Abstract
The primary function of the lung is to facilitate the transfer of molecular oxygen (O2; dioxygen) from the atmosphere to the systemic circulation. In addition to its essential role in aerobic metabolism, O2 serves as the physiologic terminal acceptor of electron transfer catalyzed by the NADPH oxidase (NOX) family of oxidoreductases. The evolution of the lungs and circulatory systems in vertebrates was accompanied by increasing diversification of NOX family enzymes, suggesting adaptive roles for NOX-derived reactive oxygen species in normal physiology. However, this adaptation may paradoxically carry detrimental consequences in the setting of overwhelming/persistent environmental stressors, both infectious and noninfectious, and during the process of aging. Here, we review current understanding of NOX enzymes in normal lung physiology and their pathophysiologic roles in a number of pulmonary diseases, including lung infections, acute lung injury, pulmonary arterial hypertension, obstructive lung disorders, fibrotic lung disease, and lung cancer. Antioxid. Redox Signal. 11, 2505–2516.
Introduction
The respiratory system brings the ambient air that we breathe into close proximity with the systemic circulation. This allows the lungs to accomplish their primary function in the exchange of carbon dioxide for oxygen (O2), essential for the maintenance of aerobic metabolism. The average adult human breathes in 9,000 to 15,000 L of air (6‱10 L/min) daily. This exposes the lungs to a variety of potentially injurious environmental agents, both infectious and noninfectious. The normal host response is to eradicate putative pathogens/injurious agents and to repair the damage caused directly by the agent or by the associated immune/inflammatory response. Human pulmonary diseases result, in large part, when the host response to the attempted eradication of the offending agent is dysregulated or when the repair/regenerative responses to ensuing tissue injury are impaired. A number of host factors, including genetic/epigenetic factors and age, may influence the susceptibility to pulmonary disease and the clinical phenotype (e.g., severity, progression) of the associated clinical syndrome.
NADPH oxidase (NOX) enzymes emerged during the evolutionary transition from unicellular to multicellular organisms, and the number of NOX/Dual oxidase (DUOX) family enzymes have increased to seven in mammals (NOX1 to 5 and DUOX1 to 2) (11, 51, 116). NOX enzymes catalyze the reduction of molecular oxygen (O2) to superoxide (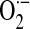 ), the typical primary product of the reaction (10, 57). Depending on the microenvironment or cellular compartment in which it is produced, spontaneous or superoxide dismutase (SOD)-catalyzed reduction of
), the typical primary product of the reaction (10, 57). Depending on the microenvironment or cellular compartment in which it is produced, spontaneous or superoxide dismutase (SOD)-catalyzed reduction of 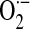 to hydrogen peroxide (H2O2) may occur in association with the generation of other reactive oxygen species (ROS). ROS function as signaling molecules and regulators of cell function when they are generated in a compartmentalized and regulated manner (126). Here, we examine the roles of these ROS-generating enzymes in cellular physiology of the lung and in the pathogenesis of pulmonary diseases (Fig. 1).
to hydrogen peroxide (H2O2) may occur in association with the generation of other reactive oxygen species (ROS). ROS function as signaling molecules and regulators of cell function when they are generated in a compartmentalized and regulated manner (126). Here, we examine the roles of these ROS-generating enzymes in cellular physiology of the lung and in the pathogenesis of pulmonary diseases (Fig. 1).
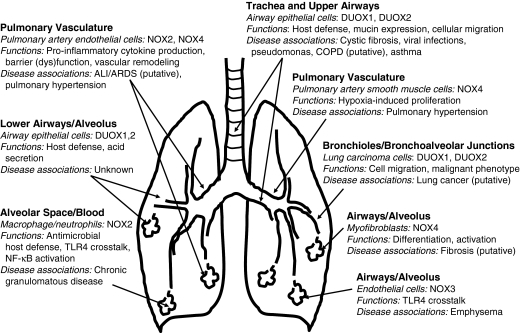
FIG. 1.
NOX Enzymes in lung cellular physiology and pulmonary disease. NOX/DUOX isoforms are expressed in a number of lung cell types, including airway/alveolar epithelial, endothelial, and mesenchymal cells, extending from the proximal trachea and large airways to terminal bronchioles and alveoli. Proposed functions of NOX isoforms in various cell types and their putative roles in diverse lung diseases are indicated. Refer to text for related references and details.
NOX Enzymes in Pulmonary Infectious Disease
The lungs are well equipped to defend against myriad microbial pathogens that may be transmitted to the lungs from inhaled air, the systemic circulation, oropharyngeal aspiration, or contiguous spread from surrounding tissues. Upper airway defense mechanisms (e.g., mucociliary clearance) work in concert with lower airway defenses (e.g., alveolar macrophages) to combat microbial pathogens. The role of the phagocytic “respiratory burst oxidase,” NOX2, in innate immune responses is well established and suggests an archaic host defense mechanism that is conserved across multiple species (25, 42, 99). Evidence demonstrates that NOX2 mediates its antimicrobial effect, at least in part, by facilitating compartmentalized protease activation within phagosomes through a transmembrane ion flux that is coupled to endosomal 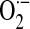 release (101).
release (101).
Chronic granulomatous disease (CGD), characterized by susceptibility to recurrent pyogenic infections, is the prototypical example of a human disease associated with inherited loss of function of genes encoding components of the NOX2 enzymatic complex. Initially characterized as a fatal granulomatous disease of childhood, the clinical course of CGD is marked by recurrent, suppurative infections and granuloma formation (15, 106). Although CGD can be associated with a defect in any of the subunits of the multicomponent NOX2 enzyme complex, the X-linked gene mutation in the catalytic NOX2 subunit, identified and positionally cloned in 1986, represents the most common site of mutations (102). Novel mutations involving the α (p22phox) and β (gp91phox) transmembrane subunits of NOX2 have been reported (13, 26, 27, 63, 64, 86, 121). Pulmonary infections remain a hallmark of the disease and the leading cause of morbidity. A national CGD registry report in 2001 noted a shift in the most common infecting organisms away from staphylococci and enteric bacteria to other pathogens, with Aspergillus pneumonia and Burkholderia cepacia infections representing the leading causes of death (49). Antimicrobial prophylaxis, interferon-γ administration, and granulocyte infusions remain the current mainstay of treatment for CGD.
A murine model of X-linked CGD with targeted deletion of the NOX2 gene encoding the 91-kDa cytochrome b subunit has been described (99). These mice display the characteristic susceptibility to Staphylococcus and Aspergillus infections and, additionally, develop a persistent inflammatory response associated with high levels of inflammatory cytokines after challenge with sterilized Aspergillus hyphae (81). Studies in mice deficient in the p47phox subunit indicate a role for NOX isoforms, requiring this subunit for enzyme activation in host defense against Pseudomonas pneumonia (103) and M. tuberculosis pneumonia (21); a potential role of NOX-derived ROS in suppressing neutrophilic inflammation also was suggested (21). Similar findings of a putative “antiinflammatory” role for p47phox/NOX2 are reported in mice challenged with intraperitoneal live Escherichia coli to induce sepsis (35), and in murine models of pneumococcal pneumonia (72), influenza pneumonia (109), and disseminated Cryptococcus neoformans infection (110).
In addition to immune defects, mice harboring mutations of p22phox develop vestibular dysfunction associated with otoconial malformation (85). A deficiency in neutrophil cytosolic factor-1, required for activation of NOX2, appears to protect from virus-induced acute lung injury (44). Together, these studies demonstrate the contextual role of NOX2 (and potentially other p47phox- and p22phox-requiring NOX enzymes) in modulating host inflammatory responses, in addition to its recognized role in antimicrobial killing.
Limited evidence supports the possibility that other NOX isoforms may also participate in host innate immune responses. Studies in gastric mucosal cells suggest a role for NOX1 in antimicrobial host defense (52, 122), although a similar role for NOX1 in the lung has yet to be demonstrated. More recently, the identification of DUOX1 and DUOX2 in salivary, tracheal, and bronchial epithelium has broadened the role of NOX homologues in host defense as “tissue-specific” generators of ROS (31, 36, 38, 82, 105, 108). DUOX enzymes and their roles in host defense of the upper airways are discussed elsewhere in this issue.
NOX Enzymes in Acute Lung Injury
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) represent clinical syndromes of varying severity and diverse causes that are first seen with a set of defined clinical–physiologic–radiologic criteria (141). The most common risk factor associated with ALI/ARDS is sepsis; other associated conditions include trauma, aspiration, pneumonia, acute pancreatitis, and transfusion of blood products (141). A unifying pathophysiologic feature involves disruption of the alveolar–capillary membrane, resulting in diffuse bilateral infiltrates on chest radiographs and arterial hypoxemia that is typically refractory to high concentrations of O2 administration (141). The generation of ROS by nonenzymatic and enzymatic mechanisms, including activation of NOX enzymes, contributes to the pathobiology of ALI/ARDS (41).
