Abstract
Olfactory ensheathing glia (OEG) express cell adhesion molecules and secrete growth factors that support newly generated olfactory axons and are a promising therapeutic treatment to facilitate axonal regeneration after spinal cord injury (SCI). To study the molecular mechanisms underlying the ability of OEG to enhance axonal outgrowth, we designed an outgrowth assay using spinal cord myelin as a substrate to mimic an injury environment. We asked if olfactory bulb-derived OEG could enhance outgrowth of dorsal root ganglion (DRG) axons on myelin. When grown on myelin alone DRG axons have limited outgrowth potential. However, when OEG are co-cultured with DRG on myelin, twice as many neurons generate axons and their average length is almost twice that grown on myelin alone. We used this OEG/DRG co-culture to determine if a cell adhesion molecule expressed by OEG, L1, and a factor secreted by OEG, brain-derived neurotrophic factor (BDNF), contribute to the ability of OEG to enhance axonal outgrowth on myelin. Using OEG and DRG from L1 mutant mice we found that L1 expression does not contribute to OEG growth promotion. However, both BDNF and its receptor, TrkB, contribute to OEG-enhanced axon regeneration as function-blocking antisera against either component significantly decreased outgrowth of DRG axons. Additional BDNF further enhanced DRG axon growth on myelin alone and on myelin co-cultured with OEG. This simple mouse outgrowth model can be used to determine the molecules that contribute to OEG-enhancement of axonal outgrowth, test therapeutic compounds, and compare the outgrowth potential of other treatments for SCI.
Keywords: OEG, BDNF, neurotrophin, L1 CAM, axon regeneration, spinal cord injury
Introduction
In general peripheral axons cannot enter the adult CNS, but the axons of the olfactory receptor neurons are an exception. Olfactory ensheathing glia (OEG) are likely to facilitate this process as they ensheath axons of the olfactory receptor neurons along their pathway from the PNS, across the cribiform plate, and into the CNS. Additionally, OEG contribute to the glia limitans, the border of glial cells at the PNS/CNS boundary that remains penetrable to olfactory receptor axons throughout adulthood (Doucette, 1990, 1991). This unique feature of the olfactory glia limitans may be due to the ability of OEG to co-mingle with CNS astrocytes (Lakatos et al., 2000) and to promote axonal outgrowth in the CNS (Ramón-Cueto and Valverde, 1995; Li et al., 1997; Ramón-Cueto et al., 2000).
Injured CNS neurons are unable to regenerate their axons through the inhibitory terrain, myelin-associated proteins, and the physical glial scar following severe spinal cord injury (Horner and Gage, 2000). Reportedly, the intrinsic characteristics of olfactory bulb-derived OEG can overcome the inhibitory environment of the CNS after injury (Ramón-Cueto and Valverde, 1995; Raisman and Li, 2007). Several studies (Ramón-Cueto and Nieto-Sampedro, 1994; Navarro et al., 1999; Li et al., 2004) demonstrated the ability of olfactory bulb-derived OEG to promote regeneration of transected dorsal root axons from the PNS into the CNS, although other reports (Goméz et al., 2003; Ramer et al., 2004; Riddell et al., 2004) found that OEG transplantation failed to stimulate significant axon regeneration across the dorsal root entry zone or recovery of function. Studies on the growth-promoting ability of OEG following complete spinal cord transection in adult rodents also varied in their results; studies transplanting olfactory bulb-derived OEG reported axon regeneration and/or functional recovery (Ramón-Cueto et al., 1998; Ramón-Cueto et al., 2000; Lopez-Vales et al., 2006; Kubasak et al., 2008) while those using lamina propria-derived OEG reported mixed results (Lu et al., 2001; Lu et al., 2006; Steward et al., 2006).
Both cell adhesion molecules (CAMs) expressed on the surface of OEG and growth factors secreted by OEG are likely to contribute to their capacity to enhance axonal outgrowth. For example, both L1 and the neural cell adhesion molecule (NCAM) are expressed on OEG processes that associate with developing and adult olfactory receptor axons, and thus may mediate olfactory axon elongation in vivo (Miragall et al., 1989). L1 is upregulated on sprouting CNS axons (Kubasak et al., 2005; Zhang et al., 2005), encourages neurite outgrowth (Mohajeri et al., 1996; Brook et al., 2000; Webb et al., 2001; Adcock et al., 2004), and is important for functional recovery after spinal cord injury (Roonprapunt et al., 2003; Becker et al., 2004; Chen et al., 2007). In addition to cell adhesion molecules, OEG secrete nerve growth factor (NGF), BDNF, and glial cell-line derived neurotrophic factor (GDNF), and display the p75 NGF receptor, the BDNF high affinity tyrosine kinase receptor trkB, and two GDNF receptors (Woodhall et al., 2001; Lipson et al., 2003). The secretion of these growth-promoting factors may facilitate the outgrowth of olfactory axons and also could aid in the regeneration of severed axons after spinal cord injury, either separately or in concert with adhesion molecules.
The goal of this study was to develop a simple in vitro assay to identify individual molecules and mechanisms that olfactory bulb-derived OEG may use to promote axonal regeneration in an inhibitory spinal cord injury-like environment. Specifically, we examined outgrowth on a strongly inhibitory substrate, purified spinal cord myelin, with or without subconfluent cultures of mouse OEG. By comparing the effects of a single gene knockout and function-blocking antibodies on OEG activity in this assay, we conclude that the secreted factor, BDNF, contributes to the OEG enhancement of axon outgrowth, whereas the prominent CAM, L1, does not play a role in this process.
Materials and Methods
Mouse olfactory bulb primary culture
Methods to prepare olfactory bulb-derived rat OEG (Ramón-Cueto et al., 2000) were adapted for mouse OEG primary cultures. The media used throughout these experiments was a 1:1 mixture of DMEM and Ham's F12 Nutrient Mixture supplemented with 10% heat inactivated fetal bovine serum and 1% Penicillin-Streptomycin (DF-media). All tissue culture reagents are from Gibco (Rockville, MD) unless otherwise specified.
Wild-type (L1+/+) and L1 mutant (Y/-; B6;129S-L1camtm1Sor; Cohen et al., 1998) mice originally obtained from Dr. Vance Lemmon (University of Miami, Miami, FL) and the Jackson Laboratory were maintained as a breeding colony at UCLA and genotyped as reported (Demyanenko et al., 1999). All procedures followed the NIH guidelines for animal use and were approved by the Chancellor's Animal Research Committee at UCLA. Following anesthesia and euthanasia, the olfactory bulbs were dissected and the pia mater removed to eliminate contaminating fibroblasts and Schwann cells associated with blood vessels and fine peripheral nerves (Doucette, 1991). The removal of the meninges greatly reduced the probability of Schwann cell contamination (Doucette, 1991) in our OEG cultures. The first two layers of the olfactory bulbs from six 8–10 week old mice of each genotype were dissected and stored in Hank's Balanced Salt Solution (HBSS) until all tissue was harvested. Following a wash in HBSS without Ca+2/Mg+2, the tissue was centrifuged at 1250 rpm for 5 min (Beckman Coulter Allegra 6KR Centrifuge). The pellet was resuspended in 0.1% trypsin made in HBSS without Ca+2/Mg+2 and placed in a 37° C water bath for 10 minutes with occasional agitation. DF-media was added to inactivate the trypsin. The olfactory bulb tissue was washed with fresh DF-media, centrifuged, and resuspended three times. Then cells were mechanically dissociated by aspiration and placed into 25-ml flasks pretreated with poly-L-lysine (Sigma; St. Louis, MO). Cells were grown in an incubator at 37° C with 5% CO2 for 7–8 days. Media was changed every 48 hours.
OEG immunopurification
In preparation for immunopanning, uncoated tissue culture dishes were pretreated with biotinylated anti-rabbit IgG antibody (1:500, Biotin-SP-conjugated AffiniPure Goat Anti-Rabbit IgG, fragment specific, Jackson Immunoresearch Laboratories; West Grove, PA) in 50 mM Tris HCl buffer overnight at 4°C. The following day the dishes were washed 4 times with 25 mM PBS, and incubated overnight in the rabbit anti-mouse p75 nerve growth factor receptor (NGFR, AB1554, 1:2000; Chemicon, Temecula, CA) antibody in 25 mM PBS at 4°C. After repeated washings, dishes were treated with PBS containing 0.5% BSA for one hour and rinsed with PBS and DMEM before immunopanning.
