelson et al., 2009). Taken together these studies, one might conclude that Chinese speakers are more likely to assimilate, but English speakers are more likely to accommodate. Of course, it is also likely that, given the differences in linguistic features, nontransparent logographic language demands accommodation, whereas transparent alphabetic language allows for accommodation. These possibilities need to be tested with a design involving native speakers of two different languages learning the same second language. So far, no imaging study of such a design has been conducted.
Integrating culture into neurolinguistics
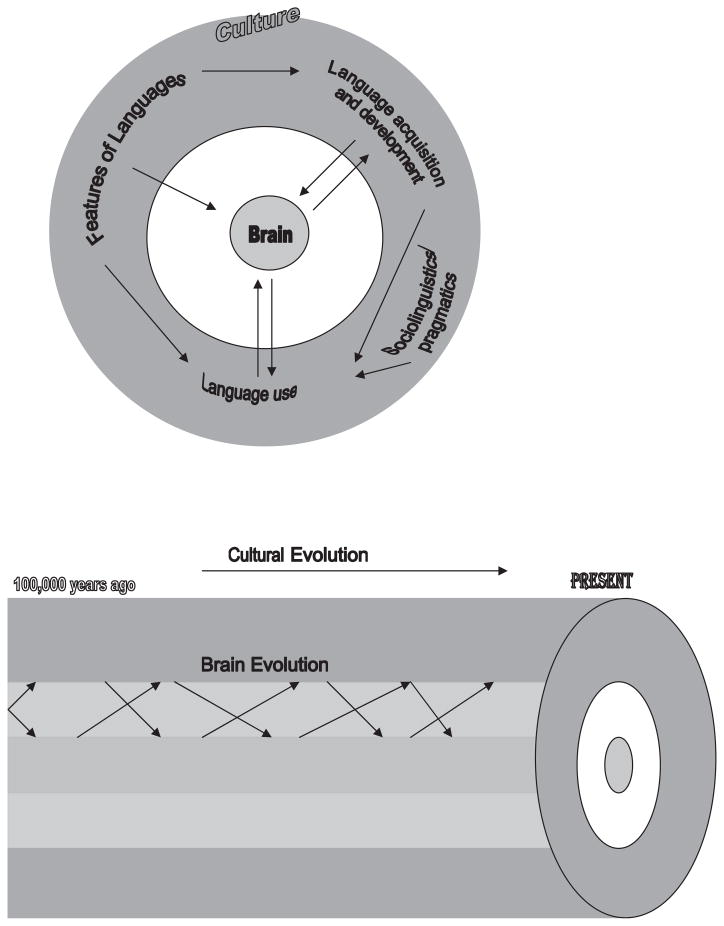
As mentioned earlier, cultural neurolinguistics is only at the beginning stage of development. Thus far, most research has focused only on the effects of language features on the brain. Much is to be done regarding other aspects of culture’s impact on the language brain. In this section, we outline a sketch of this emerging field (see Fig. 1). This field needs to address both classic and new questions such as how the interaction between the features of languages and the brain anatomy and function affect the neural basis of different languages, how first- and second-language acquisition can affect the brain (accommodation), how brain development shapes the neural mechanisms for first- and second-language acquisition and development (assimilation), how social factors (e.g., social economical status, education, vocabulary and knowledge explosion, technology use, communication style, cultural orientation, etc.) that shape the language use and experiences would shape the brain (e.g., Raizada et al., 2008), and finally how language and the brain coevolve to create the diversity in languages and the diversity in neural bases of languages.
Fig. 1.
A schematic presentation of the interrelations among culture, language, and the brain.
The last two areas are almost virgin territories. Language is not content free, so sociolinguistic factors (e.g., communication style), sociopsychological factors (e.g., self descriptions, interpersonal relations), or the use of language in other cognitive tasks (such as arithmetic) can all affect brain mechanisms. A few groups of researchers have already begun to document the effects of larger cultural contexts on brain functions. For example, Han and Northoff (2008) recently reviewed several studies showing the effect of culture on the social brain. Tang et al. (2007) showed cultural effects on the mathematical brain. Similarly, Zhou et al. (2007a, b) have systematically demonstrated that different cultures’ approach to teaching mathematics can affect the brain bases of mathematical learning. Given that language is the medium (or “tool”) of cultural representations (Vygotsky, 1934), future research needs to systematically examine how language mediates these cross-cultural differences in brain functions.