NOX-dependent ROS generation by neutrophils appears to play a major role in lung injury secondary to sepsis. Lipopolysaccharide (LPS), a constituent of the outer membrane of gram-negative bacteria, primes activation of the phagocytic NOX2 enzyme. In guinea pigs, LPS-stimulated ROS generation by neutrophils and ALI were significantly reduced with apocynin, a putative inhibitor of NOX2 (136, 140). Emerging evidence suggests the presence of crosstalk between NOX enzymes and Toll-like receptors (TLRs), which cooperatively participate in the host innate immune response. High-mobility group box 1 (HMGB1), an endogenous ligand for TLR4, activates neutrophil-associated NOX, induces neutrophilic inflammation, and results in organ failure in response to hemorrhagic shock/resuscitation in mice (29). TLRs also crosstalk with nonphagocytic NOX isoforms, as demonstrated by the finding that LPS-induced ROS generation and NF-κB activation is mediated by the interaction of TLR4 with NOX4 (93).
Endothelial barrier dysfunction in ALI/ARDS may be mediated by ROS-dependent mechanisms that involve interactions of activated neutrophils with pulmonary vascular endothelial cells (ECs) or more-direct activation of EC responses. In support of the latter concept, growing recognition indicates the expression/activation of specific NOX isoforms in vascular ECs. A role for NOX4 in LPS-induced proinflammatory responses by human aortic ECs has been reported (92); in this study, downregulation of NOX4 by transfection of NOX4 small interfering RNA (siRNA) resulted in a failure to induce ROS generation and intercellular adhesion molecule (ICAM)-1, monocyte chemoattractant protein (MCP)-1, and interleukin (IL)-8 production in response to LPS. Pulmonary ECs have been shown to generate ROS via both NOX2 and NOX4 (94, 95). Circulating blood cells from septic patients generate higher levels of phorbol ester–stimulated 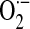 production compared with those in control subjects, and this activation is inhibited by simvastatin, a widely used cholesterol-reducing drug (28). Further, recent data indicate that simvastatin markedly decreases LPS-induced
production compared with those in control subjects, and this activation is inhibited by simvastatin, a widely used cholesterol-reducing drug (28). Further, recent data indicate that simvastatin markedly decreases LPS-induced 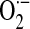 production in human pulmonary artery ECs via dual inhibitory effects on RhoA and Rac1 (17). Together, these studies suggest that excessive generation of ROS by activation of phagocytic and nonphagocytic NOX enzymes may induce endothelial damage or activation with the subsequent loss of barrier function and pulmonary edema or both, key features of ALI/ARDS. Further studies are required to characterize the relative contributions of different NOX isoforms in sepsis-induced ALI/ARDS and to determine mechanisms for the modulation of NOX activities by statins and other putative NOX inhibitors.
production in human pulmonary artery ECs via dual inhibitory effects on RhoA and Rac1 (17). Together, these studies suggest that excessive generation of ROS by activation of phagocytic and nonphagocytic NOX enzymes may induce endothelial damage or activation with the subsequent loss of barrier function and pulmonary edema or both, key features of ALI/ARDS. Further studies are required to characterize the relative contributions of different NOX isoforms in sepsis-induced ALI/ARDS and to determine mechanisms for the modulation of NOX activities by statins and other putative NOX inhibitors.
Mechanical ventilation provides life-sustaining support in critically ill patients with ALI/ARDS. However, mechanical ventilation itself may contribute to, and exacerbate, lung injury. As a result, ventilator-induced lung injury (VILI) may delay or prevent recovery from treatable clinical conditions such as sepsis. Modes of mechanical ventilation associated with greater mechanical distension/stretch enhance neutrophil infiltration and pro–inflammatory cytokine production in the lung (1, 43). Administration of N-acetylcysteine attenuates the influx of neutrophils into the alveolar space and reduces apoptosis of airway epithelial cells in rats subjected to mechanical ventilation (117). Cyclical mechanical strain of alveolar epithelial cells leads to ROS generation, via both mitochondrial and NOX-dependent pathways (16). Although the enzymatic source(s) have not been identified, ROS-mediated cellular damage or activation from biomechanical stress or both may augment lung injury and delay/prevent normal repair.
Patients with ALI/ARDS receiving mechanical ventilation often require high O2 concentrations to maintain arterial oxygenation. Supraphysiologic levels of O2 concentration (hyperoxia) and the associated generation of ROS is yet another contributor to the “biotrauma” that can worsen ALI in mechanically ventilated patients. Hyperoxia remains a particular problem in premature infants whose lungs may be ill adapted to defend against ROS (8, 144). The effect of hyperoxia on inflammation/injury has been studied in animal models and lung EC culture systems (22, 83). Exposure of mice to hyperoxia (>95% oxygen) causes lung damage characterized by inflammation, barrier dysfunction, pulmonary edema, and impaired lung function (22). The increased generation of ROS during hyperoxia may induce oxidative modifications of cellular macromolecules, including carbohydrates, nucleic acids, proteins, and lipids.
Exposure of human pulmonary artery ECs to hyperoxia (95% O2) increases ROS production that is dependent on NOX activation and independent of the mitochondrial electron transport or xanthine/xanthine oxidase systems (91, 95). NOX4, which is expressed at relatively higher levels compared with other NOX homologues, is a major source of ROS production in vascular ECs. In cultured human pulmonary artery ECs, hyperoxia increases NOX4 mRNA and protein levels by about eight- and threefold, respectively, compared with normoxia over a 24-h period (94). Activation of lung EC–associated NOX by hyperoxia is regulated, in part, by ERK-1/2 and p38 MAPKs (91, 132). More recently, a role for Src kinase in this process was demonstrated (19); in this study, exposure of lung vascular ECs to hyperoxia stimulated tyrosine phosphorylation of p47phox, which was attenuated by pharmacologic/genetic targeting of Src, suggesting Src-dependent phosphorylation of p47phox in EC-associated NOX activation. In addition, evidence for in vitro phosphorylation of p47phox by Src and interaction between Src and p47phox in hyperoxia-induced 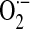 generation was demonstrated (19). Interestingly, tyrosine phosphorylation of cortactin is associated with hyperoxia-induced translocation of p47phox to the cell periphery and ROS generation in human lung ECs (131).
generation was demonstrated (19). Interestingly, tyrosine phosphorylation of cortactin is associated with hyperoxia-induced translocation of p47phox to the cell periphery and ROS generation in human lung ECs (131).
A role for NOX2 and NOX4 in hyperoxia-induced ROS generation in pulmonary vascular ECs has been demonstrated (94). Interestingly, knockdown of NOX4 or NOX2 with siRNA upregulates the mRNA and protein expression of the other homologue in human pulmonary artery ECs; a similar upregulation of NOX4 mRNA is observed in lungs of NOX2−/− mice under normoxic conditions, suggesting a compensatory mechanism (94).
The role of NOX2 in hyperoxic lung injury in mice genetically deficient in NOX2 has been investigated. Exposure of wild-type mice to hyperoxia induces pulmonary edema and neutrophil influx into the alveolar space, effects that are attenuated in NOX2−/− mice, thus suggesting a role for NOX2 in hyperoxia-mediated barrier dysfunction. However, the observed protection in NOX2−/− is incomplete, suggesting the potential involvement of other NOX isoforms, including NOX4, in alveolocapillary barrier dysfunction (94, 95).