Olfactory bulb cells were detached from the flasks with 0.25% trypsin in HBSS without Ca+2/Mg+2. Cells were washed with DF-media, centrifuged and resuspended 3 times, and then left in DF-media. Cells were plated onto anti-NGFR antibody-treated petri dishes, and placed into an incubator for 10 minutes to facilitate OEG binding. Dishes were washed with DF-media and 3 times with DMEM to remove unbound cells. Bound cells were recovered with a Costar cell scraper, subjected to a second immunopanning, and resuspended in DF-media. Rather than expanding the purified mouse OEG in a flask and treating them with trypsin one week later as is done with rat OEG (Ramón-Cueto et al., 2000; Kubasak et al., 2008), we obtained a greater yield by placing the freshly immunopurified OEG at a density of 1.6 × 104 cells/well directly into 4-well culture slides previously coated with myelin (4.0 μg/well; see next section) and slides were returned to the incubator for 5–7 days. Bovine pituitary extract (20 μg/ml) and forskolin (2 μM, Sigma) were added to the media to enhance OEG growth two days after plating.
Myelin preparation
Myelin was prepared as described previously (Colman et al., 1982; Zheng et al., 2003). Briefly, spinal cords and brainstems were dissected from 10–12 adult wild-type mice (~ 1.3 g total mass), rinsed in HBSS on ice and homogenized in 6.6 ml per gram of tissue in 0.25M sucrose-HEPES (10mM) buffer, pH 7.4, including protease inhibitors such as leupeptin (0.004 μg/ml; Roche Applied Science, Indianapolis, IN), aprotinin (0.004 μg/ml; Roche Applied Science), 5mM EDTA (Sigma), and 3mM dithiothreitol (Roche Applied Science). The homogenate was centrifuged at 1800 rpm for 2 minutes to remove cellular debris and nuclei, adjusted to 1.4M sucrose and subsequently fractionated on a sucrose gradient (2 ml of 1.9M sucrose, 9 ml in 1.4M sucrose, 1 ml 0.85M sucrose, and 0.25 ml of 0.25M sucrose on top). Following centrifugation at 40,000 rpm for 20 hours at 4°C (Beckman SW41 Ultracentrifuge), the interface between the 0.25M and 0.85M sucrose layers was collected, homogenized in 10mM HEPES (pH 7.4) containing protease inhibitors, and adjusted to 0.85M sucrose. This sample was overlaid with 0.5 ml of 0.25M sucrose in HEPES and recentrifuged at 40,000 rpm at 4°C. After 4 hours the myelin rich band at the interface was collected and subjected to two more rounds of osmotic shock and centrifugation. The final myelin fraction was collected, homogenized again in 10mM HEPES and centrifuged at 12,000 rpm for 5 minutes at 4°C (Beckman Coulter Allegra 25R Centrifuge). The pellet was resuspended in 12.5 ml of 30mM HEPES with protease inhibitors and centrifuged again. The final pellet was resuspended in 2 ml of 30mM HEPES with protease inhibitors, aliquoted and stored at −80°C. Protein concentration was determined with Bradford.
Dorsal root ganglia primary culture
After anesthesia and euthanasia 20–25 dorsal root ganglia (DRG) per mouse (P5–8) were dissected and stored in HBSS. The protocol for dissociated cultures of DRG was similar to that for the olfactory bulb primary culture with the following differences. After the initial centrifugation in HBSS, the pellet was resuspended in 0.3% collagenase type I in HBSS without Ca+2/Mg+2 and placed in a 37° C water bath for 30 minutes with occasional agitation. Trypsin (0.1%) was added to this mixture for 10 additional minutes and then deactivated.
Slide preparation for in vitro assay
The day before the immunopurified OEG were ready to be plated, two 4-well culture slides (BD Biosciences; San Jose, CA) were coated with 4.0 μg myelin per well and dried in the incubator overnight. We seeded OEG onto only one of the myelin-coated slides; the myelin alone slide (negative control) was treated identically in every way except that it lacked OEG. Five to seven days later a 4-well culture slide was coated with laminin (positive control, 10 μg/ml, Invitrogen, Carlsbad, CA) one hour before the DRG culture. Dissociated 5–8 day postnatal DRG neurons (1.2 × 105 cells/well) were plated into all 4 wells of each of the three culture slides generated for the experiment (Fig. 1A) and nerve growth factor (20 ng/ml) was added to the media. After 24 hours incubation the cultures were fixed with 4% paraformaldehyde for one hour at 4°C.
Figure 1. Schematic diagram of slide preparation (A) and analysis of the OEG/DRG inhibitory assay (B).
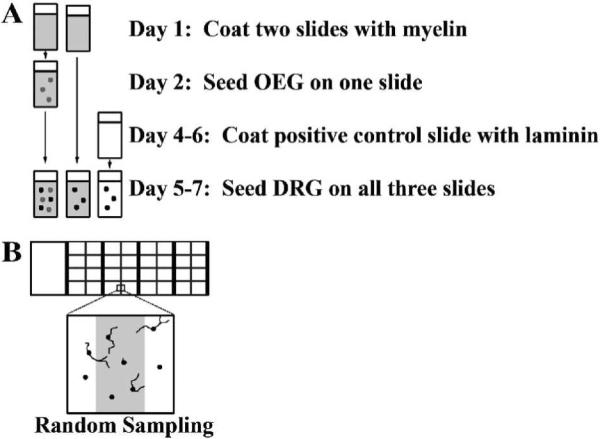
A: Time line illustrates the sequence of the culture slide preparation, and seeding of OEG and DRG. After a 24 hour growth period cultures were fixed and analyzed.
B: Only neurons with cell bodies on the designated lines were counted and their longest axons measured. Four neurons would be analyzed in this example.
BDNF perturbation experiments
To reduce BNDF activity we used two well-characterized, function-blocking antibodies: a chicken anti-human BDNF neutralization antiserum (Promega, Madison, WI; 5, 10, or 15 μg/ml) and a goat anti-mouse TrkB antiserum that interacts with the extracellular domain and blocks the receptor-ligand interaction (R & D Systems, Minneapolis, MN; 0.5, 1.0, or 2.0 μg/ml). As a third perturbation, we added exogenous BDNF (25, 100, or 1000 pg/ml) to the media on all three substrates immediately after plating the DRG neurons. As a significant number of P5–8 DRG neurons require NGF for survival (Yip et al., 1984), we were unable test if the secretion of NGF by OEG contributes to the promotion of axon outgrowth in our co-cultures.
Immunocytochemical procedures
We stained primary and purified OEG cultures with an L1 polyclonal antiserum (gift from Dr. Vance Lemmon, University of Miami; Miami, FL; Lemmon et al., 1989) as reported in Runyan et al. (2005) with the following changes: 1) 0.1% bovine serum albumin was added to the Tris buffer, and 2) standard rabbit avidin-biotin methods (Vectastain Elite Rabbit IgG kit) were visualized with diaminobenzidine or a goat anti-rabbit Alexa 594 (Molecular Probes, Eugene, OR). The NGFR antiserum used for immunopanning also identified OEG in cultures (AB 1554, 1:26000), with the protocol reported above.
To identify DRG neurons in cultures we used the neuronal class III β-tubulin polyclonal antibody (1:2500; Covance; Berkeley, CA) with the protocol described for anti-L1. In some experiments, OEG were incubated with Cell Tracker Green (10 μM; Molecular Probes) for 30 minutes prior to DRG plating. We used these cultures to analyze spatial relationships between the OEG and DRG cells with immunofluorescence.