More theoretical and empirical work is also needed to delineate the mechanisms involved in the bidirectional effects between culture/language and the brain. Some theoretical discussions have already started. For example, Fabrega (1977, 1982) has suggested three distinct ways, occurring at different stage of development, in which cultural factors might help mold the human brain. First, the ecological surroundings associated with a certain culture may selectively activate or “tune” appropriate neuronal connections. Second, cultural factors in early child learning differentially and dynamically alter brain development. Finally, life-long adaptability allows the adult brain to continuously adapt to new situations. Dehaene and Cohen (2007) recently presented a cultural recycling model, which suggests that preexisting brain circuitry places structural constraints on the brain-cognition mapping (e.g., visual words mapping onto the left fusiform gyrus across cultures), but brain plasticity allows flexibility in the specifics of the mapping.
Common across these and other models (such as Perfetti’s accommodation and assimilation model) are their emphasis on two neurobiological principles: neural plasticity and specialization. Neural plasticity allows culture to have an imprint on the brain, and neural specialization sustains cross-cultural differences in the brain. As presented earlier, language features (e.g., scripts, orthographies, and tonality) can determine the neural bases of language learning through neural specialization. Furthermore, these neural bases may carry over to second-language learning (Nakada et al., 2001; Tan et al., 2003). In a way, this discussion of neural specialization in general language learning is just an extension of the classic example of neural specialization in phonetic processing. At birth, infants are universally capable of differentiating phonetic contrasts in all languages. As a result of native language experience (or “tuning”), however, the ability to distinguish nonnative phonetic contrasts dramatically declines as early as 6 months (Kuhl and Rivera-Gaxiola, 2008; Kuhl et al., 2006; Werker and Tees, 1992). By 11 months of age, Japanese infants can no longer distinguish /ra/ from /la/, and American infants cannot distinguish Chinese sounds /chi/ and /ci/ (Kuhl et al., 2001). English speakers cannot identify Hindi phonetic contrasts that differ in voice onset time from −90 to 0 ms (Sharma and Dorman, 2000). When these speakers learn a new language, they will have “accents” (i.e., assimilation). For more discussions about this topic, readers can refer to Perceptual Assimilation Model (Best et al., 2001) and Natural Language Magnet model (Iverson and Kuhl, 1996).
In tandem with neural specialization is neural plasticity, which makes accommodation possible. For example, even though infants begin to lose sensitivity to nonnative phonemes, they can learn a new language without accents until about 10–12 years of age. Neural plasticity is at work here and it allows the brain to accommodate to foreign sounds. In fact, evidence is accumulating that language learning can change brain functions and even anatomy due to neural plasticity. For example, phonetic training can induce functional reorganization such as an expansion of existing regions and the recruitment of additional regions (Callan et al., 2003; Wang et al., 2003). Auditory training can ameliorate the dysfunction of the inferior frontal and temporoparietal regions in dyslexia (Temple et al., 2003). Braille readers who became blind early in life were found to rewire their visual cortex to respond to tactile tasks (Sadato et al., 1996). Finally, language learning can also result in permanent changes in brain structure. Bilinguals have been found to show increased gray matter density in the left inferior parietal region as compared to monolinguals (Mechelli et al., 2004). This study further revealed that the gray matter density was positively correlated with second-language proficiency and negatively correlated with age at acquisition of second language, suggesting that more learning resulted in greater structural changes in the brain.
This dynamic process of accommodation and assimilation or plasticity and specialization is likely to occur across all aspects of culture–brain connections, and across the life span. So far researchers have only uncovered a limited number of instances such as phonetic processing and visual words processing (see an earlier section). All other links in our general model (see Fig. 1) can be examined from this dynamic perspective. Beyond the links in the model, an optimistic view is that the near future will also witness the integration of culture into neurosciences at even a broader level, including molecular genetics (see Chiao and Ambady, 2007).