NOX Enzymes in Pulmonary Hypertension
Prolonged exposure to low O2 tension induces pulmonary arterial hypertension (PAH), characterized by vascular remodeling and enhanced vasoreactivity. Accumulating evidence indicates that ROS derived from NOX isoforms, in particular NOX2 and NOX4, are involved in long-term responses of the pulmonary vasculature to hypoxia (30, 33, 66, 79). ROS generation from NOX-independent sources may also contribute to hypoxia-induced vascular dysfunction; for example, hypoxia-exposed neonatal rat pups exhibit increased serum and lung xanthine oxidase (XO) activity, increased vascular XO-derived 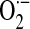 production, and vascular nitrotyrosine formation (47). A role for NOX2 in hypoxia-induced endothelial dysfunction involving intrapulmonary arteries has been demonstrated (33). In pulmonary artery adventitial fibroblasts, hypoxia significantly upregulates NOX4 expression at the mRNA and protein levels, whereas silencing of NOX4 by siRNA reduces ROS levels and decreases cellular proliferation (65). Hypoxia-dependent development of PAH in mice has been linked to increased NOX4 expression in pulmonary artery smooth muscle cells (SMCs) (79), suggesting a key role for NOX4 in the vascular remodeling associated with hypoxia-induced PAH. Hypoxia increases the expression of TGF-β (48), production of the TGF-β–activating protein, furin (76), and NOX4 expression (114). TGF-β–induced NOX4 expression and ROS production has been implicated in proliferation of human pulmonary artery SMCs (45, 114). NOX4 has also been shown to be critical for HIF-2α expression and transcriptional activation in renal carcinoma cells (71). Additionally, TGF-β/SMAD signaling may synergize with hypoxia/HIF-1α (104), thereby setting up a potential feed-forward mechanism in hypoxic vascular remodeling involving HIF-1α/HIF-2α, transforming growth factor-β (TGF-β), and NOX4.
production, and vascular nitrotyrosine formation (47). A role for NOX2 in hypoxia-induced endothelial dysfunction involving intrapulmonary arteries has been demonstrated (33). In pulmonary artery adventitial fibroblasts, hypoxia significantly upregulates NOX4 expression at the mRNA and protein levels, whereas silencing of NOX4 by siRNA reduces ROS levels and decreases cellular proliferation (65). Hypoxia-dependent development of PAH in mice has been linked to increased NOX4 expression in pulmonary artery smooth muscle cells (SMCs) (79), suggesting a key role for NOX4 in the vascular remodeling associated with hypoxia-induced PAH. Hypoxia increases the expression of TGF-β (48), production of the TGF-β–activating protein, furin (76), and NOX4 expression (114). TGF-β–induced NOX4 expression and ROS production has been implicated in proliferation of human pulmonary artery SMCs (45, 114). NOX4 has also been shown to be critical for HIF-2α expression and transcriptional activation in renal carcinoma cells (71). Additionally, TGF-β/SMAD signaling may synergize with hypoxia/HIF-1α (104), thereby setting up a potential feed-forward mechanism in hypoxic vascular remodeling involving HIF-1α/HIF-2α, transforming growth factor-β (TGF-β), and NOX4.
NOX Enzymes in Obstructive Sleep Apnea and Ischemia/Reperfusion
Obstructive sleep apnea is a clinical syndrome that is characterized by intermittent periods of hypoxemia due to partial/complete obstruction of the upper airway during sleep. Obstructive sleep apnea is also a significant cause of secondary PAH. Chronic intermittent hypoxia-induced pulmonary hypertension is associated with increased lung levels of the NOX subunits, NOX4 and p22phox, as well as activation of platelet-derived growth factor receptor-β and one of its associated downstream effectors, AKT kinase (87). In NOX2−/− mice, chronic intermittent hypoxia-induced derangements, such as increased right ventricular systolic pressure, right ventricle (RV) to left ventricle + septum weight ratio, an index of RV hypertrophy, and thickness of the RV anterior wall, as measured by echocardiography, are all attenuated (87). These findings suggest that NOX2 contributes to the development of pulmonary vascular remodeling, pulmonary hypertension, and RV remodeling induced by chronic intermittent hypoxia.
ROS also play a crucial role in ischemia/reperfusion injury after lung transplantation. Normoxic lung ischemia induces EC membrane depolarization because of acute alterations in shear stress that activates EC-associated NOX activity via a Rac1 and phosphoinositide-3-kinase (PI3K)-dependent mechanism (150). Studies using p47phox knockout mice, wild-type mice, and chimeras created by bone marrow transplantation indicate that NOX-generated ROS, specifically from bone marrow–derived cells, contribute to lung ischemia/reperfusion injury (148). Furthermore, recent studies indicate that activation of an EC-associated NOX may be the primary mechanism for ROS generation during reoxygenation after lung ischemia (5, 152). Neutrophil NOX-derived ROS also contribute to organ injury after hemorrhagic shock in mice (29). Enhanced formation of 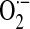 by a p47phox-requiring NOX enzyme contributes to the liver injury caused by hemorrhagic shock, and inhibitors of NOX enzymes may represent a novel therapeutic approach for the treatment of hemorrhagic shock (3, 62).
by a p47phox-requiring NOX enzyme contributes to the liver injury caused by hemorrhagic shock, and inhibitors of NOX enzymes may represent a novel therapeutic approach for the treatment of hemorrhagic shock (3, 62).
NOX Enzymes in Obstructive Lung Disorders
NOX-generated ROS have long been recognized to play key roles in the pathogenesis of a number of diverse chronic lung disorders that result in obstructive physiology, in particular asthma, cystic fibrosis, and emphysema (2, 50, 53, 73, 97, 113). With the recent identification of various NOX homologues, investigators have implicated specific NOX and DUOX isoforms in the pathogenesis of obstructive lung disorders; DUOX1, DUOX2, NOX2, and NOX4, and subunits p22phox and p47phox have been the most frequently reported. DUOX1 has been shown to be induced by the T-helper 2 (Th2) cytokines, interleukin (IL)-4 and IL-13, key effector cytokines in asthma and allergic airways disease (38). DUOX1, in addition to host defense functions, appears to promote epithelial cell migration and maintenance of barrier function (142). NOX4 has been implicated in TGF-β–mediated proliferation and hypertrophy of human airway smooth muscle, a hallmark of airway remodeling in asthmatic patients (114). NOX subunits, p22phox and p47phox, were detected in airway SMCs, and NOX-dependent ROS generation mediates TNF-α–induced airway smooth muscle hyperresponsiveness, a predictor of fatal asthma (123). Interestingly, recent haplotype studies indicate that genetic variability in the gene encoding p22phox (CYBA, 16q24.3) may contribute to the susceptibility to asthma (46).
Cystic fibrosis airway biopsy samples exhibit decreased DUOX2 expression, suggesting that the enhanced susceptibility to infections in cystic fibrosis may be linked to impaired DUOX-mediated host defense (146). In support of NOX-mediated host defense in the lung, mice deficient in NOX2 have increased susceptibility to specific strains of Burkholderia cepacia, a pathogen commonly encountered in cystic fibrosis patients (111). Pseudomonas aeruginosa, another important pathogen in cystic fibrosis, appears to inhibit DUOX1-dependent antimicrobial activity via toxin-mediated effects on airway epithelium (100).
Smokers and those with COPD exhibit differential DUOX1 and DUOX2 depending on smoking status and type of lung epithelium sampled. For example, airway epithelium of current smokers expresses decreased DUOX1 and increased DUOX2 compared with those of never smokers, whereas former smokers (all with COPD) demonstrated downregulation of both DUOX isoforms (84, 98). However, alveolar epithelial DUOX1 and DUOX2 were expressed at low levels and were unchanged regardless of smoking or COPD status (84). Knockout mouse models have enabled investigators to glean a functional role for NOX enzymes in obstructive lung processes. Mice deficient in p47phox or NOX2 exhibit increased cigarette smoke–induced lung inflammation and emphysema despite decreased ROS production compared with control mice (149). The lung responses in p47phox- and NOX2-null mice were associated with increased production of proinflammatory cytokines/chemokines via a TLR4-NF-κB pathway, indicating that NOX2 may mediate antiinflammatory functions by restraining TLR4 activation (149). However, another group reported that p47phox-null mice have less inflammation, IL-6, keratinocyte-derived chemokine (KC/CXCL1), and monocyte chemoattractant protein-1 (MCP1/CCL2) in lung-lavage specimens after cigarette-smoke exposure compared with wild-type mice (56). The differences observed by these groups may be due to variability in lung-compartment sampling, cellular distributions, and chronicity of cigarette-smoke exposure. Gene-profiling studies in lung tissues from cigarette smoke–exposed mice recently revealed upregulation of NOX organizer 1 (NOXO1), which regulates NOX1 activation, indicating that other NOX isoforms may be involved in lung responses to cigarette smoke (77). Furthermore, our ability to detect specific NOX and DUOX isoforms in different lung compartments may be dependent on whether the particular isoform undergoes transcriptional or posttranscriptional regulation (88).
More recently, unexpected roles for NOX3 in the lung are being elucidated. The emergence of NOX3 in evolution corresponds with the full-time adaptation of vertebrates to the land (51), raising potential unique roles for this isoform in the adaptive physiology of this specialized “land organ.” Previously, NOX3 was detected only in fetal tissues (18), and its only known physiologic role described in otolith biogenesis in the inner ear, as demonstrated by the head-tilt phenotype of mice deficient in functional NOX3 (9, 90). However, NOX3 is inducible in murine adult lung and lung endothelial cells, with the unexpected finding that NOX3 is regulated by TLR4 (151). Furthermore, NOX3 was found to be induced in aged mice with targeted deletion in TLR4 in association with lung destruction and emphysema, and these effects are reversed with chemical NOX inhibitors or NOX3 siRNA, suggesting a role for NOX3-generated ROS in age-related emphysema (151). These studies were confirmed by breeding TLR4-null with NOX3-null mice and demonstrating significant attenuation in susceptibility to emphysema (Patty Lee, unpublished data). In further support of the involvement of NOX3 in emphysema pathogenesis, lung-targeted, inducible NOX3 transgenic mice develop emphysema in the setting of NOX3 transgene induction (Patty Lee, unpublished data). Collectively, these results reveal a previously unappreciated role for NOX3 in the pathogenesis of emphysema.