Statistical analysis
To quantify the extent of axonal outgrowth on each substrate we analyzed a random sample of neurons using a defined grid comprising 13% of the area of each well (Fig. 1B; 0.225cm2 per 1.7 cm2 well). Measurements were made for each of the 3 substrates within an individual experiment, and the means from 3–5 separate experiments were compared for statistical significance. All β-tubulin-labeled neuron cell bodies that fell on the gridlines were counted as well as the percentage of those cells that extended an axon longer than their somal diameter. Additionally, the length of the longest axon generated by each cell was measured and the means were calculated (Fig. 1B). To analyze the effects of the TrkB and BDNF function-blocking antibodies, exogenous BDNF, and L1 we compared mean percentages of DRG neurons extending axons and their mean maximum length using factorial analysis of variance (ANOVA). Mean outgrowth on each substrate was compared to mean growth on the same substrate in the presence of varying antibody concentrations and mean outgrowth was compared across substrates at each administered concentration. Means and the corresponding pooled ANOVA based standard errors (SEs) are reported and post-hoc mean comparisons under the ANOVA model were made using the Tukey-Fisher criterion. Statistical significant was set at a two-sided P < 0.05, computed under the ANOVA model.
Results
Cultured olfactory bulb-derived mouse OEG resemble rat OEG
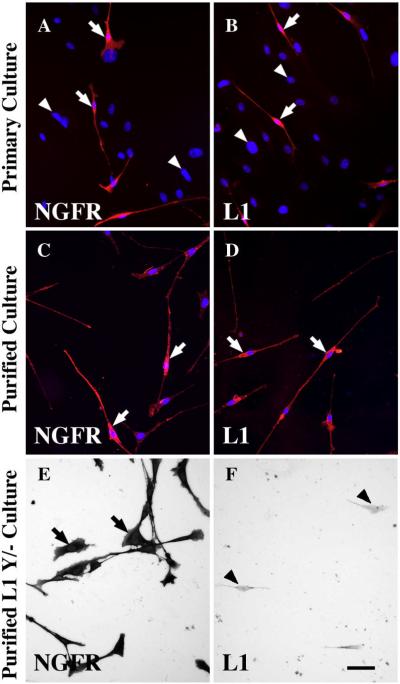
Cultured OEG derived from adult rat olfactory bulbs are well characterized as bipolar or multipolar-shaped cells that express antigens such as the p75 low affinity nerve growth factor receptor (NGFR), S100, glial fibrillary acidic protein, and L1 (Ramón-Cueto and Nieto-Sampedro, 1992; Ramón-Cueto and Valverde, 1995; Franceschini and Barnett, 1996). Initially, we characterized adult olfactory bulb-derived wild-type mouse OEG to determine if they expressed antigenic features similar to those of rat OEG. A primary culture prepared from the outer two layers of wild-type mouse olfactory bulbs contained many bipolar and multipolar OEG with long thin processes. Mouse OEG expressed the characteristic markers NGFR (Fig. 2A, arrows), L1 (Fig. 2B, arrows), and S100 (Supplemental Fig. 1A); other cells in the primary culture that did not express these molecules displayed only DAPI-labeled nuclei (Fig. 2A–B, arrowheads). To eliminate the other glial cells, we immunopurified and then cultured the NGFR-positive population. The purified mouse OEG maintained L1, NGFR, and S100 expression after being plated and grown on a myelin substrate for 5–7 days (Figs. 2C, D; Supplemental Fig. 1B). While OEG grew on myelin their growth was not robust, nor were they confluent even seven days after seeding.
Figure 2. Mouse olfactory ensheathing glia (OEG) that express both the p75 low affinity nerve growth factor receptor (NGFR) and L1 are imaged with either fluorescence (A–D) or bright field (E–F) microscopy.
A–B: OEG in primary cultures express NGFR (A, red cells at arrows) and L1 (B, red cells at arrows). Immunonegative cells are identified with only DAPI (A–B, blue nuclei at arrowheads).
C–D: Purified OEG cultures grown on a myelin substrate express NGFR (C, red cells at arrows) and L1 (D, red cells at arrows). Nuclei are DAPI-labeled (blue) and associated only with OEG.
E–F: Purified OEG cultures from L1 mutant mice grown on a myelin substrate are immunopositive for NGFR (E, arrows), but immunonegative for L1 with only cell outlines visible (F, arrowheads). Scale A–F = 50 μm.
OEG enhance axon outgrowth on an inhibitory substrate
To evaluate if mouse OEG can promote axon regeneration when grown on a simulated post-injury inhibitory environment, we compared outgrowth of DRG axons on three substrates: 1) myelin with previously seeded OEG, 2) myelin alone as a negative control, and 3) laminin alone as a positive control (Fig. 1A). The preparation of each component used in this OEG/DRG injury assay was timed carefully to ensure that each experiment could be replicated 3–5 times.
When both cells types were in close proximity, the neuronal processes frequently aligned along and closely interacted with the OEG (Fig. 3A–B). We found that more than twice as many DRG neurons extended axons (51%) with OEG present than when DRG neurons are cultured on myelin alone (23%, P < 0.0001; Fig. 4B–C, E). Additionally, the average maximal axon length (see methods) was significantly longer in the presence of OEG (96 μm) compared to the length of DRG axons cultured on myelin alone (54 μm, P < 0.0004; Fig. 4B–C, F). In contrast to growth on myelin, DRG neurons exhibited robust axonal outgrowth when grown on laminin (Fig. 4A). An average of 70% of the neurons extended axons when grown for 24 hours on laminin and their average maximal length was 145 μm (Fig. 4A, E–F). In contrast, when DRG neurons are grown in the presence of spinal cord-derived myelin, only 23% of neurons extended neurites an average of 54 μm in length (Fig. 4B, E–F). Thus the myelin substrate reduced the number of DRG neurons that regenerated axons and the maximal axonal length compared to growth on laminin (P < 0.001) whereas OEG significantly increased both parameters when DRG are grown on myelin.
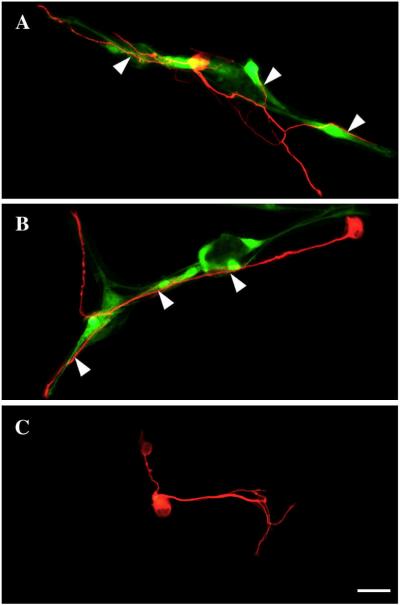
Figure 3. Spatial relationships between dorsal root ganglia (DRG, red) neurons and olfactory ensheathing glia (OEG, green) cultured on a myelin substrate.
A–B: OEG are vitally labeled with Cell Tracker Green, have long, well-differentiated processes, and are scattered on the myelin substrate. β-tubulin-labeled (red) DRG neurons exhibit cell-to-cell contact with OEG (arrowheads) derived from wild-type (A) or L1 mutant (B) olfactory bulbs.
C: A DRG neuron elaborates axons without direct contact with OEG cells, results that suggest OEG secrete factors that promote axonal outgrowth. Scale A–C = 50 μm.
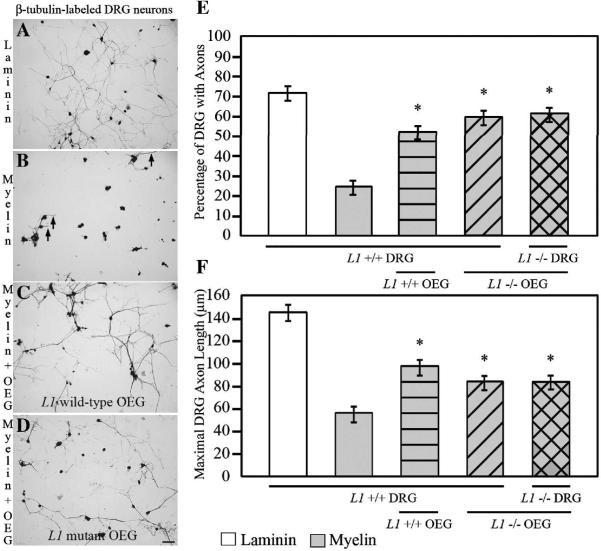
Figure 4. Addition of OEG enhances the outgrowth of DRG axons on a myelin substrate. Neurons are identified with anti-β-tubulin immunoreactivity after 24 hours in vitro.
A: For the positive control, DRG are grown on laminin and extend elaborate processes.
B: For the negative control, DRG neurons are grown alone on myelin and extend short neurites (short arrows).
