Abstract
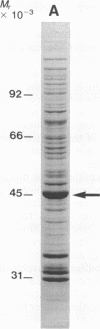
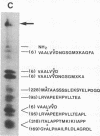
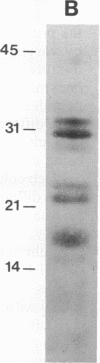
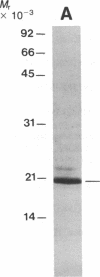
We have developed a method for obtaining partial internal amino acid sequence data from proteins isolated directly from preparative two-dimensional polyacrylamide gels. Proteins from a crude cell homogenate are separated using preparative two-dimensional polyacrylamide gel electrophoresis. Then, the gel is stained with Coomassie blue and the protein spots of interest are cut out. The in situ protein is digested with Staphylococcus aureus V8 protease in a second polyacrylamide gel and the peptides are separated by one-dimensional polyacrylamide gel electrophoresis. The peptides are then electroblotted onto a polyvinylidene difluoride membrane, visualized using Coomassie blue, cut out, and sequenced using an automated gas phase sequencer. Using this method, we have obtained amino acid sequence data for two proteins that are altered after long-term sensitization: actin and Aplysia protein 407. In addition, we have obtained amino acid sequence data for rat protein 425, a protein that appears to be homologous to Aplysia protein 407.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. A., Prahl J. M., DeLuca H. F. Partial amino acid sequence of porcine 1,25-dihydroxyvitamin D3 receptor isolated by immunoaffinity chromatography. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2454–2458. doi: 10.1073/pnas.85.8.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Kennedy T. E., Kandel E. R., Goelet P. A quantitative analysis of 2-D gels identifies proteins in which labeling is increased following long-term sensitization in Aplysia. Neuron. 1988 Jun;1(4):321–328. doi: 10.1016/0896-6273(88)90080-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collins J. H., Elzinga M. The primary structure of actin from rabbit skeletal muscle. Completion and analysis of the amino acid sequence. J Biol Chem. 1975 Aug 10;250(15):5915–5920. [PubMed] [Google Scholar]
- Cooper A. D., Crain W. R., Jr Complete nucleotide sequence of a sea urchin actin gene. Nucleic Acids Res. 1982 Jul 10;10(13):4081–4092. doi: 10.1093/nar/10.13.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau G. R. The primary structure of staphylococcal protease. Can J Biochem. 1978 Jun;56(6):534–544. doi: 10.1139/o78-082. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Eisenberg E., Korn E. D. Characterization of cytoplasmic actin isolated from Acanthamoeba castellanii by a new method. J Biol Chem. 1976 Aug 10;251(15):4778–4786. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lubit B. W., Schwartz J. H. An antiactin antibody that distinguishes between cytoplasmic and skeletal muscle actins. J Cell Biol. 1980 Sep;86(3):891–897. doi: 10.1083/jcb.86.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubit B. W., Schwartz J. H. Immunological characterization of an anti-actin antibody specific for cytoplasmic actins and its use for the immunocytological localization of actin in Aplysia nervous tissue. J Histochem Cytochem. 1983 Jun;31(6):728–736. doi: 10.1177/31.6.6404982. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Chordate muscle actins differ distinctly from invertebrate muscle actins. The evolution of the different vertebrate muscle actins. J Mol Biol. 1984 Nov 5;179(3):391–413. doi: 10.1016/0022-2836(84)90072-x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The amino acid sequence of Physarum actin. Nature. 1978 Dec 14;276(5689):720–721. doi: 10.1038/276720a0. [DOI] [PubMed] [Google Scholar]