Abstract
Long-term memory and synaptic plasticity require changes in gene expression and yet can occur in a synapse-specific manner. mRNA localization and regulated translation at synapses are thus critical for establishing synapse specificity. Using live cell microscopy of photoconvertible fluorescent protein translational reporters, we directly visualized local translation at synapses during long-term facilitation of Aplysia sensory-motor synapses. Translation of the reporter required multiple applications of serotonin, was spatially restricted to stimulated synapses, was transcript- and stimulus-specific, and occurred during long-term facilitation but not during long-term depression of sensory-motor synapses. Translational regulation only occurred in the presence of a chemical synapse and required calcium signaling in the postsynaptic motor neuron. Thus highly regulated local translation occurs at synapses during long-term plasticity and requires trans-synaptic signals.
Long-lasting learning-related synaptic plasticity requires transcription for its persistence (1–3) and yet can occur in a synapse-specific manner (4–7). One mechanism that has been proposed to mediate this spatial restriction of gene expression during neuronal plasticity involves regulated translation of localized mRNAs at stimulated synapses (8–10). Many findings support the existence of local translation at synapses. First, all of the machinery required for translation is present in neuronal processes, including polyribosomes (11, 12), translation factors (13), and a select population of mRNAs (14–18). Second, studies using protein synthesis inhibitors indicate a central role for local translation during long-lasting synaptic plasticity (5, 19, 20). Third, translation of specific transcripts has been visualized in dendrites of cultured neurons following stimulation with brain-derived neurotrophic factor BDNF (21), KCl (22), or following inhibition of sodium channels, NMDA receptor or the mTOR signaling pathway (9, 23, 24). However direct evidence that a specific mRNA undergoes spatially restricted translation at stimulated synapses during transcription-dependent synaptic plasticity has been lacking.
To directly visualize translation at the level of individual synapses during long-term, learning-related neuronal plasticity, we used the Aplysia sensory neuron (SN)-motor neuron (MN) culture system (2). The monosynaptic connection formed between SNs and MNs, a central component of the gill-withdrawal reflex in Aplysia, can be reconstituted in culture, where well-characterized stimuli elicit forms of plasticity that have direct correlates in the behavior of the animal. Specifically, five spaced applications of serotonin (5HT) induce a transcription-dependent long-term facilitation (LTF) of SN-MN synapses that parallels long-term sensitization of the gill-withdrawal reflex (2), while five spaced applications of the neuropeptide FMRFamide induce a transcription-dependent long-term depression (LTD) of SN-MN synapses that parallels long-term habituation of the gill-withdrawal reflex (25, 26). Synapse-specific, transcription-dependent LTF of SN-MN synapses is blocked by perfusion of translational inhibitors to distal SN neurites (5). Furthermore SNs only form chemical synapses with appropriate target MNs (27, 28), allowing local translation to be studied in the presence or absence of synapses.