C, D: When OEG from wild-type (C) or L1 mutant (D) mice are co-cultured with DRG neurons on myelin, a higher percentage of longer axons are detected than when DRG are grown on myelin alone (B). Scale A–D = 50 μm.
E: The highest percentage of DRG neurons extends processes on laminin and the lowest, on myelin (mean ± SEM). The presence of OEG from either wild-type or L1 mutant mice significantly enhances the percentage of DRG (wild-type and L1 mutant) that extend axons on myelin. The number of neurons sprouting axons on laminin (P < 0.0001) and OEG (P < 0.0001) substrates are significantly different from those grown alone on a myelin substrate. All are significantly different from laminin (P < 0.02). *Significantly different from myelin (P < 0.0001).
F: The average maximal axon length was shortest on myelin alone and longest on laminin. Regardless of genotype, OEG promoted significantly longer DRG axons than when grown on myelin alone. DRG processes grown on laminin (mean ± SEM; P < 0.0001) and on myelin with OEG (P < 0.02) are significantly longer than those grown on myelin alone. All are significantly different from laminin (P < 0.004). *Significantly different from myelin (P < 0.02).
Presence or absence of L1 CAM does not change axonal outgrowth
Cell-to-cell contact is one likely means by which OEG promote axonal outgrowth (Fig. 3A–B). L1 CAM can enhance axonal outgrowth through homophilic and heterophilic interactions (Kamiguchi and Lemmon, 1997; Haney et al., 1999) and both OEG and DRG express L1. Thus we asked if the presence of glial and/or neuronal L1 contributes to the ability of OEG to facilitate axonal outgrowth on a myelin substrate. First we generated purified OEG from L1 mutant mice to verify that they would grow on a myelin substrate and maintain NGFR immunoreactivity (Fig. 2E) but not express L1 (Fig. 2F). Then we prepared OEG cells from either wild-type or L1 mutant mice and grew them on myelin in the inhibitory outgrowth assay (Fig. 1A). When L1 mutant OEG are grown on myelin, the percentage of DRG neurons extending axons and their maximal length is reduced by 17% and 57%, respectively, compared to outgrowth on laminin (Fig. 4D–F). However, compared to growth on myelin alone, the presence of OEG derived from L1 mutant mice more than doubled the percentage of DRG neurons that extended axons, and these processes were on average 1.5 times longer (P < 0.02; Fig. 4D–F). Therefore OEG from wild-type or L1 mutant mice did not differ in their ability to enhance the extension of DRG axons (51% versus 58%) or in their average maximum length (96 μm versus 82 μm; Fig. 4E–F). Finally, to rule out a possible role of neuronal L1 expressed by the DRG, we co-cultured both OEG and DRG from L1 mutant mice. In these experiments the OEG still enhanced the percentage of DRG neurons that display axonal outgrowth (60%; P < 0.0001) and their average length (83 μm; P < 0.02) compared to myelin alone (Fig. 4E–F). As this level of enhancement is similar to that seen with wild-type OEG and DRG, L1 is not required for OEG-enhanced axon outgrowth in this inhibitory outgrowth assay.
Blocking BDNF reduces OEG growth promotion
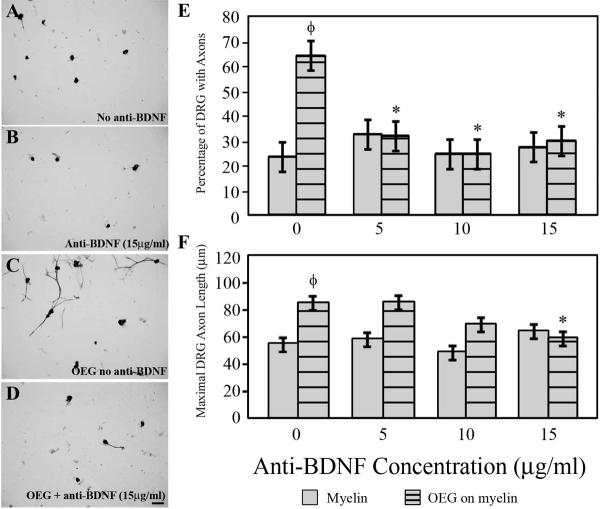
When in close proximity DRG axons course along the surfaces of OEG (Fig. 3A–B), but other DRG neurons elaborate processes on a myelin substrate without contacting OEG (Fig. 3C), an observation consistent with reports that OEG secrete growth factors that enhance process outgrowth (Kafitz and Greer, 1999; Woodhall et al., 2001; Lipson et al., 2003; Chung et al., 2004) in the absence of, or in combination with cell-to-cell contact. To determine if the reported secretion of BDNF by OEG contributes to OEG growth promotion, we applied a function-blocking anti-BDNF antiserum (5, 10, or 15 μg/ml) to the media in the inhibitory co-culture model depicted in Fig. 1A. The concentration of anti-BDNF did not affect the percentage of DRG that extended axons when grown on laminin (maintained at ~92%) or the maximum length of their processes (varied between 123–137 μm). Similarly, the percentage of DRG neurons that exhibited axons when grown on myelin alone or their average maximum length did not change significantly in the presence of different concentrations of anti-BDNF (Fig. 5A–B, E–F). In contrast, addition of anti-BDNF antiserum significantly reduced both the maximal length (33% decrease, P < 0.006) and the percentage of neurons extending neurites (63% decrease, P < 0.001) on myelin in the presence of OEG compared to the control (P < 0.001; Fig. 5). Thus, blocking BDNF diminishes the positive effects of OEG on both the percentage of DRG neurons with axons and the maximal length of their processes.
Figure 5. Anti-BDNF blocks the growth-promotion ability of OEG when co-cultured with DRG on myelin. The DRG neurons are identified by anti-β-tubulin.
A–B: Addition of anti-BDNF (B; 15 μg/ml) did not alter the percent of neurons that extended axons or their length compared to outgrowth on myelin alone (A).
C–D: In DRG/OEG co-cultures grown on myelin, both the percentage of neurons that regenerate axons and their maximal lengths are reduced in the presence of anti-BDNF antibodies (D; 15 μg/ml). Scale A–D = 50 μm.
E: The presence of anti-BDNF significantly reduces the enhancement that OEG have on the percentage of regenerating neurons, but it does not change outgrowth on myelin alone (values are means ± SEM). *Significantly different from OEG without anti-BDNF (P < 0.001). ΦSignificantly different from myelin without anti-BDNF (P < 0.0001).
F: Anti-BDNF at 15 μg/ml causes a significant decrease in the average maximal length of axons in OEG/DRG co-cultures, but does not change their lengths on myelin alone.
*Significantly different from OEG without anti-BDNF (P < 0.006). ΦSignificantly different from myelin without anti-BDNF (P < 0.002).
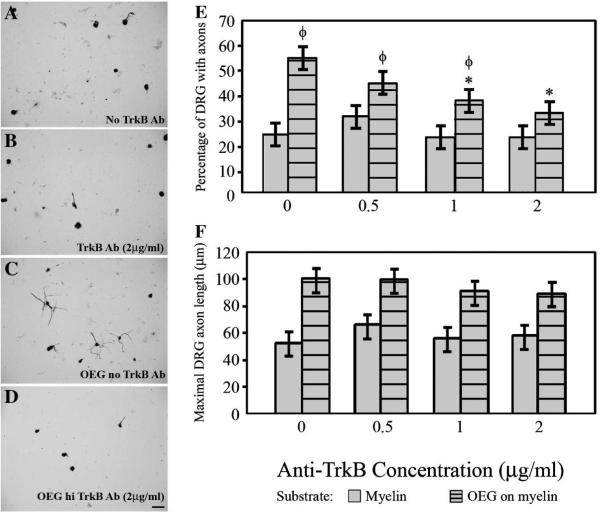
Blocking TrkB decreases the percentage of DRG with axons in OEG-enhanced cultures
If BDNF secretion plays a role in the growth promoting ability of OEG, we would expect that blocking the high affinity BDNF receptor, TrkB, also would reduce DRG axon outgrowth on myelin. We added a function-blocking TrkB antiserum (0.5, 1.0, or 2.0 μg/ml) to the culture media in the OEG/DRG injury assay. The presence of the antibody did not significantly alter the percentage of DRG neurons extending axons or their maximal length on either the laminin (88–92% of DRG extend axons that average 143–154 μm long) or the myelin controls (Fig. 6A–B, E–F). In contrast, the percentage of neurons with OEG-enhanced axonal outgrowth decreased in a dose-dependent manner in the presence of increasing concentrations of TrkB antiserum (from 55% to 32%; Fig. 6C–E). In the presence of OEG the two highest anti-TrkB concentrations (1 and 2 μg/ml) significantly reduced the percentage of DRG neurons extending processes (P < 0.01), and the highest concentration reduced it to a level similar to growth on myelin alone (Fig. 6D, E). While the average longest axon in the co-cultures decreased in length with increasing antibody concentrations, these changes were not significant (Fig. 6C–D, F). Therefore, blocking the TrkB receptor in the presence of OEG decreased the percentage of DRG extending axons on myelin.
