Abstract
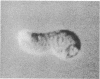
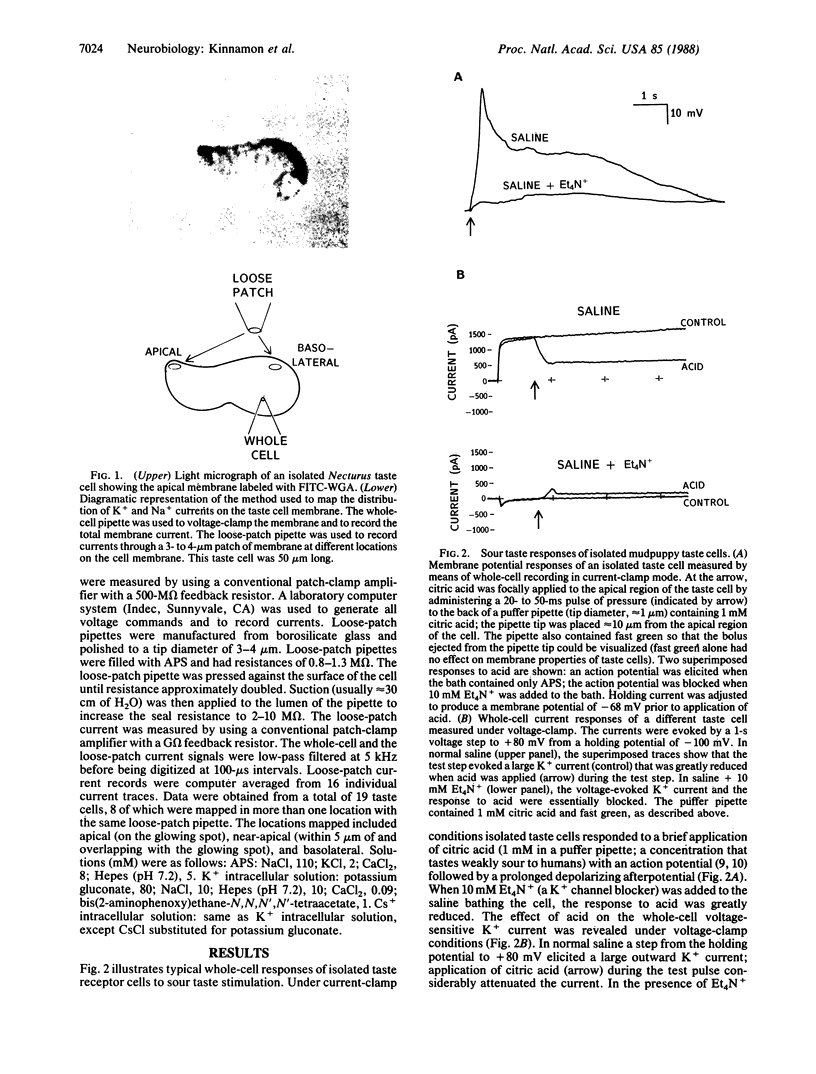
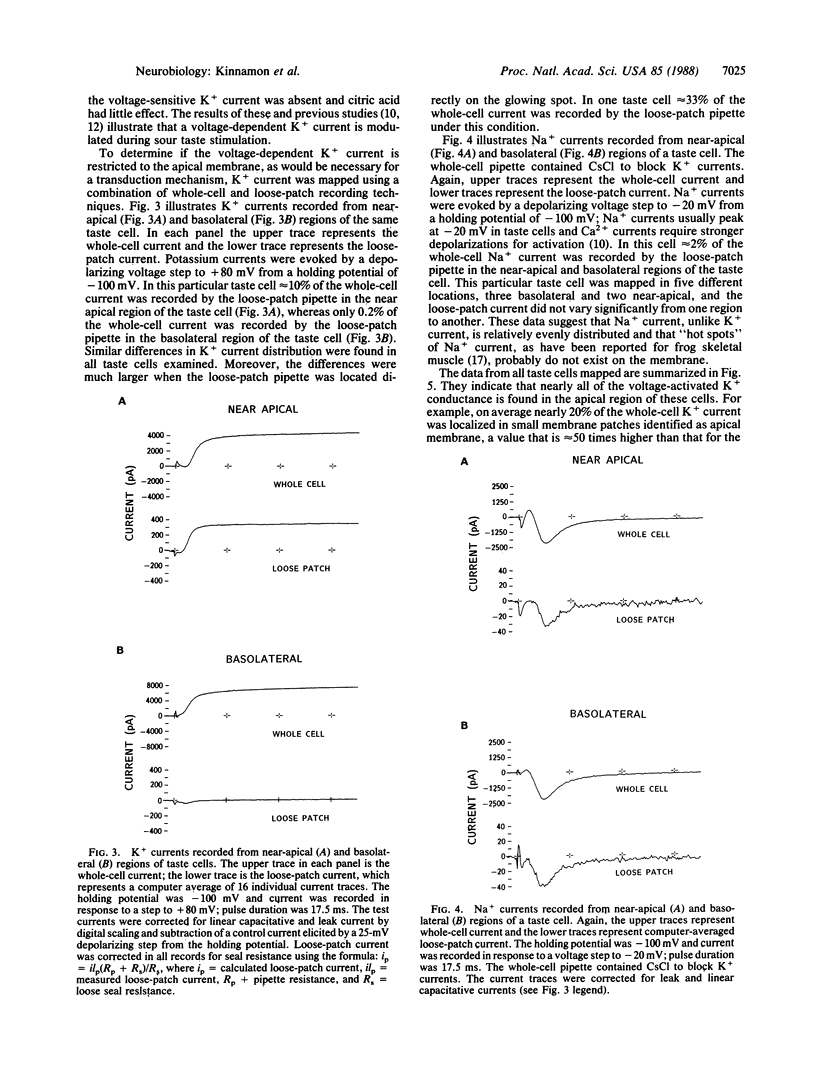
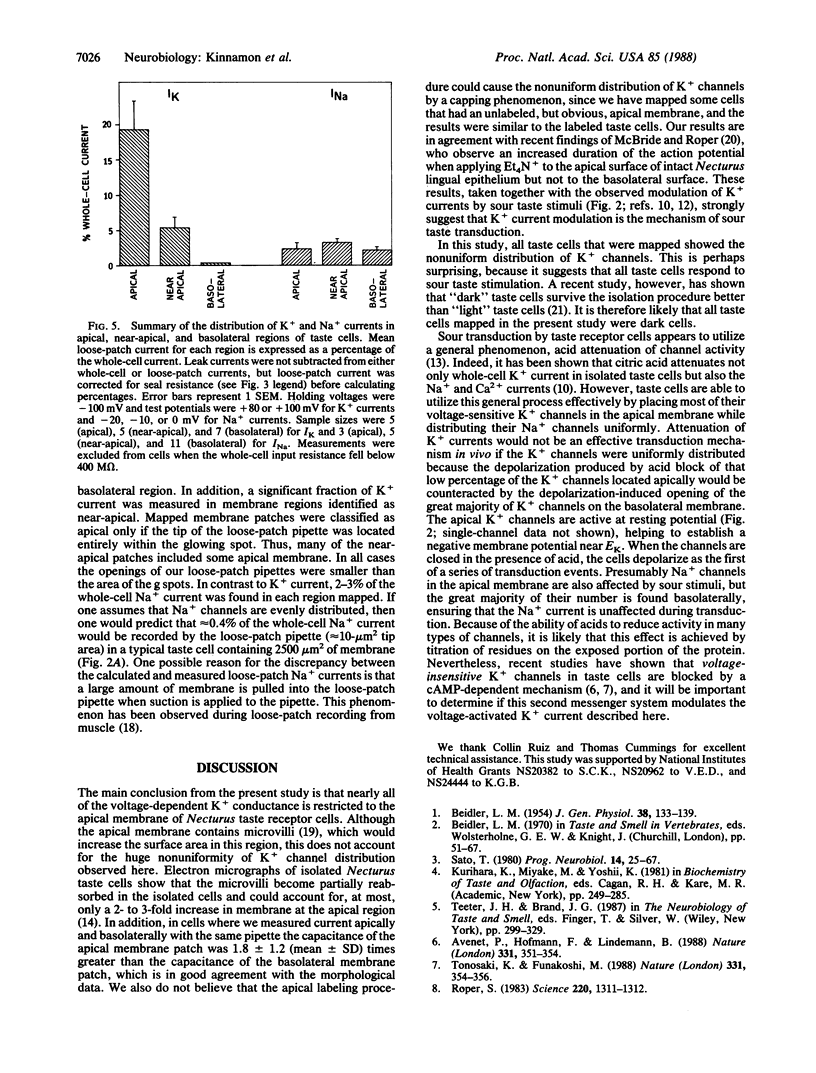
Previous studies have shown that mudpuppy taste receptor cells respond to sour taste stimuli (weak acids) with depolarizing receptor potentials or action potentials that are blocked by the K+ channel blocker tetraethylammonium. Voltage-clamp recordings from isolated taste cells indicated that taste receptor cells exhibit a variety of voltage-dependent conductances and that acids reduce a voltage-dependent K+ current. Since taste stimuli are restricted to the apical surface of the intact tongue, only 1-2% of the taste receptor cell surface is exposed to chemical stimuli. Thus, modification of a K+ conductance would be an effective transduction mechanism in receptor cells only if the majority of K+ channels were located on the apical membrane. We have used a combination of "loose-patch" and whole-cell recording methods to map the distribution of voltage-sensitive K+ and Na+ channels on dissociated Necturus maculosus taste