Sensorin translational reporter
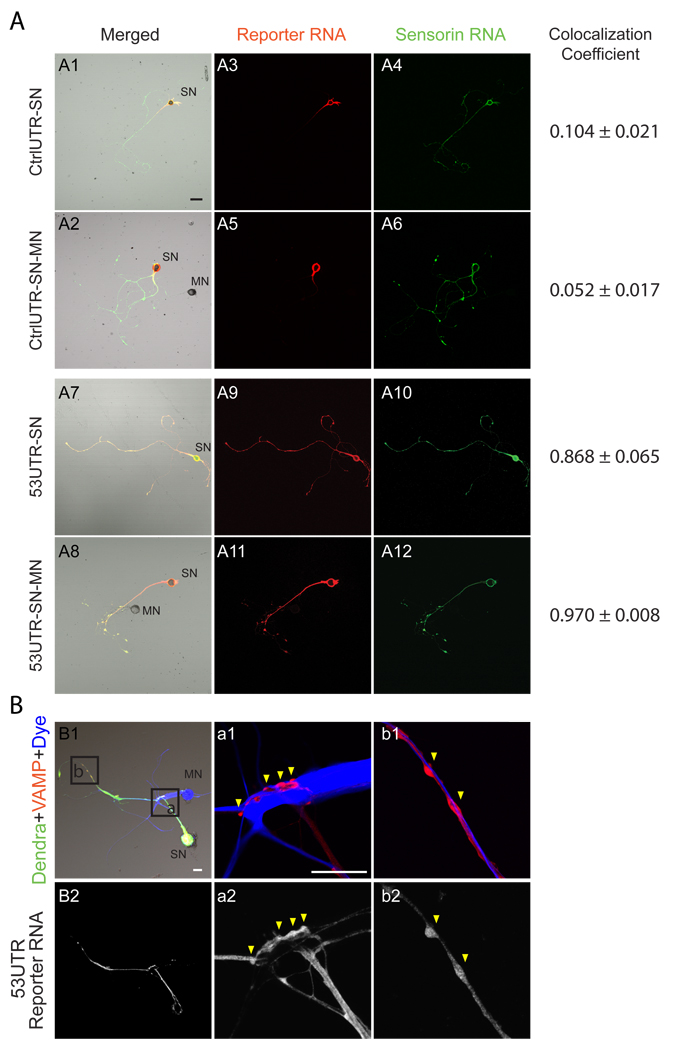
To monitor translation at SN-MN synapses during neuronal plasticity, we generated translational reporters of sensorin, a SN-specific peptide neurotransmitter whose mRNA localizes to distal neuronal processes and concentrates at synapses in SNs paired with MNs (15, 27, 29). Sensorin translation is required for synapse stabilization between SNs and MNs (27, 30) and both sensorin translation and secretion are required for 5HT-induced LTF of Aplysia SN-MN synapses (31). To generate sensorin translational reporters, we fused the 5’ and 3’ UTRs of sensorin to the coding region of the photoconvertible fluorescent protein dendra2 (32). Dendra2 switches fluorescence irreversibly from green to red following UV illumination, allowing newly synthesized proteins (green) to be differentiated from proteins synthesized prior to photoconversion (red). Addition of the 5’ and 3’UTRs of sensorin to the dendra2 coding sequence generated a reporter whose mRNA localization was indistinguishable from endogenous sensorin mRNA (Fig. 1A; S1, S2). Specifically, the reporter mRNA localized to neurites of isolated SNs, and concentrated at SN-MN synapses as indicated by ectopic labeling of presynaptic terminals by expression of VAMP-mCherry and labeling of MNs with Alexa647 (Fig. 1B). Reporters containing either the 3’UTR or the 5’UTR alone revealed that the 3’UTR was sufficient for localization to neurites, while addition of the 5’UTR was required for targeting to synapses (Fig. S2). Thus distinct cis-acting elements mediate neuritic and synaptic mRNA localization.
Figure 1. Translation reporter mRNA colocalizes with endogenous sensorin mRNA at synapses.
(A) Colocalization of 5’3’UTR sensorin reporter mRNA and endogenous sensorin mRNA. Expression vectors encoding the control (CtrlUTR) or sensorin (5’3’UTR) translational reporter were microinjected into DIV2 Aplysia SNs (isolated or paired with MNs). Cultures were fixed 48 hrs later and processed for double-label FISH using DIG-labeled dendra2 riboprobes and biotin-labeled sensorin riboprobes. Representative confocal images of DIC/merged (A1, 2, 7 and 8), dendra reporter mRNA in red (A3, 5, 9 and 11) and endogenous sensorin mRNA in green (A4, 6, 10 and 12). FISH signals are shown in isolated SNs (A1, 3, 4, 7, 9 and 10) and in SNs paired with MNs (A2, 5, 6, 8, 11 and 12). Scale bar: 50µm. Colocalization was quantified as colocalization coefficient (0, no colocalization; 1, perfect colocalization); (B) VAMP-mCherry and reporter were co-expressed in SN; MN was labeled with Alexa647. Synapses are detected as VAMP-mCherry-positive varicosities contacting the MN. B1: Low magnification images of reporter protein (green), VAMP-mCherry (red) and MN (blue) with boxes of higher magnification images in a1 and b1. Cells were fixed after imaging and processed for reporter mRNA FISH in B2, a2 and b2. Scale bar: 50µm.