Figure 6. Blocking the BDNF receptor, TrkB, reduces the percentage of DRG that extend axons on myelin. DRG neurons are labeled with anti-β-tubulin.
A–B: Minimal neurite outgrowth characterizes DRG grown on myelin in the absence (A) or presence of anti-TrkB (B; 2 μg/ml).
C–D: OEG-enhanced axonal outgrowth on myelin (C) is reduced with the addition of anti-trkB (D; 2 μg/ml). Scale A–D = 50 μm.
E: Application of anti-TrkB does not alter the percentage of neurons that grow processes on a myelin substrate alone. Anti-TrkB at the two highest anti-TrkB doses (1 and 2 μg/ml) causes a dose-dependent decrease in the OEG enhancement of the percentage of neurons that extend axons. Data presented as mean values ± SEM. *Significantly different from OEG without TrkB blocker (P < 0.01). ΦSignificantly different from myelin without TrkB blocker (P < 0.03).
F: The average maximal axon length is not altered by the presence of anti-TrkB.
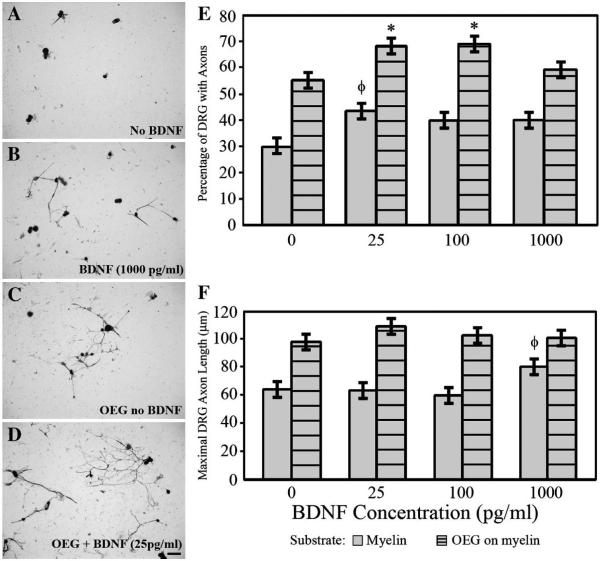
Addition of BDNF enhances neurite outgrowth
Given the results of the anti-BDNF and TrkB blocking experiments, we then asked if adding low levels of exogenous BDNF (25, 100, or 1000 pg/ml) could promote further axonal outgrowth in the OEG/DRG injury model. Addition of BDNF to DRG neurons cultured on a laminin substrate did not alter the percent of neurons extending processes (90%) or their maximal length (132–138 μm) when compared to cultures with no additional BNDF. All concentrations of BDNF increased the percentage of neurons that extended axons on a myelin substrate alone, but only the 25 pg/ml concentration of BDNF reached significance (P < 0.02; Fig. 7A–B, E). The maximal length of neuronal processes grown on myelin alone remained stable at the lower BDNF concentrations (25 and 100 pg/ml), but it increased significantly at the highest level examined (1000 pg/ml; P < 0.02; Fig. 7B, F). In OEG/DRG co-cultures on a myelin substrate, exogenous BDNF increased the percent of neurons with axons (25 and 100 pg/ml; P < 0.02; Fig. 7C–E), but did not change the maximal length of the processes (Fig. 7F). At the highest BDNF concentration (1000 pg/ml), both the percent of neurons displaying axons and their maximal length were similar to control cultures (Fig. 7E–F). Thus addition of BDNF can enhance the percentage of DRG neurons with axonal processes when grown on a myelin substrate with or without OEG.
Figure 7. Addition of BDNF promotes DRG axon outgrowth on myelin.
A–B: Exogenous BDNF (B; 1000 pg/ml) increased axonal outgrowth compared to outgrowth without BDNF on a myelin substrate (A). DRG are identified with anti-β-tubulin.
C–D: Addition of low levels of BDNF (D; 25 pg/ml) to OEG/DRG co-cultures enhanced the percent of neurons that extended processes. Scale A–D = 50 μm.
E: BDNF significantly increased the percentage of neurons growing processes on myelin alone (25 pg/ml) and in the presence of OEG (25 and 100 pg/ml). Data represent mean values ± SEM.
*Addition of BDNF (25 and 100 pg/ml) in OEG/DRG co-cultures is significantly different from those without additional BDNF (P < 0.02). ΦAddition of BDNF (25 pg/ml) on a myelin substrate is significantly different from myelin without additional BDNF (P < 0.01).
F: Adding BDNF does not significantly alter the average maximal axon length on myelin with or without OEG compared to no additional BDNF. ΦAddition of BDNF (1000 pg/ml) on myelin alone is significantly different from myelin with the addition of 100 pg/ml of BDNF (P < 0.02).
Discussion
We first developed an in vitro model to test the ability of olfactory bulb-derived OEG to facilitate axon regeneration of dissociated DRG neurons on a purified CNS myelin substrate. Then we used this injury model to demonstrate that OEG promote a higher percentage of DRG neurons to extend axons and grow longer processes than when placed in a myelin-rich environment alone. These findings provide clear evidence that OEG are not only viable but also can promote axon regeneration in a strongly inhibitory environment. We tested our model to determine if either a cell adhesion molecule expressed by OEG (L1) or a factor secreted by OEG (BDNF) contribute to the mechanism by which OEG promote axonal outgrowth. We found that neither axonal nor glial L1 is required for OEG enhancement of DRG axon outgrowth. However, when either BDNF or its receptor, TrkB, are blocked in vitro, the ability of OEG to stimulate axonal outgrowth is impaired. Conversely, when low levels of exogenous BDNF are added to the OEG/DRG inhibitory assay, the percentage of regenerating DRG axons increased. These findings imply that BDNF contributes significantly to the axonal growth-promoting effects of OEG.
OEG enhance neurite outgrowth in vitro
While results from many in vitro experiments suggest that OEG facilitate neurite outgrowth (Chuah and Au, 1994; Kafitz and Greer, 1999; Sonigra et al., 1999; Woodhall et al., 2001; Lipson et al., 2003; Chung et al., 2004; Leaver et al., 2006), only Chung et al., (2004) challenged OEG with an inhibitory environment –a scratch wound. Using cortical-meningeal co-cultures these authors found that OEG acted as a physical substrate and secreted soluble factors that stimulated neurite sprouting. One of the strengths of our OEG/DRG injury model is that purified spinal cord myelin more closely replicates the severity of the inhibition produced by the glial scar than a scratch wound (Chung et al., 2004). While myelin is only one aspect of the gliotic scar and cystic formation following SCI, it clearly creates a hostile environment for axon regeneration. In addition we used p75-immunopurified, olfactory bulb-derived OEG in the co-cultures as these cells are more effective in promoting functional recovery following complete spinal cord transection (Ramón-Cueto et al., 2000; Kubasak et al., 2008) than OEG derived from the nasal epithelium (Lu et al., 2006; Steward et al., 2006). Furthermore, we studied adult OEG that differentiate into well-established phenotypes (Sonigra et al., 1999; Leaver et al., 2006) and are the cells transplanted after complete spinal cord transection studies that demonstrate measurable functional improvement (Ramón-Cueto et al., 2000; Kubasak et al., 2008). Additionally, we evaluated axonal outgrowth using DRG neurons, rather than retinal ganglion cells (Sonigra et al., 1999; Leaver et al., 2006; Pastrana et al., 2007), olfactory neurons (Chuah and Au, 1994; Kafitz and Greer, 1999), embryonic cortical neurons (Kafitz and Greer, 1999; Chung et al., 2004) or explanted peripheral ganglia (Lipson et al., 2003). There are two advantages of testing neurite outgrowth with DRG neurons. First, DRG neurons have no dendrites and thus all process outgrowth represents axon regeneration. Second, after spinal cord injury the regeneration of DRG axons through the glial scar is necessary and relevant for the recovery of sensory function. A final advantage of the OEG/DRG inhibitory assay is that we used mouse rather than rat cells (Chuah and Au, 1994; Kafitz and Greer, 1999; Sonigra et al., 1999; Woodhall et al., 2001; Lipson et al., 2003; Chung et al., 2004; Leaver et al., 2006) in order to test OEG and DRG from mice with single-gene deletions.