We speculate that NOX3, because of its potentially deleterious effects in the lung, requires tight suppression in adulthood (e.g., by TLR4); however, aberrant or pathologic states of TLR4 deficiency may allow unrestrained NOX3 activity and ROS generation. Interestingly, recent human studies reported that aging and cigarette smoke are associated with depressed TLR4 function (69, 134), supporting the theory of a disrupted TLR–NOX3 axis in human emphysema. Additional studies in human subjects with emphysema are required to elucidate/confirm these findings further. Furthermore, the development of in vivo, cell-specific targeting of NOX isoforms will reveal the extent to which tissue distribution and cell specificity determine NOX-mediated responses in obstructive lung disorders.
NOX Enzymes in Pulmonary Fibrosis
Pulmonary fibrosis is a specifc type of tissue-remodeling response to known (e.g., environmental exposures, drugs, connective tissue diseases) or unknown (i.e., idiopathic) injury that is typically recurrent or chronic in nature. Tissue-remodeling responses in fibrosis are characterized by the accumulation of activated mesenchymal cells and the deposition of excellular matrix (ECM) (40). A subset of activated mesenchymal cells, referred to as myofibroblasts, are key effector cells in tissue remodeling and fibrotic reactions in diverse organ systems, including the lung (128). Myofibroblast differentiation is critically dependent on the action of TGF-β1 (24, 127). In addition to the multiple fibrogenic actions, myofibroblasts generate ROS in response to TGF-β1 (23, 125, 138). NOX4 has been identified as a source of TGF-β1–induced ROS production in cardiac myofibroblasts and is implicated in the induction of myofibroblast differentiation (23). Although the cellular localization/compartmentalization of NOX4 has not been clarified in myofibroblasts, a unique feature of NOX4 activity is its capacity for constitutive generation of extracellular H2O2 (74, 107, 137). Extracellular generation of H2O2 by lung myofibroblasts may mediate additional fibrogenic effects in tissues by inducing epithelial cell apoptosis by a paracrine mechanism (138), or by inducing matrix-crosslinking reactions in the presence of extracellular heme peroxidases (59).
Currently limited published studies are available on in vivo roles for NOX4 in lung fibrosis; however, studies in kidney fibrosis (12, 89, 120, 143), vascular-remodeling/fibrosis associated with chronic hypertension (4), cardiac fibrosis (39, 112, 139), and pancreatic fibrosis (75) suggest a role for the NOX4 isoform in the fibrogenic process. Other NOX isoforms that are reported to contribute to tissue fibrosis in nonpulmonary organ systems include NOX1 (4, 75, 112, 139) and NOX2 (67, 75, 89, 112, 143). A p47phox-requiring NOX isoform is required for the development of fibrosis in a murine lung-injury model that is inflammation dependent, and the observed protection in p47phox−/− mice is associated with enhanced neutrophilic inflammation and matrix metalloproteinase (MMP)-9 activity (70). Significant crosstalk occurs between the renin–angiotensin–aldosterone system and TGF-β1 in organ fibrosis (130, 147), and this effect is, at least in part, mediated by induction/activation of NOX1, NOX2, NOX4, or a combination of these (4, 12, 112, 120, 139, 143).
NOX Enzymes in Lung Cancer
Tumorigenesis entails a series of cellular/tissue alterations that promote cell survival and growth while usurping normal, homeostatic controls. For the malignant potential of cells to be fully realized, it has been proposed that cancer cells develop five essential capabilities: unrelenting cell proliferation, resistance to apoptosis, intrinsic growth signaling with limited response to growth-inhibitory factors, continual neovascularization (angiogenesis), and capacity for migration and tissue invasion (37). ROS have been implicated in the signaling/regulation of the malignant phenotype (34, 58), and oxidative stress–mediated epigenetic changes are increasingly recognized (32).
Early studies indicated the generation of ROS by a NOX-like flavoenzyme in several different cancer cells, although the identity of the enzymatic source(s) was not known (119). With the discovery of the different homologues of NOX/DUOX enzymes, specific isoforms have been identified in a variety of human malignancies, including colon (60, 96, 118), gastric (129), pancreatic (61, 80, 135), and prostate (14) cancers. The tumorigenic potential of NOX enzymes was first demonstrated with athymic murine models. NOX1-transfected cells produce phenotypically aggressive tumors in athymic mice (115). In a similar murine model, injection of NOX1-expressing cells resulted in rapid cell growth and tumor formation, whereas injection of cells coexpressing NOX1 and catalase failed to promote a mitogenic response or tumor formation in vivo (7). Recently, NOX1 was found to mediate malignant transformation of the Ras oncogene (78); in this study, RNA interference–mediated knockdown of NOX1 in K-Ras transformed cells reduced NOX1-dependent 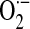 production, resulting in abrogation of anchorage-independent cell growth and capacity for tumor formation in vivo (78).
production, resulting in abrogation of anchorage-independent cell growth and capacity for tumor formation in vivo (78).
NOX1 was originally referred to as the “mitogenic oxidase” (7, 115); however, its effects on specific cell types are likely contextual and include other cellular functions. Comparing pathologic specimens of human gastric adenocarcinomas and chronic atrophic gastritis, the presence of NOX1 was demonstrated by mRNA expression and immunohistochemical staining within adenocarcinomas, whereas it was notably absent in control samples, supporting the utility of NOX1 as a marker of malignant transformation (129).
In addition to propagation of tumor cell growth, evidence supports a role for NOX1 in angiogenesis (6). Vascular endothelial growth factor (VEGF) functions as a key mediator of neovascularization within tumors. VEGF is upregulated by H2O2 and was found to enhance tumor cell proliferation and promote a migratory phenotype (20). Additionally, Ras-induced VEGF transcription is dependent on Sp1 phosphorylation/activation, which is mediated by a NOX1/Ras/ERK-MAPK pathway (55). Other studies support a role for the NOX2 isoform in angiogenesis (133).
Resistance to apoptosis is another hallmark of cancer cells (37). NOX4-generated ROS confer an apoptosis-resistant phenotype in pancreatic cancer cells (135). Similarly, NOX5 is expressed in DU-145 prostate cancer cells and was found to mediate ROS production, cell proliferation, and resistance to apoptosis (14). A potential mechanism by which the NOX isoforms promote apoptosis resistance may involve ROS-mediated inactivation of protein tyrosine phosphatases (PTPs). In human pancreatic adenocarcinoma tissue samples, NOX4 colocalized with low-molecular-weight PTPs in the cytoplasm of tumor cells (61); furthermore, these investigators showed that NOX4-dependent ROS production mediates PTP inactivation and sustained phosphorylation of JAK2, a prosurvival kinase (61). In human pancreatic adenocarcinoma cells, the PI3K/AKT and apoptosis signal-regulating kinase 1 pathway has been shown to mediate NOX4-induced prosurvival signaling (80).
Recent evidence also indicates a role for epigenetic mechanisms in the regulation of NOX/DUOX enzymes in cancer cells. DUOX1 and DUOX2 and their maturation factors were found to be downregulated by promoter methylation in primary lung carcinomas (68). In this study, restoration of a functional DUOX1 altered the phenotypic profile of the lung cancer cell lines, supporting a homeostatic function for DUOX and providing the first evidence that epigenetic modification of this family of enzymes may promote malignant potential (68). An interesting aspect to the role of NOX/DUOX biology in carcinogenesis is the number and diversity of the isoforms involved in promoting the malignant phenotype, as illustrated by the finding that two different NOX enzymes function to mediate the same malignant trait—apoptosis resistance—in two different cancers (14, 135).
Conclusions
The respiratory and the cardiovascular systems co-evolved to allow transport of atmospheric O2 to cells/tissues of internal organs in larger organisms that became dependent on aerobic metabolism, but were limited by the diffusibility of O2 across multiple cell layers (124). The chemical properties of O2 and ROS appear to have been exploited further in the emergence of biologic complexity; an increasing number of NOX isoforms have emerged with mammalian evolution (11, 51, 116). Gene targeting of specific NOX isoforms or related subunits in mice illustrates the complexity of these enzymatic systems in vivo. Although NOX enzymes serve physiologic functions in the lung, they may also contribute to disease pathogenesis. The varying roles of NOX isoforms in emphysema illustrate this pleiotropic nature. A deficiency in p47phox or NOX2 appears to enhance cigarette smoke–induced emphysema (149), whereas a deficiency in NOX3 is predicted to confer protection against emphysema (151). Although such observed differences may be model system–dependent or isoform specific or both, these studies raise the intriguing possibility of “antagonistic pleiotropy” of NOX genes (58). In antagonistic pleiotropy, potentially harmful genes (e.g., NOX3) are retained during evolution because they confer an early survival advantages, but their deleterious effects accrue with age and result in age-related, chronic disease (54, 58, 145). The lungs may be particularly susceptible to toxic effects of NOX activation because O2 concentrations in lung tissues are generally higher than those in other organs systems.