To visualize local translation of the reporter during LTF induced by bath application of 5X5HT, we expressed the reporter in SNs paired with MNs, removed the SN soma, and photoconverted dendra2 (Fig. S1, S3, SOM). Newly synthesized protein (green) had to result from local translation in the neurite because the soma was no longer present. While 5x5HT induces transcription-dependent LTF, 24 hr (but not 48 hr) facilitation occurs in a translation-dependent manner in the absence of a SN soma, indicating that the initial events involved in persistent LTF can be monitored in SNs lacking cell bodies (33). We imaged red and green channels before the first application and immediately after the fifth application of 5HT. Control cultures were stimulated with five applications of vehicle (5X artificial seawater, ASW), or were untreated. Very little green signal but robust red signal was detected following UV illumination, indicating efficient photoconversion (Fig. 2A). After the fifth pulse of 5HT, green dendra2 signal increased at multiple sites within the neurite, and this was completely blocked by the translational inhibitor anisomycin (10 µM, Fig. 2B, S4). Modest increases in green dendra2 fluorescence were observed in control cultures following application of 5XASW, which were also blocked by anisomycin (10 µM, Fig. 2B, S4). This modest increase in green dendra2 fluorescence represents basal translation because it was also observed in untreated cultures (Fig. 2B, S4). Immediately following imaging, we fixed the cells and performed FISH for the reporter mRNA. 143 of 147 (97%) of sites with new translation contained concentrated reporter mRNA. Thus the subcellular localization of new translation correlated with the subcellular localization of reporter mRNA with remarkable accuracy. Because the reporter mRNA specifically concentrated in VAMP-positive synapses (Fig. 1, S2, (27)), we conclude that the reporter was translated at synapses.
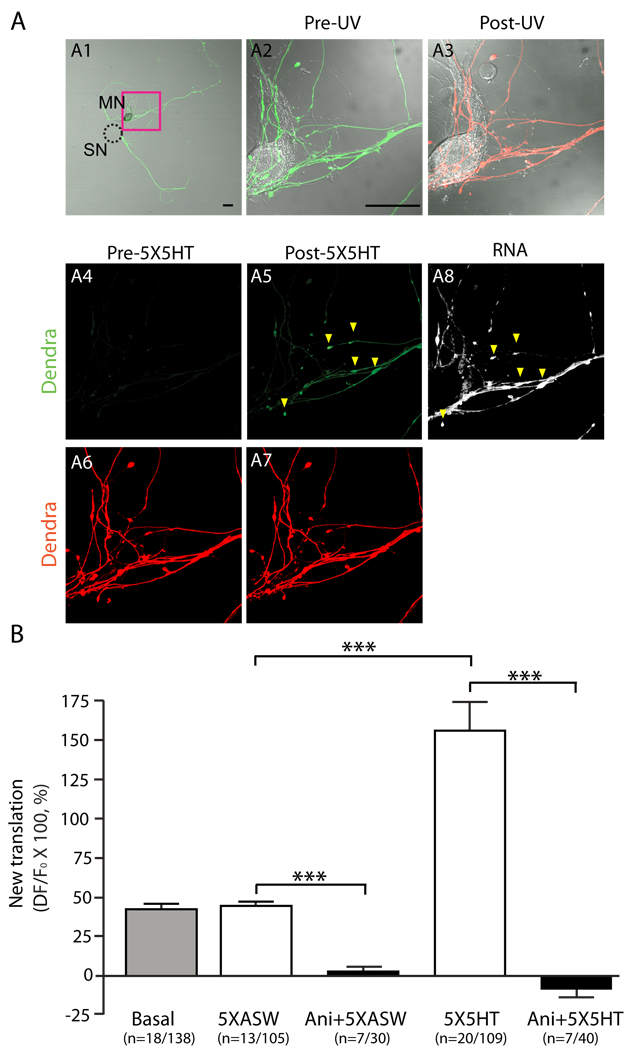
Figure 2. Bath application of 5X5HT stimulates translation of reporter mRNA at synapses.