Our results demonstrate that DRG axons contact OEG directly (Fig. 3A, B), confirming the frequent physical interaction between these glia and growing axons in vitro (Chuah and Au, 1994; Sonigra et al., 1999; Lipson et al., 2003; Chung et al., 2004; Leaver et al., 2006). However, DRG also regenerate axons without direct glial contact in an inhibitory environment (Fig. 3C), a finding consistent with secreted molecules contributing to the ability of OEG to promote axonal outgrowth. While some culture studies proposed that OEG secrete molecules that enhance neurite outgrowth (Kafitz and Greer, 1999; Woodhall et al., 2001; Lipson et al., 2003; Chung et al., 2004; Pastrana et al., 2007), others argued against a role for secreted factors based on negative results from OEG-conditioned media experiments (Chuah and Au, 1994; Sonigra et al., 1999; Lipson et al., 2003; Leaver et al., 2006). The present OEG/DRG co-culture produced clear-cut evidence demonstrating that OEG secrete a factor that provides a significant level of growth stimulation.
L1 CAM is not required for OEG-stimulated growth promotion
L1 is down-regulated on most axons during postnatal development (Akopians et al., 2003), but is maintained on adult small-diameter DRG nociceptors (Haney et al., 1999; Runyan et al., 2005; Runyan et al., 2007) and on the Schwann cells that ensheath these unmyelinated axons (Haney et al., 1999). Using L1-deficient mice Haney et al. (1999) found that Schwann cells did not maintain ensheathment of DRG axons, due to the loss of axonal L1. Furthermore, many studies report that L1 encourages neurite outgrowth (Mohajeri et al., 1996; Brook et al., 2000; Webb et al., 2001; Adcock et al., 2004; Kubasak et al., 2005; Zhang et al., 2005) and promotes recovery following spinal cord injury (Roonprapunt et al., 2003; Becker et al., 2004; Chen et al., 2007). Despite evidence that favored a contact-mediated role for L1 in OEG-enhanced regeneration, OEG stimulated axonal outgrowth regardless of the presence or absence of L1 in our assay. Our findings are consistent with those using the L1 mutant mouse model that report L1 expression is not essential for DRG sprouting following unilateral deafferentation (Runyan et al., 2007) or growth of the corticospinal tract into a contusion injury (Jakeman et al., 2006). These results suggest that other cell adhesion molecules or extracellular matrix proteins expressed by OEG, such as PSA-NCAM, must participate in contact-mediated growth promotion.
BDNF and TrkB are important in OEG enhancement of axonal outgrowth
BDNF supports the survival of developing DRG neurons (Kalcheim et al., 1987), mice that lack trkB receptors have 30% fewer DRG neurons (Klein et al., 1993), and 27% of adult rat DRG express trkB receptors (McMahon et al., 1994). In addition to its role in survival, BDNF can modulate axon length, the extent of axonal arborization, and has a role in synapse formation (Cohen-Cory and Fraser, 1995; Vicario-Abejón et al., 2002). Additionally, a gradient of BDNF can either attract, or if combined with cAMP inhibitors, repulse growth cones from Xenopus spinal neurons (Song et al., 1997). Pastrana et al. (2006) listed BDNF as a candidate molecule in genetic expression profiles of OEG and found that BDNF secretion by OEG contributed to axonal regeneration of retinal ganglion neurons (Pastrana et al., 2007). Now the results from our study highlight the importance of the BDNF secreted by OEG in overcoming myelin inhibition in an in vitro model of spinal cord injury. Combined, data from Pastrana et al. (2007) and the present study support the conclusion that OEG-secreted BDNF functions to enhance axonal outgrowth despite disparate reports on the effects of OEG-conditioned media on outgrowth (Chuah and Au, 1994; Kafitz and Greer, 1999; Sonigra et al., 1999; Woodhall et al., 2001; Lipson et al., 2003; Chung et al., 2004; Leaver et al., 2006; Pastrana et al., 2007). Perhaps neurons require specific concentrations or a gradient of BDNF in close proximity to elicit a significant change as demonstrated with developing growth cones (Song et al., 1997). As a subpopulation of DRG neurons can synthesize and secrete BDNF (Zhou and Rush, 1996), they may contribute to the observed effect, although there were no differences in our positive (DRG cultured on laminin) or negative (DRG cultured on myelin) controls after treatments with anti-BDNF or anti-TrkB neutralizing antisera. We conclude that one means by which OEG stimulate DRG neurons to extend longer processes when presented with an inhibitory substrate is by BDNF secretion.
While the growth-promoting effects of OEG can be reduced by BDNF or trkB function-blocking antisera, these reagents had no effect on the percentage of DRG neurons extending axons or their average maximal length when grown alone on myelin or laminin. In contrast, the addition of BDNF increased axonal outgrowth in the myelin only controls, but never reached the levels detected in OEG/DRG co-cultures. It is unlikely that this difference is due to low BDNF concentrations, as the benefits of exogenous BDNF on neurite outgrowth reportedly diminish at higher concentrations (Deumens et al., 2006), and we found a similar trend. Most likely, OEG use additional factors, in combination with BDNF, to facilitate DRG axon regeneration on a myelin substrate. Therefore, we suggest that BDNF contributes to, but is not the sole factor responsible for, the enhanced axonal outgrowth stimulated by OEG.
Previous studies report conflicting results on whether or not BDNF stimulates adult DRG regeneration after injury (Bradbury et al., 1999; Gavazzi et al., 1999; Song et al., 2008). A recent series of experiments by Song et al. (2008) concluded that BDNF promotes the regeneration of adult DRG axons after a dorsal column crush injury. In addition, they used BDNF antiserum to inhibit the intrinsic outgrowth ability of DRG neurons stimulated by a conditioning lesion (the peripheral DRG axon is cut one week before the central branch is injured). As the upregulation of cyclic AMP (cAMP) induces the regeneration of the central DRG axon following a conditioning lesion (Neumann et al., 2002; Qui et al., 2002), Song et al. (2008) further demonstrated that BDNF promotes the central axon regeneration by increasing cAMP. An upregulation of cAMP also could facilitate axon outgrowth in our assay because we cultured P5–8 mouse DRG, an age when rat DRG axons are inhibited by myelin-associated glycoprotein, a major component of myelin (Cai et al., 2001). Future studies will test this possibility in our mouse model and determine if there are species differences. In addition, however, BDNF may work in concert with other secreted, extracellular matrix, or adhesion-type molecules to stimulate young postnatal axonal regeneration.
Currently there are a number of cell types (neural stem cells, Schwann cells and their precursors, OEG), combinations of cells (OEG and meningeal fibroblasts), as well as genetically engineered cells proposed as repair strategies for transplantation into the injured spinal cord. By testing each of the cell types or combinations in the same in vitro model, the information comparing their abilities to enhance axon outgrowth in an inhibitory environment should help evaluate and better understand the use of cell transplantation strategies for treatment of SCI.
Supplementary Material
A: Primary cultures prepared from the olfactory bulb contain S100-positive OEG (arrows). The nuclei of other cell types (arrowheads) are just visible in the background.
B: After immunopurification and seeding on a myelin substrate, the OEG express S100 (red cells at arrows) and have the characteristic bipolar or multipolar shape. DAPI-labeled (blue) nuclei are only associated with S100-labeled OEG. Scale A–B = 50 μm.