Acknowledgments
This manuscript was prepared with the support from the National Science Foundation (grant numbers BCS-0823624 and BCS 0823495), the National Institute of Health (grant number HD057884-01A2), and the 111 Project of China (B07008).
References
- Best C, McRoberts G, Goodell E. Discrimination of non-native consonant contrasts varying in perceptual assimilation to the listener’s native phonological system. The Journal of the Acoustical Society of America. 2001;109:775–794. doi: 10.1121/1.1332378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: Universal structures plus writing system variation. Human Brain Mapping. 2005;25:92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Price C, Friston K. A multimodal language region in the ventral visual pathway. Nature. 1998;394:274–277. doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Burton ML, Moore CC, Whiting JWM, Romney AK, Aberle DF, Barcelo JA, et al. Regions based on social structure. Current Anthropology. 1996;37:87–123. [Google Scholar]
- Callan DE, Tajima K, Callan AM, Kubo R, Masaki S, Akahane-Yamada R. Learning-induced neural plasticity associated with improved identification performance after training of a difficult second-language phonetic contrast. NeuroImage. 2003;19:113–124. doi: 10.1016/s1053-8119(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza LL, Menozzi P, Piazza A. The history and geography of human genes. Princeton University Press; 1994. [Google Scholar]
- Chee MW, Tan EW, Thiel T. Mandarin and English single word processing studied with functional magnetic resonance imaging. Journal of Neuroscience. 1999a;19:3050–3056. doi: 10.1523/JNEUROSCI.19-08-03050.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Caplan D, Soon CS, Sriram N, Tan EWL, Thiel T, et al. Processing of visually presented sentences in Mandarin and English studied with fMRI. Neuron. 1999b;23:127–137. doi: 10.1016/s0896-6273(00)80759-x. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Soon CS, Lee HL. Common and segregated neuronal networks for different languages revealed using functional magnetic resonance adaptation. Journal of Cognitive Neuroscience. 2003;15:85–97. doi: 10.1162/089892903321107846. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Weekes B, Lee KM, Soon CS, Schreiber A, Hoon JJ, et al. Overlap and dissociation of semantic processing of Chinese characters, English words, and pictures: Evidence from fMRI. NeuroImage. 2000;12:392–403. doi: 10.1006/nimg.2000.0631. [DOI] [PubMed] [Google Scholar]
- Chen C, Xue G, Dong Q, Jin Z, Li T, Xue F, et al. Sex determines the neurofunctional predictors of visual word learning. Neuropsychologia. 2007;45:741–747. doi: 10.1016/j.neuropsychologia.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fu S, Iversen SD, Smith SM, Matthews PM. Testing for dual brain processing routes in reading: A direct contrast of Chinese character and Pinyin reading using fMRI. Journal of Cognitive Neuroscience. 2002;14:1088–1098. doi: 10.1162/089892902320474535. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Ambady N. Cultural neuroscience: Parsing universality and diversity across levels of analysis. In: Kitayama S, Cohen D, editors. Handbook of cultural psychology. New York: Guilford Press; 2007. [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: The case for the visual word form area. NeuroImage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56:384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Dupoux E, Mehler J, Cohen L, Paulesu E, Perani D, et al. Anatomical variability in the cortical representation of first and second language. NeuroReport. 1997;8:3809–3815. doi: 10.1097/00001756-199712010-00030. [DOI] [PubMed] [Google Scholar]
- Dong Q, Mei L, Xue G, Chen C, Li T, Xue F, et al. Sex-dependent neurofunctional predictors of long-term maintenance of visual word learning. Neuroscience Letters. 2008;430:87–91. doi: 10.1016/j.neulet.2007.09.078. [DOI] [PubMed] [Google Scholar]
- Fabrega H. Culture, behavior, and the nervous system. Annual Review of Anthropology. 1977;6:419–455. [Google Scholar]