Sensorin translational reporter was expressed in Aplysia SNs co-cultured with MNs, the SN soma was removed and dendra2 was UV photoconverted from green to red throughout the neuronal arbor. (A1) Low magnification image of dendra-reporter SN-MN co-culture. The dashed circle outlines the removed soma. (A2 and A3) High magnification DIC/merged image of area in the red box in A1, before (A2) and after (A3) UV photoconversion. (A4 and A5) Green dendra2 signal following photoconversion, before (A4) and after (A5) serotonin stimulation (5X5HT). Lack of green dendra2 indicates efficient photoconversion; new green dendra2 reveals significant new translation post 5x5HT. (A6 and A7) Photoconverted red dendra2 signal before (A6) and after (A7) 5x5HT shows SN volume. A8: Cells were fixed at the end of the experiment and processed for FISH with antisense dendra2 riboprobes. Arrowheads in A5 and A8 point to the sites of new (green) dendra2 protein synthesis colocalizing with reporter mRNA clusters. Scale bar: 50µm. See fig. S4 for Basal, 5XASW, Ani+5X5HT, Ani+5XASW results. (B) Quantification of new translation as ΔF/F, ***p<0.001, ANOVA and Bonferroni multiple comparison test.
We quantified new translation as ΔF/F (x100 = % change), normalized to the red dendra2 signal (which serves as a volume control, SOM, Fig 2B). When stimuli were applied in the presence of anisomycin, there was no significant change in green signal, and thus no translation (2.3±3.4% for Ani+5XASW; −8.9±4.9% for Ani+5X5HT). In untreated or vehicle-treated cultures, we observed a 41.2±3.7% and 43.5±3.6% increase in translation. Following stimulation with 5X5HT, we observed a 155.9±18.7% increase in translation. Thus a basal level of sensorin translation occurs in neurites, which is significantly increased following stimuli that induce LTF (***p<0.001, ANOVA with Bonferroni’s multiple comparison test).
Spatial specificity of translation
To determine the spatial specificity of stimulus-induced local translation, we perfused 5X5HT locally onto a subset of synapses (Fig. 3, S5 and S6). This protocol produces synapse-specific LTF of SN-MN synapses (5, 7). Images were acquired from both stimulated (perfused) and unstimulated (non-perfused) sites within the same SN at the beginning and end of local perfusion. In vehicle-treated cultures, we detected modest increases in newly synthesized green dendra2 at both perfused and non-perfused synapses (27.4±5.6% and 20.2±8.2%, respectively). However, when 5X5HT was perfused onto a subset of synapses, robust increases in new green dendra2 signal were detected at stimulated sites (54.7±10.9%), with only modest increases at non-perfused sites (16.6±4.9%). For each neuron, we calculated the ratio of the ΔF/F at the perfused site to the average ΔF/F at non-perfused sites. In vehicle-treated cultures, (Fig. 3D, S7), this ratio was 1.0±0.1, indicating no significant difference between perfused and non-perfused sites. In contrast, in cultures locally perfused with 5X5HT (Fig. 3A), the ratio was 1.3±0.1, indicating a significant increase in new translation at perfused as compared to non-perfused sites (***p<0.001, paired Student’s t-test). Thus local serotoninergic stimulation induced spatially restricted translation within an individual neuron. FISH analysis revealed equivalent reporter mRNA at all synapses under all conditions (Fig. 3, S7, S10). No significant difference in basal translation was observed at non-perfused synapses between 5XASW and 5X5HT groups (fig. S10). Finally, 5x5HT did not induce significant structural changes between the initial and final imaging times (fig. S6).
Figure 3. Synapse-, stimulus- and transcript-specific translation.
Sensorin translational reporter was expressed in SNs paired with MNs, SN soma was removed and dendra2 was UV photoconverted from green to red. Stimuli were locally applied to subsets of SN-MN synapses using perfusion electrodes (see fig S3). (A, B, C) Low magnification image of dendra2-reporter expressing coculture. Dashed circle indicates removed SN soma; blue arrowheads indicate direction of perfusion; black squares denote regions imaged before and after local perfusion; white squares denote imaged non-perfused regions. (A-C panels 1–4) Pseudocolored images of green dendra2 signal after photoconversion and before (pre) and after (post) local perfusion. (A-C panels 5 and 6) reporter mRNA FISH images. Arrowheads in A2 and A5 point to new translation colocalizing with reporter mRNA. A: 5’3’UTR reporter, local perfusion of 5x5HT. B: 5’3’UTR reporter, local perfusion of 5xFMRFa. C: 3’UTR, local perfusion of 5x5HT. See fig S6–9 for images of pre-UV green and post-UV red photoconverted dendra2 signal, 5XASW controls, and 1X5HT stimulation. Scale bar: 50µm; (D) Quantification of translation as ratio of ΔF/F at perfused compared to non-perfused sites reveals translation occurs only with 5x5HT. ***p<0.0001, Wilcoxon-Mann Whitney test (also see fig S10 for group data).