Future studies on the physiological roles of specific NOX isoforms in the lung will provide important insights into their pathologic roles in pulmonary disease and opportunities for therapeutic targeting. Because of their cell-specific and contextual effects, studies of NOX function in vivo will be more informative when the gene can be conditionally deleted in specific cell types (e.g., by inducible Cre recombinase under the control of a cell-specific promoter to delete a targeted/floxed gene). Animal models do not faithfully recapitulate human disease expression/phenotype because of a multitude of factors, including species-specific differences, disease heterogeneity, chronicity, and environmental influences that may result in epigenetic alterations. This highlights the importance of studying the expression and localization of NOX isoforms in lung cells/tissues derived from patients with specific lung disorders.
Abbreviations Used
- ALI
Acute lung injury
- ARDS
acute respiratory distress syndrome
- CGD
chronic granulomatous disease
- DUOX
dual oxidase
- EC
endothelial cell
- ECM
extracellular matrix
- HIF
hypoxia-inducible factor
- HMGB1
high-mobility group box 1
- H2O2
hydrogen peroxide
- ICAM
intercellular adhesion molecule
- IL
interleukin
- LPS
lipopolysaccharide
- MCP
monocyte chemoattractant protein
- MMP
matrix metalloproteinase
- NOX
NADPH oxidase
- O2
oxygen
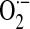
superoxide anion
- PAH
pulmonary arterial hypertension
- PI3K
phosphoinositide-3-phosphate
- ROS
reactive oxygen species
- RV
right ventricle
- SMC
smooth muscle cell
- SOD
superoxide dismutase
- TGF-β
transforming growth factor-β
- Th2
T-helper 2
- TLR
Toll-like receptor
- VEGF
vascular endothelial growth factor
- VILI
ventilator-induced lung injury
- XO
xanthine oxidase
Acknowledgments
This work is supported in part by National Institutes of Health grants, R01 HL08533 (V.N.), P01 HL58064 (V.N.), R01 HL071595 (P.J.L), and R01 HL67967 (V.J.T.).
References
- 1.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Abdala-Valencia H. Earwood J. Bansal S. Jansen M. Babcock G. Garvy B. Wills-Karp M. Cook-Mills JM. Nonhematopoietic NADPH oxidase regulation of lung eosinophilia and airway hyperresponsiveness in experimentally induced asthma. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1111–L1125. doi: 10.1152/ajplung.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelrahman M. Mazzon E. Bauer M. Bauer I. Delbosc S. Cristol JP. Patel NS. Cuzzocrea S. Thiemermann C. Inhibitors of NADPH oxidase reduce the organ injury in hemorrhagic shock. Shock. 2005;23:107–114. doi: 10.1097/01.shk.0000151028.15377.f7. [DOI] [PubMed] [Google Scholar]
- 4.Akasaki T. Ohya Y. Kuroda J. Eto K. Abe I. Sumimoto H. Iida M. Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: involvement of the renin-angiotensin system. Hypertens Res. 2006;29:813–820. doi: 10.1291/hypres.29.813. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mehdi AB. Zhao G. Dodia C. Tozawa K. Costa K. Muzykantov V. Ross C. Blecha F. Dinauer M. Fisher AB. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+ Circ Res. 1998;83:730–737. doi: 10.1161/01.res.83.7.730. [DOI] [PubMed] [Google Scholar]
- 6.Arbiser JL. Petros J. Klafter R. Govindajaran B. McLaughlin ER. Brown LF. Cohen C. Moses M. Kilroy S. Arnold RS. Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold RS. Shi J. Murad E. Whalen AM. Sun CQ. Polavarapu R. Parthasarathy S. Petros JA. Lambeth JD. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asikainen TM. White CW. Pulmonary antioxidant defenses in the preterm newborn with respiratory distress and bronchopulmonary dysplasia in evolution: implications for antioxidant therapy. Antioxid Redox Signal. 2004;6:155–167. doi: 10.1089/152308604771978462. [DOI] [PubMed] [Google Scholar]
- 9.Banfi B. Malgrange B. Knisz J. Steger K. Dubois-Dauphin M. Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 10.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 11.Bedard K. Lardy B. Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie. 2007;89:1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Block K. Eid A. Griendling KK. Lee DY. Wittrant Y. Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem. 2008;283:24061–24076. doi: 10.1074/jbc.M803964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolscher BG. de Boer M. de Klein A. Weening RS. Roos D. Point mutations in the beta-subunit of cytochrome b558 leading to X-linked chronic granulomatous disease. Blood. 1991;77:2482–2487. [PubMed] [Google Scholar]
- 14.Brar SS. Corbin Z. Kennedy TP. Hemendinger R. Thornton L. Bommarius B. Arnold RS. Whorton AR. Sturrock AB. Huecksteadt TP. Quinn MT. Krenitsky K. Ardie KG. Lambeth JD. Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 15.Bridges RA. Berendes H. Good RA. A fatal granulomatous disease of childhood; the clinical, pathological, and laboratory features of a new syndrome. AMA J Dis Child. 1959;97:387–408. [PubMed] [Google Scholar]
- 16.Chapman KE. Sinclair SE. Zhuang D. Hassid A. Desai LP. Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L834–L841. doi: 10.1152/ajplung.00069.2005. [DOI] [PubMed] [Google Scholar]
- 17.Chen W. Pendyala S. Natarajan V. Garcia JG. Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am J Physiol Lung Cell Mol Physiol. 2008;295:L575–L583. doi: 10.1152/ajplung.00428.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng G. Cao Z. Xu X. van Meir EG. Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury AK. Watkins T. Parinandi NL. Saatian B. Kleinberg ME. Usatyuk PV. Natarajan V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem. 2005;280:20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- 20.Chua CC. Hamdy RC. Chua BH. Upregulation of vascular endothelial growth factor by H2O2 in rat heart endothelial cells. Free Radic Biol Med. 1998;25:891–897. doi: 10.1016/s0891-5849(98)00115-4. [DOI] [PubMed] [Google Scholar]
- 21.Cooper AM. Segal BH. Frank AA. Holland SM. Orme IM. Transient loss of resistance to pulmonary tuberculosis in p47phox−/− mice. Infect Immun. 2000;68:1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol. 1986;48:721–731. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- 23.Cucoranu I. Clempus R. Dikalova A. Phelan PJ. Ariyan S. Dikalov S. Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 24.Desmouliere A. Geinoz A. Gabbiani F. Gabbiani G. Transforming growth factor-beta1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinauer MC. The respiratory burst oxidase and the molecular genetics of chronic granulomatous disease. Crit Rev Clin Lab Sci. 1993;30:329–369. doi: 10.3109/10408369309082591. [DOI] [PubMed] [Google Scholar]
- 26.Dinauer MC. Pierce EA. Bruns GA. Curnutte JT. Orkin SH. Human neutrophil cytochrome b light chain (p22phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest. 1990;86:1729–1737. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinauer MC. Pierce EA. Erickson RW. Muhlebach TJ. Messner H. Orkin SH. Seger RA. Curnutte JT. Point mutation in the cytoplasmic domain of the neutrophil p22-phox cytochrome b subunit is associated with a nonfunctional NADPH oxidase and chronic granulomatous disease. Proc Natl Acad Sci U S A. 1991;88:11231–11235. doi: 10.1073/pnas.88.24.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durant R. Klouche K. Delbosc S. Morena M. Amigues L. Beraud JJ. Canaud B. Cristol JP. Superoxide anion overproduction in sepsis: effects of vitamin E and simvastatin. Shock. 2004;22:34–39. doi: 10.1097/01.shk.0000129197.46212.7e. [DOI] [PubMed] [Google Scholar]
- 29.Fan J. Li Y. Levy RM. Fan JJ. Hackam DJ. Vodovotz Y. Yang H. Tracey KJ. Billiar TR. Wilson MA. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 30.Fike CD. Slaughter JC. Kaplowitz MR. Zhang Y. Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L881–L888. doi: 10.1152/ajplung.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forteza R. Salathe M. Miot F. Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32:462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- 32.Franco R. Schoneveld O. Georgakilas AG. Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 33.Fresquet F. Pourageaud F. Leblais V. Brandes RP. Savineau JP. Marthan R. Muller B. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. Br J Pharmacol. 2006;148:714–723. doi: 10.1038/sj.bjp.0706779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fruehauf JP. Meyskens FL., Jr. Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 35.Gao XP. Standiford TJ. Rahman A. Newstead M. Holland SM. Dinauer MC. Liu QH. Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J Immunol. 2002;168:3974–3982. doi: 10.4049/jimmunol.168.8.3974. [DOI] [PubMed] [Google Scholar]