- Fabrega H. Brain, culture, and neuropsychiatric illness. In: Al-Issa I, editor. Culture and psychopathology. Baltimore, MD: University Park Press; 1982. pp. 361–385. [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Chen Y, Smith S, Iversen S, Matthews PM. Effects of word form on brain processing of written Chinese. NeuroImage. 2002;17:1538–1548. doi: 10.1006/nimg.2002.1155. [DOI] [PubMed] [Google Scholar]
- Gandour J. Neurophonetics of tone. In: Brown K, editor. Encyclopedia of language and linguistics. 2. Oxford, UK: Elsevier; 2005. [Google Scholar]
- Gandour J, Dzemidzic M, Wong D, Lowe M, Tong Y, Hsieh L, et al. Temporal integration of speech prosody is shaped by language experience: An fMRI study. Brain and Language. 2003;84:318–336. doi: 10.1016/s0093-934x(02)00505-9. [DOI] [PubMed] [Google Scholar]
- Gandour J, Wong D, Hsieh L, Weinzapfel B, Van Lancker D, Hutchins GD. A crosslinguistic PET study of tone perception. Journal of Cognitive Neuroscience. 2000;12:207–222. doi: 10.1162/089892900561841. [DOI] [PubMed] [Google Scholar]
- Gandour J, Wong D, Hutchins G. Pitch processing in the human brain is influenced by language experience. NeuroReport. 1998;9:2115–2119. doi: 10.1097/00001756-199806220-00038. [DOI] [PubMed] [Google Scholar]
- Gandour J, Wong D, Lowe M, Dzemidzic M, Satthamnuwong N, Tong Y, et al. A cross-linguistic FMRI study of spectral and temporal cues underlying phonological processing. Journal of Cognitive Neuroscience. 2002;14:1076–1087. doi: 10.1162/089892902320474526. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K. Semantic versus perceptual priming in fusiform cortex. Trends in Cognitive Sciences. 2001;5:227–228. doi: 10.1016/s1364-6613(00)01665-x. [DOI] [PubMed] [Google Scholar]
- Han S, Northoff G. Culture-sensitive neural substrates of human cognition: A transcultural neuroimaging approach. Nature Reviews Neuroscience. 2008;9:646–654. doi: 10.1038/nrn2456. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Iverson P, Kuhl P. Influences of phonetic identification and category goodness on American listeners’ perception of /r/ and /l/ The Journal of the Acoustical Society of America. 1996;99:1130–1140. doi: 10.1121/1.415234. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kim KH, Relkin NR, Lee KM, Hirsch J. Distinct cortical areas associated with native and second languages. Nature. 1997;388:171–174. doi: 10.1038/40623. [DOI] [PubMed] [Google Scholar]
- Klein D. A positron emission tomography study of presurgical language mapping in a bilingual patient with a left posterior temporal cavernous angioma. Journal of Neurolinguistics. 2003;16:417–427. [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC. The neural substrates underlying word generation: A bilingual functional-imaging study. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:2899–2903. doi: 10.1073/pnas.92.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Zhao V, Nikelski J. Cerebral organization in bilinguals: A PET study of Chinese–English verb generation. NeuroReport. 1999;10:2841–2846. doi: 10.1097/00001756-199909090-00026. [DOI] [PubMed] [Google Scholar]
- Klein D, Zatorre RJ, Milner B, Zhao V. A cross-linguistic PET study of tone perception in Mandarin Chinese and English speakers. NeuroImage. 2001;13:646–653. doi: 10.1006/nimg.2000.0738. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Fox P, Lancaster J, Tan LH, Amunts K, Zilles K, et al. Localized morphological brain differences between English-speaking Caucasians and Chinese-speaking Asians: New evidence of anatomical plasticity. NeuroReport. 2003;14:961–964. doi: 10.1097/01.wnr.0000075417.59944.00. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: Evidence from a parametric fMRI study. NeuroImage. 2004;21:946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Kuhl P, Rivera-Gaxiola M. Neural substrates of language acquisition. Annual Review of Neuroscience. 2008;31:511–534. doi: 10.1146/annurev.neuro.30.051606.094321. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Stevens E, Hayashi A, Deguchi T, Kiritani S, Iverson P. Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Developmental Science. 