Stimulus specificity
Five spaced applications of the peptide neurotransmitter FMRFamide produce transcription-dependent LTD of Aplysia SN-MN synapses, and local perfusion of 5xFMRFamide has been shown to produce synapse-specific LTD (34). To ask whether the 5HT-induced translation of the reporter was stimulus-specific, we locally perfused 5xFMRFamide and monitored reporter translation. The ratio of translation between 5xFMRFamide perfused and non-perfused sites was 1.0±0.1, indicating that FMRFamide did not stimulate sensorin translation (Fig. 3C), consistent with previous studies (35). In addition, 1X5HT, which induces translation-independent STF (short-term facilitation), did not stimulate translation (Fig. 3D, S8, S10). Basal levels of translation at non-perfused synapses, and concentrations of reporter RNA were equivalent to those in cultures locally perfused with 5x5HT or 5xASW (Fig. 3, S7, S10). Thus, translational regulation of the sensorin reporter is stimulus-specific.
Transcript specificity
Global translation in sensory neurites increases by three-fold in response to 5HT (5). To ask whether 5HT stimulates global increases in translation in neurites, or whether it regulates translation of a specific subset of transcripts, we constructed a reporter containing the 3’UTR of sensorin (and 5’UTR of SV40) which localized to distal sensory neurites and is present but not concentrated in synapses (Fig. S2). We expressed this reporter in SNs making synapses with MNs, and asked whether local perfusion of 5x5HT stimulated its translation. We did not detect any increase in translation of this reporter at synapses stimulated with 5X5HT (ratio of ΔF/F at perfused versus non-perfused sites = 1.0 ± 0.1) (Fig 3).
Importantly, the 3’UTR reporter was present in SN processes and translation was quantified at sites containing equivalent amounts of 3’UTR or 5’3’UTR reporter (fig. S10). Thus, 5HT does not regulate the translation of all process-localized mRNAs.
Translational regulation requires a synapse
We next investigated the role of synaptic connectivity in translational regulation by 5HT. Specifically, we asked whether local perfusion of 5x5HT stimulated translation of the reporter in isolated SNs (which do not form chemical synapses) or in SNs paired with a non-target MN, with which it does not form chemical synapses. Local perfusion of 5x5HT did not increase translation of the reporter in isolated SNs (ratio of perfused to non-perfused = 1.0 ± 0.1), despite abundant reporter mRNA (Fig. 4, S11). Notably, basal translation of the reporter in isolated SN was not significantly different from basal translation in SNs paired with MNs (Fig. S12 and S13). To further test the requirement for chemical synapses, we cultured SNs with a non-target MN, L11, with which SNs fasciculate but do not make chemical synapses (27, 28). Local perfusion of 5x5HT did not increase reporter translation (ratio of perfused to non-perfused sites = 1.0 ± 0.1, Fig. 4). Reporter mRNA was localized at perfused sites (Fig 4, and fig. S13) and basal translation of the reporter did not differ from that in SNs paired with target MNs (Fig. S12 and S13). Thus, 5HT stimulates sensorin reporter translation only in the context of a chemical synapse.
Figure 4. Local stimulation with 5X5HT does not stimulate translation of sensorin reporter in isolated SNs or in SNs paired with non-target MNs.
Sensorin translational reporter was expressed in (A) isolated Aplysia SNs,or (B) SNs cocultured with nontarget L11 MNs. Local perfusion of 5X5HT was as described in Fig 3. (A, B) Low magnification image of dendra2-reporter expressing SNs. Dashed circle indicates removed soma; blue arrowheads indicate direction of perfusion; black squares denote regions imaged before and after local perfusion; white squares denote imaged non-perfused regions. (A and B panels 1–4) Pseudocolored images of green dendra2 signal after photoconversion and before (pre) and after (post) local perfusion. (A and B panels 5 and 6) reporter mRNA FISH. See fig S9 for images of red photoconverted dendra2 signal. Scale bar: 50µm; (C) Quantification of translation as ratio of ΔF/F at perfused compared to non-perfused sites reveals no increase in translation with either 5X5HT or 5XASW (Wilcoxon-Mann-Whitney test; also see group data and RNA intensity quantification in S13).