- 36.Geiszt M. Witta J. Baffi J. Lekstrom K. Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 37.Hanahan D. Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 38.Harper RW. Xu C. Eiserich JP. Chen Y. Kao CY. Thai P. Setiadi H. Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Henderson BC. Sen U. Reynolds C. Moshal KS. Ovechkin A. Tyagi N. Kartha GK. Rodriguez WE. Tyagi SC. Reversal of systemic hypertension-associated cardiac remodeling in chronic pressure overload myocardium by ciglitazone. Int J Biol Sci. 2007;3:385–392. doi: 10.7150/ijbs.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinz B. Phan SH. Thannickal VJ. Galli A. Bochaton-Piallat ML. Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoidal JR. Xu P. Huecksteadt T. Sanders KA. Pfeffer K. Sturrock AB. Lung injury and oxidoreductases. Environ Health Perspect. 1998;106(suppl 5):1235–1239. doi: 10.1289/ehp.98106s51235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes B. Page AR. Good RA. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967;46:1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imai Y. Kawano T. Miyasaka K. Takata M. Imai T. Okuyama K. Inflammatory chemical mediators during conventional ventilation and during high frequency oscillatory ventilation. Am J Respir Crit Care Med. 1994;150:1550–1554. doi: 10.1164/ajrccm.150.6.7952613. [DOI] [PubMed] [Google Scholar]
- 44.Imai Y. Kuba K. Neely GG. Yaghubian-Malhami R. Perkmann T. van Loo G. Ermolaeva M. Veldhuizen R. Leung YH. Wang H. Liu H. Sun Y. Pasparakis M. Kopf M. Mech C. Bavari S. Peiris JS. Slutsky AS. Akira S. Hultqvist M. Holmdahl R. Nicholls J. Jiang C. Binder CJ. Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ismail S. Sturrock A. Wu P. Cahill B. Norman K. Huecksteadt TP. Sanders KA. Kennedy TP. Hoidal JR. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of TGF-β1 and IGFBP-3. Am J Physiol Lung Cell Mol Physiol. 2009;296:L489–L499. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Izakovicova Holla L. Kankova K. Znojil V. Haplotype analysis of the NADPH oxidase p22 phox gene in patients with bronchial asthma. Int Arch Allergy Immunol. 2009;148:73–80. doi: 10.1159/000151508. [DOI] [PubMed] [Google Scholar]
- 47.Jankov RP. Kantores C. Pan J. Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2008;294:L233–L245. doi: 10.1152/ajplung.00166.2007. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Y. Dai A. Li Q. Hu R. Hypoxia induces transforming growth factor-beta1 gene expression in the pulmonary artery of rats via hypoxia-inducible factor-1alpha. Acta Biochim Biophys Sin (Shanghai) 2007;39:73–80. doi: 10.1111/j.1745-7270.2007.00249.x. [DOI] [PubMed] [Google Scholar]
- 49.Johnston RB., Jr. Clinical aspects of chronic granulomatous disease. Curr Opin Hematol. 2001;8:17–22. doi: 10.1097/00062752-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Joseph BZ. Routes JM. Borish L. Activities of superoxide dismutases and NADPH oxidase in neutrophils obtained from asthmatic and normal donors. Inflammation. 1993;17:361–370. doi: 10.1007/BF00918997. [DOI] [PubMed] [Google Scholar]
- 51.Kawahara T. Quinn MT. Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawahara T. Teshima S. Oka A. Sugiyama T. Kishi K. Rokutan K. Type I Helicobacter pylori lipopolysaccharide stimulates Toll-like receptor 4 and activates mitogen oxidase 1 in gastric pit cells. Infect Immun. 2001;69:4382–4389. doi: 10.1128/IAI.69.7.4382-4389.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kilpatrick LE. Jakabovics E. McCawley LJ. Kane LH. Korchak HM. Cromolyn inhibits assembly of the NADPH oxidase and superoxide anion generation by human neutrophils. J Immunol. 1995;154:3429–3436. [PubMed] [Google Scholar]
- 54.Kirkwood TB. Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Phil Trans R Soc Lond B Biol Sci. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- 55.Komatsu D. Kato M. Nakayama J. Miyagawa S. Kamata T. NADPH oxidase 1 plays a critical mediating role in oncogenic Ras-induced vascular endothelial growth factor expression. Oncogene. 2008;27:4724–4732. doi: 10.1038/onc.2008.102. [DOI] [PubMed] [Google Scholar]
- 56.Lagente V. Planquois JM. Leclerc O. Schmidlin F. Bertr CP. Oxidative stress is an important component of airway inflammation in mice exposed to cigarette smoke or lipopolysaccharide. Clin Exp Pharmacol Physiol. 2008;35:601–605. doi: 10.1111/j.1440-1681.2007.04848.x. [DOI] [PubMed] [Google Scholar]
- 57.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 58.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larios JM. Budhiraja R. Fanburg BL. Thannickal VJ. Oxidative protein cross-linking reactions involving L-tyrosine in transforming growth factor-beta1-stimulated fibroblasts. J Biol Chem. 2001;276:17437–17441. doi: 10.1074/jbc.M100426200. [DOI] [PubMed] [Google Scholar]
- 60.Laurent E. McCoy JW., 3rd Macina RA. Liu W. Cheng G. Robine S. Papkoff J. Lambeth JD. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int J Cancer. 2008;123:100–107. doi: 10.1002/ijc.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JK. Edderkaoui M. Truong P. Ohno I. Jang KT. Berti A. Pandol SJ. Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 62.Lehnert M. Arteel GE. Smutney OM. Conzelmann LO. Zhong Z. Thurman RG. Lemasters JJ. Dependence of liver injury after hemorrhage/resuscitation in mice on NADPH oxidase-derived superoxide. Shock. 2003;19:345–351. doi: 10.1097/00024382-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Leusen JH. de Boer M. Bolscher BG. Hilarius PM. Weening RS. Ochs HD. Roos D. Verhoeven AJ. A point mutation in gp91-phox of cytochrome b558 of the human NADPH oxidase leading to defective translocation of the cytosolic proteins p47phox and p67phox. J Clin Invest. 1994;93:2120–2126. doi: 10.1172/JCI117207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leusen JH. Meischl C. Eppink MH. Hilarius PM. de Boer M. Weening RS. Ahlin A. Sanders L. Goldblatt D. Skopczynska H. Bernatowska E. Palmblad J. Verhoeven AJ. van Berkel WJ. Roos D. Four novel mutations in the gene encoding gp91-phox of human NADPH oxidase: consequences for oxidase assembly. Blood. 2000;95:666–673. [PubMed] [Google Scholar]
- 65.Li S. Tabar SS. Malec V. Eul BG. Klepetko W. Weissmann N. Grimminger F. Seeger W. Rose F. Hanze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal. 2008;10:1687–1698. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 66.Liu JQ. Zelko IN. Erbynn EM. Sham JS. Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 67.Looi YH. Grieve DJ. Siva A. Walker SJ. Anilkumar N. Cave AC. Marber M. Monaghan MJ. Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–325. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 68.Luxen S. Belinsky SA. Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 2008;68:1037–1045. doi: 10.1158/0008-5472.CAN-07-5782. [DOI] [PubMed] [Google Scholar]
- 69.MacRedmond RE. Greene CM. Dorscheid DR. McElvaney NG. O'Neill SJ. Epithelial expression of TLR4 is modulated in COPD and by steroids, salmeterol and cigarette smoke. Respir Res. 2007;8:84. doi: 10.1186/1465-9921-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manoury B. Nenan S. Leclerc O. Guenon I. Boichot E. Planquois JM. Bertrand CP. Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res. 2005;6:11. doi: 10.1186/1465-9921-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maranchie JK. Zhan Y. Nox4 is critical for hypoxia-inducible factor 2-alpha transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Res. 2005;65:9190–9193. doi: 10.1158/0008-5472.CAN-05-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marriott HM. Jackson LE. Wilkinson TS. Simpson AJ. Mitchell TJ. Buttle DJ. Cross SS. Ince PG. Hellewell PG. Whyte MK. Dockrell DH. Reactive oxygen species regulate neutrophil recruitment and survival in pneumococcal pneumonia. Am J Respir Crit Care Med. 2008;177:887–895. doi: 10.1164/rccm.200707-990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martinez-Losa M. Cortijo J. Juan G. O'Connor JE. Sanz MJ. Santangelo F. Morcillo EJ. Inhibitory effects of N-acetylcysteine on the functional responses of human eosinophils in vitro. Clin Exp Allergy. 2007;37:714–722. doi: 10.1111/j.1365-2222.2007.02694.x. [DOI] [PubMed] [Google Scholar]