2006;9:F13–F21. doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Tsao FM, Liu HM, Zhang Y, Boer B. Language/culture/mind/brain. Annals of the New York Academy of Sciences. 2001;935:136–174. [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Duann JR, Wu YT, Ho LT, Hung D, et al. A left-lateralized network for reading Chinese words: A 3T fMRI study. NeuroReport. 2001;12:3997–4001. doi: 10.1097/00001756-200112210-00029. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee CY, Wu YT, Chou CC, Ho LT, et al. Frequency effects of Chinese character processing in the brain: An event-related fMRI study. NeuroImage. 2003;18:720–730. doi: 10.1016/s1053-8119(03)00015-6. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee JR, Chen LF, Lee PL, Chen SS, et al. Orthographic and phonological processing of Chinese characters: An fMRI study. NeuroImage. 2004;21:1721–1731. doi: 10.1016/j.neuroimage.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Lee CY, Tsai JL, Kuo WJ, Yeh TC, Wu YT, Ho LT, et al. Neuronal correlates of consistency and frequency effects on Chinese character naming: An event-related fMRI study. NeuroImage. 2004;23:1235–1245. doi: 10.1016/j.neuroimage.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Lee SY, Uttal DH, Chen C. Writing systems and acquisition of reading in American, Chinese, and Japanese first-graders. In: Taylor I, Olson D, editors. Scripts and literacy: Reading and learning to read alphabets, syllabaries, and characters. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 247–263. [Google Scholar]
- Li P, Jin Z, Tan LH. Neural representations of nouns and verbs in Chinese: An fMRI study. NeuroImage. 2004;21:1533–1541. doi: 10.1016/j.neuroimage.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Liu L, Peng D, Ding G, Jin Z, Zhang L, Li K, et al. Dissociation in the neural basis underlying Chinese tone and vowel production. NeuroImage. 2006;29:515–523. doi: 10.1016/j.neuroimage.2005.07.046. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dunlap S, Fiez J, Perfetti C. Evidence for neural accommodation to a writing system following learning. Human Brain Mapping. 2007;28:1223–1234. doi: 10.1002/hbm.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JC, Newcombe F. Patterns of paralexia: A psycholinguistic approach. Journal of Psycholinguistic Research. 1973;2:175–199. doi: 10.1007/BF01067101. [DOI] [PubMed] [Google Scholar]
- McBride-Chang C, Cho JR, Liu H, Wagner RK, Shu H, Zhou A, et al. Changing models across cultures: Associations of phonological awareness and morphological structure awareness with vocabulary and word recognition in second graders from Beijing, Hong Kong, Korea, and the United States. Journal of Experimental Child Psychology. 2005;92:140–160. doi: 10.1016/j.jecp.2005.03.009. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, Mechelli A, Frith U, Price CJ. More than words: A common neural basis for reading and naming deficits in developmental dyslexia? Brain. 2005;128:261–267. doi: 10.1093/brain/awh340. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, et al. Neurolinguistics: Structural plasticity in the bilingual brain. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Mei L, Chen C, Xue G, He Q, Li T, Xue F, et al. Neural predictors of auditory word learning. NeuroReport. 2008;19:215–219. doi: 10.1097/WNR.0b013e3282f46ea9. [DOI] [PubMed] [Google Scholar]
- Meng X, Sai X, Wang C, Wang J, Sha S, Zhou X. Auditory and speech processing and reading development in Chinese school children: Behavioural and ERP evidence. Dyslexia. 2005;11:292–310. doi: 10.1002/dys.309. [DOI] [PubMed] [Google Scholar]
- Nakada T, Fujii Y, Kwee IL. Brain strategies for reading in the second language are determined by the first language. Neuroscience Research. 2001;40:351–358. doi: 10.1016/s0168-0102(01)00247-4. [DOI] [PubMed] [Google Scholar]