cells. We report here that the K+ conductance is approximately equal to 50-fold greater on apical membrane than on basolateral membrane, whereas the Na+ conductance is distributed evenly. The marked nonuniformity of the voltage-sensitive K+ conductance, together with the block of this conductance by sour stimuli, indicates that K+ current modulation is the mechanism of sour taste transduction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Stanfield P. R., Stühmer W. Lateral distribution of sodium and potassium channels in frog skeletal muscle: measurements with a patch-clamp technique. J Physiol. 1983 Mar;336:261–284. doi: 10.1113/jphysiol.1983.sp014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenet P., Hofmann F., Lindemann B. Transduction in taste receptor cells requires cAMP-dependent protein kinase. Nature. 1988 Jan 28;331(6154):351–354. doi: 10.1038/331351a0. [DOI] [PubMed] [Google Scholar]
- BEIDLER L. M. A theory of taste stimulation. J Gen Physiol. 1954 Nov 20;38(2):133–139. doi: 10.1085/jgp.38.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings T. A., Delay R. J., Roper S. D. Ultrastructure of apical specializations of taste cells in the mudpuppy, Necturus maculosus. J Comp Neurol. 1987 Jul 22;261(4):604–615. doi: 10.1002/cne.902610411. [DOI] [PubMed] [Google Scholar]
- Kinnamon S. C., Roper S. D. Membrane properties of isolated mudpuppy taste cells. J Gen Physiol. 1988 Mar;91(3):351–371. doi: 10.1085/jgp.91.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnamon S. C., Roper S. D. Passive and active membrane properties of mudpuppy taste receptor cells. J Physiol. 1987 Feb;383:601–614. doi: 10.1113/jphysiol.1987.sp016431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper S. Regenerative impulses in taste cells. Science. 1983 Jun 17;220(4603):1311–1312. doi: 10.1126/science.6857254. [DOI] [PubMed] [Google Scholar]
- Sato T. Recent advances in the physiology of taste cells. Prog Neurobiol. 1980;14(1):25–67. doi: 10.1016/0301-0082(80)90003-9. [DOI] [PubMed] [Google Scholar]
- Tonosaki K., Funakoshi M. Cyclic nucleotides may mediate taste transduction. Nature. 1988 Jan 28;331(6154):354–356. doi: 10.1038/331354a0. [DOI] [PubMed] [Google Scholar]