- 74.Martyn KD. Frederick LM. von Loehneysen K. Dinauer MC. Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 75.Masamune A. Watanabe T. Kikuta K. Satoh K. Shimosegawa T. NADPH oxidase plays a crucial role in the activation of pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G99–G108. doi: 10.1152/ajpgi.00272.2007. [DOI] [PubMed] [Google Scholar]
- 76.McMahon S. Grondin F. McDonald PP. Richard DE. Dubois CM. Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: impact on the bioactivation of proproteins. J Biol Chem. 2005;280:6561–6569. doi: 10.1074/jbc.M413248200. [DOI] [PubMed] [Google Scholar]
- 77.Meng QR. Gideon KM. Harbo SJ. Renne RA. Lee MK. Brys AM. Jones R. Gene expression profiling in lung tissues from mice exposed to cigarette smoke, lipopolysaccharide, or smoke plus lipopolysaccharide by inhalation. Inhal Toxicol. 2006;18:555–568. doi: 10.1080/08958370600686226. [DOI] [PubMed] [Google Scholar]
- 78.Mitsushita J. Lambeth JD. Kamata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 2004;64:3580–3585. doi: 10.1158/0008-5472.CAN-03-3909. [DOI] [PubMed] [Google Scholar]
- 79.Mittal M. Roth M. Konig P. Hofmann S. Dony E. Goyal P. Selbitz AC. Schermuly RT. Ghofrani HA. Kwapiszewska G. Kummer W. Klepetko W. Hoda MA. Fink L. Hanze J. Seeger W. Grimminger F. Schmidt HH. Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 80.Mochizuki T. Furuta S. Mitsushita J. Shang WH. Ito M. Yokoo Y. Yamaura M. Ishizone S. Nakayama J. Konagai A. Hirose K. Kiyosawa K. Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–3707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 81.Morgenstern DE. Gifford MA. Li LL. Doerschuk CM. Dinauer MC. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med. 1997;185:207–218. doi: 10.1084/jem.185.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moskwa P. Lorentzen D. Excoffon KJ. Zabner J. McCray PB., Jr Nauseef WM. Dupuy C. Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murray LA. Knight DA. McAlonan L. Argentieri R. Joshi A. Shaheen F. Cunningham M. Alexopolou L. Flavell RA. Sarisky RT. Hogaboam CM. Deleterious role of TLR3 during hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. 2008;178:1227–1237. doi: 10.1164/rccm.200807-1020OC. [DOI] [PubMed] [Google Scholar]
- 84.Nagai K. Betsuyaku T. Suzuki M. Nasuhara Y. Kaga K. Kondo S. Nishimura M. Dual oxidase 1 and 2 expression in airway epithelium of smokers and patients with mild/moderate chronic obstructive pulmonary disease. Antioxid Redox Signal. 2008;10:705–714. doi: 10.1089/ars.2007.1941. [DOI] [PubMed] [Google Scholar]
- 85.Nakano Y. Longo-Guess CM. Bergstrom DE. Nauseef WM. Jones SM. Banfi B. Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J Clin Invest. 2008;118:1176–1185. doi: 10.1172/JCI33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Newburger PE. Skalnik DG. Hopkins PJ. Eklund EA. Curnutte JT. Mutations in the promoter region of the gene for gp91phox in X-linked chronic granulomatous disease with decreased expression of cytochrome b558. J Clin Invest. 1994;94:1205–1211. doi: 10.1172/JCI117437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nisbet RE. Graves AS. Kleinhenz DJ. Rupnow HL. Reed AL. Fan TH. Mitchell PO. Sutliff RL. Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol. 2009;40:601–609. doi: 10.1165/rcmb.2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noguera A. Batle S. Miralles C. Iglesias J. Busquets X. MacNee W. Agusti AG. Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax. 2001;56:432–437. doi: 10.1136/thorax.56.6.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohshiro Y. Ma RC. Yasuda Y. Hiraoka-Yamamoto J. Clermont AC. Isshiki K. Yagi K. Arikawa E. Kern TS. King GL. Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes. 2006;55:3112–3120. doi: 10.2337/db06-0895. [DOI] [PubMed] [Google Scholar]
- 90.Paffenholz R. Bergstrom RA. Pasutto F. Wabnitz P. Munroe RJ. Jagla W. Heinzmann U. Marquardt A. Bareiss A. Laufs J. Russ A. Stumm G. Schimenti JC. Bergstrom DE. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parinandi NL. Kleinberg MA. Usatyuk PV. Cummings RJ. Pennathur A. Cardounel AJ. Zweier JL. Garcia JG. Natarajan V. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L26–L38. doi: 10.1152/ajplung.00123.2002. [DOI] [PubMed] [Google Scholar]
- 92.Park HS. Chun JN. Jung HY. Choi C. Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res. 2006;72:447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 93.Park HS. Jung HY. Park EY. Kim J. Lee WJ. Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 94.Pendyala S. Gorshkova IA. Usatyuk P. He D. Pennathur A. Lambeth JD. Thannickal VJ. Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal. doi: 10.1089/ars.2008.2203. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pendyala S. Usatyuk P. Gorshkova IA. Garcia JG. Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal. doi: 10.1089/ars.2008.2231. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perner A. Andresen L. Pedersen G. Rask-Madsen J. Superoxide production and expression of NAD(P)H oxidases by transformed and primary human colonic epithelial cells. Gut. 2003;52:231–236. doi: 10.1136/gut.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peters EA. Hiltermann JT. Stolk J. Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic Biol Med. 2001;31:1442–1447. doi: 10.1016/s0891-5849(01)00725-0. [DOI] [PubMed] [Google Scholar]
- 98.Pierrou S. Broberg P. O'Donnell RA. Pawlowski K. Virtala R. Lindqvist E. Richter A. Wilson SJ. Angco G. Moller S. Bergstrand H. Koopmann W. Wieslander E. Stromstedt PE. Holgate ST. Davies DE. Lund J. Djukanovic R. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:577–586. doi: 10.1164/rccm.200607-931OC. [DOI] [PubMed] [Google Scholar]
- 99.Pollock JD. Williams DA. Gifford MA. Li LL. Du X. Fisherman J. Orkin SH. Doerschuk CM. Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 100.Rada B. Lekstrom K. Damian S. Dupuy C. Leto TL. The pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol. 2008;181:4883–4893. doi: 10.4049/jimmunol.181.7.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reeves EP. Lu H. Jacobs HL. Messina CG. Bolsover S. Gabella G. Potma EO. Warley A. Roes J. Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 102.Royer-Pokora B. Kunkel LM. Monaco AP. Goff SC. Newburger PE. Baehner RL. Cole FS. Curnutte JT. Orkin SH. Cloning the gene for an inherited human disorder—chronic granulomatous disease—on the basis of its chromosomal location. Nature. 1986;322:32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- 103.Sadikot RT. Zeng H. Yull FE. Li B. Cheng DS. Kernodle DS. Jansen ED. Contag CH. Segal BH. Holland SM. Blackwell TS. Christman JW. p47phox deficiency impairs NF-kappa B activation and host defense in Pseudomonas pneumonia. J Immunol. 2004;172:1801–1808. doi: 10.4049/jimmunol.172.3.1801. [DOI] [PubMed] [Google Scholar]
- 104.Sanchez-Elsner T. Botella LM. Velasco B. Corbi A. Attisano L. Bernabeu C. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–38535. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- 105.Schwarzer C. Machen TE. Illek B. Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- 106.Segal BH. Leto TL. Gallin JI. Malech HL. Holl SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 107.Serrander L. Cartier L. Bedard K. Banfi B. Lardy B. Plastre O. Sienkiewicz A. Forro L. Schlegel W. Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shao MX. Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2005;102:767–772. doi: 10.1073/pnas.0408932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Snelgrove RJ. Edwards L. Rae AJ. Hussell T. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur J Immunol. 2006;36:1364–1373. doi: 10.1002/eji.200635977. [DOI] [PubMed] [Google Scholar]
- 110.Snelgrove RJ. Edwards L. Williams AE. Rae AJ. Hussell T. In the absence of reactive oxygen species, T cells default to a Th1 phenotype and mediate protection against pulmonary Cryptococcus neoformans infection. J Immunol. 2006;177:5509–5516. doi: 10.4049/jimmunol.177.8.5509. [DOI] [PubMed] [Google Scholar]