- Nelson JR, Liu Y, Fiez J, Perfetti CA. Assimilation and accommodation patterns in ventral occipitotemporal cortex in learning a second writing system. Human Brain Mapping. 2009;30:810–820. doi: 10.1002/hbm.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, et al. A cultural effect on brain function. Nature Neuroscience. 2000;3:91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Peng D-l, Ding GS, Perry C, Xu D, Jin Z, Luo Q, et al. fMRI evidence for the automatic phonological activation of briefly presented words. Cognitive Brain Research. 2004;20:156–164. doi: 10.1016/j.cogbrainres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Peng D-l, Xu D, Jin Z, Luo Q, Ding GS, Perry C, et al. Neural basis of the non-attentional processing of briefly presented words. Human Brain Mapping. 2003;18:215–221. doi: 10.1002/hbm.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Paulesu E, Galles NS, Dupoux E, Dehaene S, Bettinardi V, et al. The bilingual brain. Proficiency and age of acquisition of the second language. Brain. 1998;121:1841–1852. doi: 10.1093/brain/121.10.1841. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Liu Y, Fiez J, Nelson J, Bolger DJ, Tan LH. Reading in two writing systems: Accommodation and assimilation of the brain’s reading network. Bilingualism: Language and Cognition. 2007;10:131–146. [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the processing of singe words. Journal of Cognitive Neuroscience. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ. The functional anatomy of word comprehension and production. Trends in Cognitive Sciences. 1998;2:281–288. doi: 10.1016/s1364-6613(98)01201-7. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: Contributions from functional neuroimaging. Journal of Anatomy. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. NeuroImage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Functional ontologies for cognition: The systematic definition of structure and function. Cognitive Neuropsychology. 2005;22:262–275. doi: 10.1080/02643290442000095. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Frackowiak RSJ. Demonstrating the implicit processing of visually presented words and pseudowords. Cerebral Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Raizada RDS, Richards TL, Meltzoff A, Kuhl PK. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. NeuroImage. 2008;40:1392–1401. doi: 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, Dricot L, Devolder A, Bodart JM, Crommelinck M, Gelder BD, et al. Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 2000;12:793–802. doi: 10.1162/089892900562606. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafmani J, Ibanez V, Deiber MP, Dold G, et al. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Sapir E. Language: An introduction to the study of speech. New York: Harcourt; 1921. [Google Scholar]
- Sharma A, Dorman MF. Neurophysiologic correlates of cross-language phonetic perception. The Journal of the Acoustical Society of America. 2000;107:2697–2703. doi: 10.1121/1.428655. [DOI] [PubMed] [Google Scholar]
- Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH. A structural-functional basis for dyslexia in the cortex of Chinese readers. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5561–5566. doi: 10.1073/pnas.0801750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH. Biological abnormality of impaired reading is constrained by culture. Nature. 2004;431:71–76. doi: 10.1038/nature02865. [DOI] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta-analysis. Human Brain Mapping. 2005;25:83–91. doi: 10.1002/hbm.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, Gao JH. The neural system underlying Chinese logograph reading. NeuroImage. 2001;13:836–846. doi: 10.1006/nimg.2001.0749. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong J, et al. Neural systems of second language reading are shaped by native language. Human Brain Mapping. 2003;18:158–166. doi: 10.1002/hbm.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Gao JH, Liu HL, Perfetti CA, Xiong J, et al. Brain activation in the processing of Chinese characters and words: A functional MRI study. Human Brain Mapping. 2000;10:16–27. doi: 10.1002/(SICI)1097-0193(200005)10:1<16::AID-HBM30>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhang W, Chen K, Feng S, Ji Y, Shen J, et al. Arithmetic processing in the brain shaped by culture. Proceedings of National Academy of Sciences. 2007;103:10775–10780. doi: 10.1073/pnas.0604416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, et al. Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Jobard G, Mazoyer B, Tzourio-Mazoyer N. Word and non-word reading: What role for the visual word form area? NeuroImage. 2005;27:694–705. doi: 10.1016/j.neuroimage.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Vygotsky LV. In: Thought and language. Hanfmann E, Vakar G, translators and editors. MIT Press; 1934. [Google Scholar]