Translational regulation requires post-synaptic calcium
To begin to explore the nature of the trans-synaptic signal provided by the MN, we asked whether it involved calcium signaling in the postsynaptic compartment. To do so, we microinjected the membrane-impermeable calcium chelator BAPTA (50mM) into the MN and, 1–2 hrs later, monitored reporter translation induced by 5X5HT in the SN. BAPTA injection blocks LTF of SN-MN synapses as well as increases in sensorin immunoreactivity in the SN, without affecting basal synaptic transmission (36). Calcium imaging (with Fluo-4) confirmed that BAPTA injection blocked depolarization-induced increases in intracellular calcium in the MN; FISH analysis revealed that it did not alter endogenous sensorin or reporter mRNA localization (Fig. S14). BAPTA injection, however, completely blocked 5HT-induced translation of the reporter in the SN (ratio of ΔF/F at perfused versus non-perfused sites = 1.0 ±0.0, Fig 5, S15). Vehicle injection into the MN did not inhibit translation in the SN (ratio of ΔF/F at perfused versus non-perfused sites = 1.2 ± 0.0, Fig 5, S16). Thus, a calcium-dependent trans-synaptic signal is required for translational regulation of the reporter in the presynaptic neuron by 5HT.
Figure 5. Calcium signaling in motor neuron is required for 5HT-induced translation of reporter in sensory neuron.
Sensorin translational reporter was expressed in Aplysia SNs co-cultured with target LFS MNs. The SN soma was removed, either vehicle (A) or BAPTA (50mM, B) was microinjected into the MN, dendra2 was photoconverted, and 5X5HT was locally perfused. (A, B) Low magnification image of dendra2-reporter expressing coculture. Dashed circle indicates removed soma; blue arrowheads indicate direction of perfusion; black squares denote regions imaged before and after local perfusion; white squares denote imaged non-perfused regions. (A and B panels 1–4) Pseudocolored images of green dendra2 signal after photoconversion and before (pre) and after (post) local perfusion. (A and B panels 5 and 6) Reporter mRNA FISH images. See fig S13 for images of pre-UV and photoconverted dendra2 signal. Scale bar: 50µm; (D) Quantification of translation as ratio of ΔF/F at perfused compared to non-perfused sites reveals that BAPTA injection into the MN blocks 5HT-induced translation in the SN (***p<0.0005, t-test).
Our results provide direct evidence that local translation occurs at synapses in response to stimuli that induce transcription-dependent, learning-related synaptic plasticity. Spatially restricted translation and release of sensorin may function to spatially restrict the growth and/or stabilization of new synaptic growth, and to thereby promote LTF precisely at the stimulated sites (5, 27, 31). Our results provide a number of additional insights into the regulation of local translation in neurons. First, translational regulation of the reporter is stimulus specific, occurring during 5HT-induced LTF but not during FMRFamide-induced LTD. Second, 5HT does not regulate translation of all localized transcripts (because translation of the 3’UTR reporter is not stimulated), indicating that translational regulation is transcript-specific. Finally, stimulus-induced translation of the reporter requires calcium signaling in the postsynaptic MN, suggesting that a trans-synaptic retrograde signal is required for the regulation of local translation during neuronal plasticity.