- 111.Sousa SA. Ulrich M. Bragonzi A. Burke M. Worlitzsch D. Leitao JH. Meisner C. Eberl L. Sa-Correia I. Doring G. Virulence of Burkholderia cepacia complex strains in gp91phox−/− mice. Cell Microbiol. 2007;9:2817–2825. doi: 10.1111/j.1462-5822.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 112.Stas S. Whaley-Connell A. Habibi J. Appesh L. Hayden MR. Karuparthi PR. Qazi M. Morris EM. Cooper SA. Link CD. Stump C. Hay M. Ferrario C. Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology. 2007;148:3773–3780. doi: 10.1210/en.2006-1691. [DOI] [PubMed] [Google Scholar]
- 113.Stolk J. Rossie W. Dijkman JH. Apocynin improves the efficacy of secretory leukocyte protease inhibitor in experimental emphysema. Am J Respir Crit Care Med. 1994;150:1628–1631. doi: 10.1164/ajrccm.150.6.7952625. [DOI] [PubMed] [Google Scholar]
- 114.Sturrock A. Cahill B. Norman K. Huecksteadt TP. Hill K. Sanders K. Karwande SV. Stringham JC. Bull DA. Gleich M. Kennedy TP. Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 115.Suh YA. Arnold RS. Lassegue B. Shi J. Xu X. Sorescu D. Chung AB. Griendling KK. Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 116.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 117.Syrkina O. Jafari B. Hales CA. Quinn DA. Oxidant stress mediates inflammation and apoptosis in ventilator-induced lung injury. Respirology. 2008;13:333–340. doi: 10.1111/j.1440-1843.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- 118.Szanto I. Rubbia-Brandt L. Kiss P. Steger K. Banfi B. Kovari E. Herrmann F. Hadengue A. Krause KH. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol. 2005;207:164–176. doi: 10.1002/path.1824. [DOI] [PubMed] [Google Scholar]
- 119.Szatrowski TP. Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 120.Taira M. Toba H. Murakami M. Iga I. Serizawa R. Murata S. Kobara M. Nakata T. Spironolactone exhibits direct renoprotective effects and inhibits renal renin-angiotensin-aldosterone system in diabetic rats. Eur J Pharmacol. 2008;589:264–271. doi: 10.1016/j.ejphar.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 121.Teimourian S. Zomorodian E. Badalzadeh M. Pouya A. Kannengiesser C. Mansouri D. Cheraghi T. Parvaneh N. Characterization of six novel mutations in CYBA: the gene causing autosomal recessive chronic granulomatous disease. Br J Haematol. 2008;141:848–851. doi: 10.1111/j.1365-2141.2008.07148.x. [DOI] [PubMed] [Google Scholar]
- 122.Teshima S. Tsunawaki S. Rokutan K. Helicobacter pylori lipopolysaccharide enhances the expression of NADPH oxidase components in cultured guinea pig gastric mucosal cells. FEBS Lett. 1999;452:243–246. doi: 10.1016/s0014-5793(99)00636-5. [DOI] [PubMed] [Google Scholar]
- 123.Thabut G. El-Benna J. Samb A. Corda S. Megret J. Leseche G. Vicaut E. Aubier M. Boczkowski J. Tumor necrosis factor-alpha increases airway smooth muscle oxidants production through a NADPH oxidase-like system to enhance myosin light chain phosphorylation and contractility. J Biol Chem. 2002;277:22814–22821. doi: 10.1074/jbc.M200315200. [DOI] [PubMed] [Google Scholar]
- 124.Thannickal VJ. Oxygen in the evolution of complex life and the price we pay. Am J Respir Cell Mol Biol. 2009;40:507–510. doi: 10.1165/rcmb.2008-0360PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thannickal VJ. Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270:30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 126.Thannickal VJ. Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 127.Thannickal VJ. Lee DY. White ES. Cui Z. Larios JM. Chacon R. Horowitz JC. Day RM. Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 128.Tomasek JJ. Gabbiani G. Hinz B. Chaponnier C. Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 129.Tominaga K. Kawahara T. Sano T. Toida K. Kuwano Y. Sasaki H. Kawai T. Teshima-Kondo S. Rokutan K. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radic Biol Med. 2007;43:1627–1638. doi: 10.1016/j.freeradbiomed.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 130.Uhal BD. Kim JK. Li X. Molina-Molina M. Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages. Curr Pharm Des. 2007;13:1247–1256. doi: 10.2174/138161207780618885. [DOI] [PubMed] [Google Scholar]
- 131.Usatyuk PV. Romer LH. He D. Parinandi NL. Kleinberg ME. Zhan S. Jacobson JR. Dudek SM. Pendyala S. Garcia JG. Natarajan V. Regulation of hyperoxia-induced NADPH oxidase activation in human lung endothelial cells by the actin cytoskeleton and cortactin. J Biol Chem. 2007;282:23284–23295. doi: 10.1074/jbc.M700535200. [DOI] [PubMed] [Google Scholar]
- 132.Usatyuk PV. Vepa S. Watkins T. He D. Parinandi NL. Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal. 2003;5:723–730. doi: 10.1089/152308603770380025. [DOI] [PubMed] [Google Scholar]
- 133.Ushio-Fukai M. Tang Y. Fukai T. Dikalov SI. Ma Y. Fujimoto M. Quinn MT. Pagano PJ. Johnson C. Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 134.van Duin D. Allore HG. Mohanty S. Ginter S. Newman FK. Belshe RB. Medzhitov R. Shaw AC. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J Infect Dis. 2007;195:1590–1597. doi: 10.1086/516788. [DOI] [PubMed] [Google Scholar]
- 135.Vaquero EC. Edderkaoui M. Pandol SJ. Gukovsky I. Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 136.Vejrazka M. Micek R. Stipek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta. 2005;1722:143–147. doi: 10.1016/j.bbagen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 137.von Lohneysen K. Noack D. Jesaitis AJ. Dinauer MC. Knaus UG. Mutational analysis reveals distinct features of the Nox4-p22phox complex. J Biol Chem. 2008;283:35273–35282. doi: 10.1074/jbc.M804200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Waghray M. Cui Z. Horowitz JC. Subramanian IM. Martinez FJ. Toews GB. Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 139.Wang P. Tang F. Li R. Zhang H. Chen S. Liu P. Huang H. Contribution of different Nox homologues to cardiac remodeling in two-kidney two-clip renovascular hypertensive rats: effect of valsartan. Pharmacol Res. 2007;55:408–417. doi: 10.1016/j.phrs.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 140.Wang W. Suzuki Y. Tanigaki T. Rank DR. Raffin TA. Effect of the NADPH oxidase inhibitor apocynin on septic lung injury in guinea pigs. Am J Respir Crit Care Med. 1994;150:1449–1452. doi: 10.1164/ajrccm.150.5.7952574. [DOI] [PubMed] [Google Scholar]
- 141.Ware LB. Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 142.Wesley UV. Bove PF. Hristova M. McCarthy S. van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem. 2007;282:3213–3220. doi: 10.1074/jbc.M606533200. [DOI] [PubMed] [Google Scholar]
- 143.Whaley-Connell A. Habibi J. Nistala R. Cooper SA. Karuparthi PR. Hayden MR. Rehmer N. DeMarco VG. Andresen BT. Wei Y. Ferrario C. Sowers JR. Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension. 2008;51:474–480. doi: 10.1161/HYPERTENSIONAHA.107.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wilborn AM. Evers LB. Canada AT. Oxygen toxicity to the developing lung of the mouse: role of reactive oxygen species. Pediatr Res. 1996;40:225–232. doi: 10.1203/00006450-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 145.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 146.Wright JM. Merlo CA. Reynolds JB. Zeitlin PL. Garcia JG. Guggino WB. Boyle MP. Respiratory epithelial gene expression in patients with mild and severe cystic fibrosis lung disease. Am J Respir Cell Mol Biol. 2006;35:327–336. doi: 10.1165/rcmb.2005-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang Z. Sharma AK. Marshall M. Kron IL. Laubach VE. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. Am J Respir Cell Mol Biol. 2009;40:375–381. doi: 10.1165/rcmb.2008-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yao H. Edirisinghe I. Yang SR. Rajendrasozhan S. Kode A. Caito S. Adenuga D. Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol. 2008;172:1222–1237. doi: 10.2353/ajpath.2008.070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang Q. Chatterjee S. Wei Z. Liu WD. Fisher AB. Rac and PI3 kinase mediate endothelial cell-reactive oxygen species generation during normoxic lung ischemia. Antioxid Redox Signal. 2008;10:679–689. doi: 10.1089/ars.2007.1521. [DOI] [PubMed] [Google Scholar]
- 151.Zhang X. Shan P. Jiang G. Cohn L. Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao G. al-Mehdi AB. Fisher AB. Anoxia-reoxygenation versus ischemia in isolated rat lungs. Am J Physiol. 1997;273:L1112–L1117. doi: 10.1152/ajplung.1997.273.6.L1112. [DOI] [PubMed] [Google Scholar]