Acknowledgements
The authors are grateful to Dr. Vance Lemmon for generously providing L1 breeding pairs and the L1 polyclonal antisera, Drs. Rachelle Crosbie and Angela Peter for advice and sharing equipment, Dr. Binhai Zheng for guidance on the myelin preparation, Dr. David Ginty for helpful discussions, Tiffany Yap for animal care and genotyping, and Katherine Moore for assistance with the supplemental figure. We thank Drs. Tama Hasson, Susana Cohen-Cory, Ina Wanner, and Stephanie White and Aya Takeoka for helpful suggestions on the manuscript.
Grant sponsors: Partial support provided by National Institute of Neurological Disorders and Stroke R21 NS42000; R01NS54159; Molecular, Cellular, and Integrative Physiology training grant 5 T32 GM065823
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock KH, Brown DJ, Shearer MC, Shewan D, Schachner M, Smith GM, Geller HM, Fawcett JW. Axon behaviour at Schwann cell - astrocyte boundaries: manipulation of axon signalling pathways and the neural adhesion molecule L1 can enable axons to cross. Eur. J. Neurosci. 2004;20:1425–1435. doi: 10.1111/j.1460-9568.2004.03573.x. [DOI] [PubMed] [Google Scholar]
- Akopians A, Runyan SA, Phelps PE. Expression of L1 decreases during postnatal development of rat spinal cord. J. Comp. Neurol. 2003;467:375–388. doi: 10.1002/cne.10956. [DOI] [PubMed] [Google Scholar]
- Becker CG, Lieberoth BC, Morellini F, Feldner J, Becker T, Schachner M. L1.1 is involved in spinal cord regeneration in adult zebrafish. J. Neurosci. 2004;24:7837–7842. doi: 10.1523/JNEUROSCI.2420-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Khemani S, King VR, Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci. 1999;11:3873–3883. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- Brook GA, Houweling DA, Gieling RG, Hermanns T, Joosten EAJ, Bär DPR, Gipsen WH, Schmitt AB, Leprince P, Noth J, Nacimiento W. Attempted endogenous tissue repair following experimental spinal cord injury in the rat: Involvement of cell adhesion molecules L1 and NCAM? Eur. J. Neurosci. 2000;12:3224–3238. doi: 10.1046/j.1460-9568.2000.00228.x. [DOI] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J. Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wu J, Apostolova I, Skup M, Irintchev A, Kügler S, Schachner M. Adeno-associated virus-mediated L1 expression after spinal cord injury. Brain. 2007;130:954–969. doi: 10.1093/brain/awm049. [DOI] [PubMed] [Google Scholar]
- Chuah MI, Au C. Olfactory cell cultures on ensheathing cell monolayers. Chem. Senses. 1994;19:25–34. doi: 10.1093/chemse/19.1.25. [DOI] [PubMed] [Google Scholar]
- Chung RS, Woodhouse A, Fung S, Dickson TC, West AK, Vickers JC, Chuah MI. Olfactory ensheathing cells promote neurite sprouting of injured axons in vitro by direct cellular contact and secretion of soluble factors. Cell Mol. Life Sci. 2004;61:1238–1245. doi: 10.1007/s00018-004-4026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NR, Taylor JSH, Scott LB, Guillery RW, Soriano P, Furley AJW. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Current Biol. 1998;8:26–33. doi: 10.1016/s0960-9822(98)70017-x. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- Colman DR, Kreibich G, Frey AB, Sabatini DD. Synthesis and incorporation of myelin polypeptides into CNS myelin. J. Cell Biol. 1982;95:598–608. doi: 10.1083/jcb.95.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanenko GP, Tsai AY, Maness PF. Abnormalities in neuronal process extension, hippocampal development, and the ventricular system of L1 knockout mice. J. Neurosci. 1999;19:4907–4920. doi: 10.1523/JNEUROSCI.19-12-04907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deumens R, Koopmans GC, Jaken RJP, Morren K, Comhair T, Kosar S, Steinbusch HWM, Den Bakker CGJ, Joosten EAJ. Stimulation of neurite outgrowth on neonatal cerebral astrocytes is enhanced in the presence of BDNF. Neurosci. Lett. 2006;407:268–273. doi: 10.1016/j.neulet.2006.08.059. [DOI] [PubMed] [Google Scholar]
- Doucette R. Glial influences on axonal growth in the primary olfactory system. Glia. 1990;3:433–449. doi: 10.1002/glia.440030602. [DOI] [PubMed] [Google Scholar]
- Doucette R. PNS-CNS transition zone of the first cranial nerve. J. Comp. Neurol. 1991;312:451–466. doi: 10.1002/cne.903120311. [DOI] [PubMed] [Google Scholar]
- Franceschini IA, Barnett SC. Low-affinity NGF-receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Dev. Biol. 1996;173:327–343. doi: 10.1006/dbio.1996.0027. [DOI] [PubMed] [Google Scholar]
- Gavazzi I, Kumar RDC, McMahon SB, Cohen J. Growth responses of different subpopulations of adult sensory neurons to neurotrophic factors in vitro. Eur J Neurosci. 1999;11:3405–3414. doi: 10.1046/j.1460-9568.1999.00756.x. [DOI] [PubMed] [Google Scholar]
- Goméz VM, Averill S, King V, Yang Q, Pérez ED, Chacón SC, Ward R, Nieto-Sampedro M, Priestley J, Taylor J. Transplantation of olfactory ensheathing cells fails to promote significant axonal regeneration from dorsal roots into the rat cervical cord. J Neurocyto. 2003;32:53–70. doi: 10.1023/a:1027328331832. [DOI] [PubMed] [Google Scholar]
- Haney CA, Sahenk Z, Li C, Lemmon VP, Roder J, Trapp BD. Heterophilic binding of L1 on unmyelinated sensory axons mediates Schwann cell adhesions and is required for axonal survival. J. Cell Biol. 1999;146:1173–1183. doi: 10.1083/jcb.146.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Chen Y, Lucin KM, McTigue DM. Mice lacking L1 cell adhesion molecule have deficits in locomotion and exhibit enhanced corticospinal tract sprouting following mild contusion injury to the spinal cord. Eur. J. Neurosci. 2006;23:1997–2011. doi: 10.1111/j.1460-9568.2006.04721.x. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Greer CA. Olfactory ensheathing cells promote neurite extension from embryonic olfactory receptor cells in vitro. Glia. 1999;25:99–110. [PubMed] [Google Scholar]
- Kalcheim C, Barde Y-A, Thoenen H, Le Douarin NM. In vivo effect of brain-derived neurotrophic factor on the survival of developing dorsal root ganglion cells. EMBO J. 1987;6:2871–2873. doi: 10.1002/j.1460-2075.1987.tb02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. Neural cell adhesion molecule L1: signaling pathways and growth cone motility. J. Neurosci. Res. 1997;49:1–8. doi: 10.1002/(sici)1097-4547(19970701)49:1<1::aid-jnr1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- Kubasak MD, Hedlund E, Roy RR, Carpenter EM, Edgerton VR, Phelps PE. L1 CAM expression is increased surrounding the lesion site in rats with complete spinal cord transection as neonates. Exp. Neurol. 2005;194:363–375. doi: 10.1016/j.expneurol.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kubasak MD, Jindrich DL, Zhong H, Takeoka A, McFarland KC, Muñoz-Quiles C, Roy RR, Edgerton VR, Ramón-Cueto A, Phelps PE. OEG implantation and step training enhance hindlimb stepping ability in adult spinal transected rats. Brain. 2008;131:264–276. doi: 10.1093/brain/awm267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos A, Franklin RJM, Barnett SC. Olfactory ensheathing cells and Schwann cells differ in their in vitro interactions with astrocytes. Glia. 2000;32:214–225. doi: 10.1002/1098-1136(200012)32:3<214::aid-glia20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Leaver SG, Harvey AR, Plant GW. Adult olfactory ensheathing glia promote the long-distance growth of adult retinal ganglion cell neurites in vitro. Glia. 2006;53:467–476. doi: 10.1002/glia.20311. [DOI] [PubMed] [Google Scholar]
- Lemmon V, Farr KL, Lagenaur C. L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron. 1989;2:1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Carlstedt T, Berthold CH, Raisman G. Interaction of transplanted olfactory-ensheathing cells and host astrocytic processes provides a bridge for axons to regenerate across the dorsal root entry zone. Exp. Neurol. 2004;188:300–308. doi: 10.1016/j.expneurol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- Lipson AC, Widenfalk J, Lindqvist E, Ebendal T, Olson L. Neurotrophic properties of olfactory ensheathing glia. Expt. Neurol. 2003;180:167–171. doi: 10.1016/s0014-4886(02)00058-4. [DOI] [PubMed] [Google Scholar]