- Wang Y, Sereno JA, Jongman A, Hirsch J. fMRI evidence for cortical modification during learning of Mandarin lexical tone. Journal of Cognitive Neuroscience. 2003;15:1019–1027. doi: 10.1162/089892903770007407. [DOI] [PubMed] [Google Scholar]
- Werker JF, Tees RC. The organization and reorganization of human speech perception. Annual Review of Neuroscience. 1992;15:377–402. doi: 10.1146/annurev.ne.15.030192.002113. [DOI] [PubMed] [Google Scholar]
- Whorf B. In: Language, thought, and reality: Selected writings of Benjamin Lee Whorf. Carroll J, editor. Cambridge, MA: MIT Press; 1956. [Google Scholar]
- Wong PC, Parsons LM, Martinez M, Diehl RL. The role of the insular cortex in pitch pattern perception: The effect of linguistic contexts. Journal of Neuroscience. 2004;24:9153–9160. doi: 10.1523/JNEUROSCI.2225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PCM. Hemispheric specialization of linguistic pitch patterns. Brain Research Bulletin. 2002;59:83–95. doi: 10.1016/s0361-9230(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Wong PCM, Perrachione TK, Parrish TB. Neural characteristics of successful and less successful speech and word learning in adults. Human Brain Mapping. 2007;28:995–1006. doi: 10.1002/hbm.20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Gandour J, Talavage T, Wong D, Dzemidzic M, Tong Y, et al. Activation of the left planum temporale in pitch processing is shaped by language experience. Human Brain Mapping. 2006;27:173–183. doi: 10.1002/hbm.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Chen C, Jin Z, Dong Q. Cerebral asymmetry in the fusiform areas predicted the efficiency of learning a new writing system. Journal of Cognitive Neuroscience. 2006a;18:923–931. doi: 10.1162/jocn.2006.18.6.923. [DOI] [PubMed] [Google Scholar]
- Xue G, Chen C, Jin Z, Dong Q. Language experience shapes fusiform activation when processing a logographic artificial language: An fMRI training study. NeuroImage. 2006b;31:1315–1326. doi: 10.1016/j.neuroimage.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Chen K, Jin Z, Chen C, Zeng Y, et al. Cerebral asymmetry in children when reading Chinese characters. Cognitive Brain Research. 2005;24:206–214. doi: 10.1016/j.cogbrainres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Jin Z, Chen C. Mapping of verbal working memory in nonfluent Chinese–English bilinguals with functional MRI. NeuroImage. 2004a;22:1–10. doi: 10.1016/j.neuroimage.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Jin Z, Zhang L, Wang Y. An fMRI study with semantic access in low proficiency second language learners. NeuroReport. 2004b;15:791–796. doi: 10.1097/00001756-200404090-00010. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E. Neural mechanisms underlying melodic perception and memory for pitch. Journal of Neuroscience. 1994;14:1908–1919. doi: 10.1523/JNEUROSCI.14-04-01908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Zhuang J, Ma L, Yu W, Peng D, Ding G, et al. Semantic processing of Chinese in left inferior prefrontal cortex studied with reversible words. NeuroImage. 2004;23:975–982. doi: 10.1016/j.neuroimage.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Zhou X, Chen C, Zang Y, Dong Q, Chen C, Qiao S, et al. Dissociated brain organization for single-digit addition and multiplication. NeuroImage. 2007a;35:871–880. doi: 10.1016/j.neuroimage.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Zhou X, Chen C, Zhang H, Chen C, Zhou R, Dong Q. The operand-order effect in single-digit multiplication: An ERP study of Chinese adults. Neuroscience Letters. 2007b;414:41–44. doi: 10.1016/j.neulet.2006.06.078. [DOI] [PubMed] [Google Scholar]