Supplementary Material
Footnotes
References and Notes
- 1.Alberini CM. J Exp Biol. 1999;202:2887. doi: 10.1242/jeb.202.21.2887. [DOI] [PubMed] [Google Scholar]
- 2.Kandel ER. Science. 2001;294:1030. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 3.Greer PL, Greenberg ME. Neuron. 2008;59:846. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen PV, Abel T, Kandel ER. Science. 1994;265:1104. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 5.Martin KC, et al. Cell. 1997;91:927. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- 6.Frey U, Morris RG. Nature. 1997;385:533. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 7.Casadio A, et al. Cell. 1999;99:221. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- 8.Martin KC. Curr Opin Neurobiol. 2004;14:305. doi: 10.1016/j.conb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Sutton MA, Schuman EM. Cell. 2006;127:49. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Govindarajan A, Kelleher RJ, Tonegawa S. Nat Rev Neurosci. 2006;7:575. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- 11.Steward O, Levy WB. J Neurosci. 1982;2:284. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostroff LE, Fiala JC, Allwardt B, Harris KM. Neuron. 2002;35:535. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- 13.Tang SJ, et al. Proc Natl Acad Sci U S A. 2002;99:467. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberwine J, Belt B, Kacharmina JE, Miyashiro K. Neurochem Res. 2002;27:1065. doi: 10.1023/a:1020956805307. [DOI] [PubMed] [Google Scholar]
- 15.Moccia R, et al. J Neurosci. 2003;23:9409. doi: 10.1523/JNEUROSCI.23-28-09409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong J, Zhang T, Bloch LM. BMC Neurosci. 2006;7:17. doi: 10.1186/1471-2202-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poon MM, Choi SH, Jamieson CA, Geschwind DH, Martin KC. J Neurosci. 2006;26:13390. doi: 10.1523/JNEUROSCI.3432-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki T, Tian QB, Kuromitsu J, Kawai T, Endo S. Neurosci Res. 2007;57:61. doi: 10.1016/j.neures.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Kang H, Schuman EM. Science. 1996;273:1402. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 20.Huber KM, Roder JC, Bear MF. J Neurophysiol. 2001;86:321. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- 21.Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Neuron. 2001;30:489. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 22.Job C, Eberwine J. Nat Rev Neurosci. 2001;2:889. doi: 10.1038/35104069. [DOI] [PubMed] [Google Scholar]
- 23.Ju W, et al. Nat Neurosci. 2004;7:244. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- 24.Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Science. 2006;314:144. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- 25.Castellucci VF, Schacher S. Prog Brain Res. 1990;86:105. doi: 10.1016/s0079-6123(08)63170-2. [DOI] [PubMed] [Google Scholar]
- 26.Mackey SL, et al. Proc Natl Acad Sci U S A. 1987;84:8730. doi: 10.1073/pnas.84.23.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyles V, Zhao Y, Martin KC. Neuron. 2006;49:349. doi: 10.1016/j.neuron.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 28.Glanzman DL, Kandel ER, Schacher S. Neuron. 1989;3:441. doi: 10.1016/0896-6273(89)90203-1. [DOI] [PubMed] [Google Scholar]
- 29.Brunet JF, Shapiro E, Foster SA, Kandel ER, Iino Y. Science. 1991;252:856. doi: 10.1126/science.1840700. [DOI] [PubMed] [Google Scholar]
- 30.Hu JY, Chen Y, Schacher S. J Neurosci. 2007;27:11712. doi: 10.1523/JNEUROSCI.3305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu JY, Wu F, Schacher S. J Neurosci. 2006;26:1026. doi: 10.1523/JNEUROSCI.4258-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurskaya NG, et al. Nat Biotechnol. 2006;24:461. doi: 10.1038/nbt1191. [DOI] [PubMed] [Google Scholar]
- 33.Liu K, Hu JY, Wang D, Schacher S. J Neurobiol. 2003;56:275. doi: 10.1002/neu.10242. [DOI] [PubMed] [Google Scholar]
- 34.Guan Z, et al. Cell. 2002;111:483. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- 35.Santarelli L, Montarolo P, Schacher S. J Neurobiol. 1996;31:297. doi: 10.1002/(SICI)1097-4695(199611)31:3<297::AID-NEU3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Cai D, Chen S, Glanzman DL. Curr Biol. 2008;18:920. doi: 10.1016/j.cub.2008.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.We thank S. Braslow and K. Cadenas for assistance with image analysis, R. Grambo for assistance with figures, D. Black, C. Heusner, E. Meer and L. Zipursky for critical reading of the manuscript, G. Weinmaster and Martin lab members for helpful discussions. This work was supported by NIH grant NS045324, a W. M. Keck Foundation Young Scholar Award, and Eleanor Leslie Term Chair from the UCLA Brain Research Institute (to KCM), Canadian Institute of Health Research grant MT-15121 (to WSS), and a fellowship from the Nakajima Foundation (to SKM).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.