- Lopez-Vales R, Fores J, Verdu E, Navarro X. Acute and delayed transplantation of olfactory ensheathing cells promote partial recovery after complete transection of the spinal cord. Neurobiol. Dis. 2006;21:57–68. doi: 10.1016/j.nbd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Lu J, Féron F, Ho SM, Mackay-Sim A, Waite PME. Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic rats. Brain Res. 2001;889:344–357. doi: 10.1016/s0006-8993(00)03235-2. [DOI] [PubMed] [Google Scholar]
- Lu P, Yang H, Culbertson M, Graham L, Roskams AJ, Tuszynski MH. Olfactory ensheathing cells do not exhibit unique migratory or axonal growth-promoting properties after spinal cord injury. J. Neurosci. 2006;26:11120–11130. doi: 10.1523/JNEUROSCI.3264-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Miragall F, Kadmon G, Schachner M. Expression of L1 and N-CAM cell adhesion molecules during development of the mouse olfactory system. Dev. Biol. 1989;135:272–286. doi: 10.1016/0012-1606(89)90179-6. [DOI] [PubMed] [Google Scholar]
- Mohajeri M, H., Bartsch U, van der Putten H, Sansig G, Mucke L, Schachner M. Neurite outgrowth on non-permissive substrates in vitro is enhanced by ectopic expression of the neural adhesion molecule L1 by mouse astrocytes. Eur. J. Neurosci. 1996;8:1085–1097. doi: 10.1111/j.1460-9568.1996.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Navarro X, Valero A, Gudiño G, Forés J, Rodríguez FJ, Verdú E, Pascual R, Cuadras J, Nieto-Sampedro M. Ensheathing glia transplants promote dorsal root regeneration and spinal reflex restitution after multiple lumbar rhizotomy. Annals of Neurology. 1999;45:207–215. doi: 10.1002/1531-8249(199902)45:2<207::aid-ana11>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injuried spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Pastrana E, Moreno-Flores MT, Gurzov EN, Avila J, Wandosell F, Diaz-Nido J. Genes associated with adult axon regeneration promoted by olfactory ensheathing cells: a new role for matrix metalloproteinase 2. J. Neurosci. 2006;26:5347–5359. doi: 10.1523/JNEUROSCI.1111-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E, Moreno-Flores MT, Avila J, Wandosell F, Minichiello L, Diaz-Nido J. BDNF production by olfactory ensheathing cells contributes to axonal regeneration of cultured adult CNS neurons. Neurochem. Intl. 2007;50:491–498. doi: 10.1016/j.neuint.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Qui J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Raisman G, Li Y. Repair of neural pathways by olfactory ensheathing cells. Nat. Rev. Neurosci. 2007;8:312–319. doi: 10.1038/nrn2099. [DOI] [PubMed] [Google Scholar]
- Ramer LM, Richter MW, Roskams AJ, Tetzlaff W, Ramer MS. Peripherally-derived olfactory ensheathing cells do not promote primary afferent regeneration following dorsal root injury. Glia. 2004;47:189–206. doi: 10.1002/glia.20054. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Nieto-Sampedro M. Glial cells from adult rat olfactory bulb: Immunocytochemical properties of pure cultures of ensheathing cells. Neurosci. 1992;47:213–220. doi: 10.1016/0306-4522(92)90134-n. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Nieto-Sampedro M. Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exp. Neurol. 1994;127:232–244. doi: 10.1006/exnr.1994.1099. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J. Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón-Cueto A, Valverde F. Olfactory bulb ensheathing glia: A unique cell type with axonal growth-promoting properties. Glia. 1995;14:163–173. doi: 10.1002/glia.440140302. [DOI] [PubMed] [Google Scholar]
- Riddell JS, Enriquez-Denton M, Toft A, Fairless R, Barnett SC. Olfactory ensheathing cell grafts have minimal influence on regeneration at the dorsal root entry zone following rhizotomy. Glia. 2004;47:150–167. doi: 10.1002/glia.20041. [DOI] [PubMed] [Google Scholar]
- Roonprapunt C, Huang W, Grill R, Friedlander D, Grumet M, Chen S, Schachner M, Young W. Soluble cell adhesion molecule L1-Fc promotes locomotor recovery in rats after spinal cord injury. J. Neurotrauma. 2003;20:871–882. doi: 10.1089/089771503322385809. [DOI] [PubMed] [Google Scholar]
- Runyan SA, Roy R, Zhong H, Phelps PE. L1 CAM expression in the superficial dorsal horn is derived from the dorsal root ganglion. J. Comp. Neurol. 2005;485:267–279. doi: 10.1002/cne.20479. [DOI] [PubMed] [Google Scholar]
- Runyan SA, Roy RR, Zhong H, Phelps PE. L1 cell adhesion molecule is not required for small-diameter primary afferent sprouting after deafferentation. Neurosci. 2007;150:959–969. doi: 10.1016/j.neuroscience.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H-J, Ming G-I, Poo M-M. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Song X-Y, Li F, Zhang F-H, Zhong J-H, Zhou X-F. Peripherally-derived BDNF promotes regeneration of ascending sensory neurons after spinal cord injury. PLoS ONE. 2008;3:e1707. doi: 10.1371/journal.pone.0001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigra RJ, Brighton PC, Jacoby J, Hall S, Wigley CB. Adult rat olfactory nerve ensheathing cells are effective promoters of adult central nervous system neurite outgrowth in coculture. Glia. 1999;25:256–269. doi: 10.1002/(sici)1098-1136(19990201)25:3<256::aid-glia6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Steward O, Sharp K, Selvan G, Hadden A, Hofstadter M, Au E, Roskams J. A re-assessment of the consequences of delayed transplantation of olfactory lamina propria following complete spinal cord transection in rats. Exp. Neurol. 2006;198:483–499. doi: 10.1016/j.expneurol.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejón C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nature Reviews Neuroscience. 2002;3:965–974. doi: 10.1038/nrn988. [DOI] [PubMed] [Google Scholar]
- Webb K, Budko E, Neuberger TJ, Chen S, Schachner M, Tresco PA. Substrate-bound human recombinant L1 selectively promotes neuronal attachment and outgrowth in the presence of astrocytes and fibroblasts. Biomaterials. 2001;22:1017–1028. doi: 10.1016/s0142-9612(00)00353-7. [DOI] [PubMed] [Google Scholar]
- Woodhall E, West AK, Chuah MI. Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Mol. Brain Res. 2001;88:203–213. doi: 10.1016/s0169-328x(01)00044-4. [DOI] [PubMed] [Google Scholar]
- Yip HK, Rich KM, Lampe PA, Johnson EM., Jr. The effects of nerve growth factor and its antiserum on the postnatal development and survival after injury of sensory neurons in rat dorsal root ganglia. J. Neurosci. 1984;4:2986–2992. doi: 10.1523/JNEUROSCI.04-12-02986.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bo X, Schoepfer R, Holtmaat AJDG, Verhaagen J, Emson PC, Lieberman AR, Anderson PN. Growth-associated protein GAP-43 and L1 act synergistically to promote regenerative growth of Purkinje cell axons in vivo. Proc. Natl. Acad. Sci. USA. 2005;102:14883–14888. doi: 10.1073/pnas.0505164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- Zhou X-F, Rush RA. Endogenous brain-derived neurotrophic factor is anterogradely transported in primary sensory neurons. Neurosci. 1996;74:945–951. doi: 10.1016/0306-4522(96)00237-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Primary cultures prepared from the olfactory bulb contain S100-positive OEG (arrows). The nuclei of other cell types (arrowheads) are just visible in the background.
B: After immunopurification and seeding on a myelin substrate, the OEG express S100 (red cells at arrows) and have the characteristic bipolar or multipolar shape. DAPI-labeled (blue) nuclei are only associated with S100-labeled OEG. Scale A–B = 50 μm.