Abstract
Mitochondria are the primary intracellular site of oxygen consumption and the major source of reactive oxygen species (ROS), most of them originating from the mitochondrial respiratory chain. Among the arsenal of antioxidants and detoxifying enzymes existing in mitochondria, mitochondrial glutathione (mGSH) emerges as the main line of defense for the maintenance of the appropriate mitochondrial redox environment to avoid or repair oxidative modifications leading to mitochondrial dysfunction and cell death. mGSH importance is based not only on its abundance, but also on its versatility to counteract hydrogen peroxide, lipid hydroperoxides, or xenobiotics, mainly as a cofactor of enzymes such as glutathione peroxidase or glutathione-S-transferase (GST). Many death-inducing stimuli interact with mitochondria, causing oxidative stress; in addition, numerous pathologies are characterized by a consistent decrease in mGSH levels, which may sensitize to additional insults. From the evaluation of mGSH influence on different pathologic settings such as hypoxia, ischemia/reperfusion injury, aging, liver diseases, and neurologic disorders, it is becoming evident that it has an important role in the pathophysiology and biomedical strategies aimed to boost mGSH levels. Antioxid. Redox Signal. 11, 2685–2700.
Introduction
The tripeptide glutathione (γ-l-glutamyl-l-cysteinyl-glycine, GSH), the main nonprotein thiol found in cells, is synthesized in the cytosol in two steps that require ATP. First is the formation of γ-glutamylcysteine from glutamate and cysteine, by the activity of the γ-glutamylcysteine synthetase, followed by the formation of GSH by the activity of GSH synthetase, which uses γ-glutamylcysteine and glycine as substrates (Fig. 1). The formation of γ-glutamylcysteine is the rate-limiting reaction in GSH synthesis and is feedback inhibited by GSH itself, a mechanism responsible for the regulation of cellular GSH concentration. Virtually all mammalian cells have the capacity to synthesize GSH. Because of the presence of cysteine in its backbone, GSH is essential in the regulation of disulfide bonds of proteins and in the disposal of electrophiles and oxidants (29, 51). Thus, the antioxidant function of GSH is mediated by this redox-active thiol group that becomes oxidized when GSH reduces target molecules. The importance of GSH as a cellular redox buffer is underscored by the fact that GSH displays a low redox potential (E'0 = −240 mV) and is found in high concentration in the cells (∼10–15 mM) (52, 72, 95).
FIG. 1.
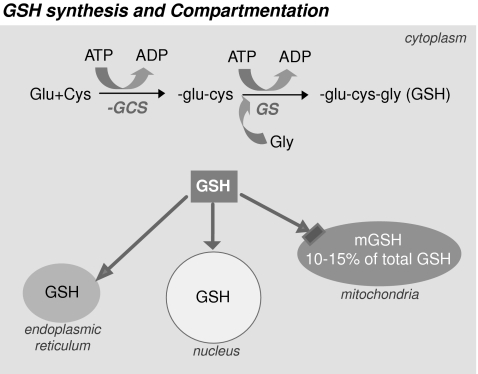
GSH synthesis and compartmentation. GSH is synthesized in the cytoplasm by the action of γ-glutamylcysteine synthetase (γ-GCS) and glutathione synthetase (GS), both enzymes requiring ATP. Once synthesized, GSH is distributed in the endoplasmic reticulum, nucleus, and mitochondria.
Although GSH was initially described as a potent antioxidant, many other cellular functions have been ascribed to GSH. At present, it is widely accepted that GSH acts not only as a reducing agent and a major antioxidant within the cells, but also as a mediator of many other physiologic reactions including metabolism of xenobiotics, thiol disulfide exchange reactions, and cellular signaling (cell-cycle regulation, proliferation, and apoptosis).
Despite its exclusive synthesis in the cytosol, GSH is distributed in intracellular organelles, including the endoplasmic reticulum (ER), nucleus, and mitochondria (Fig. 1). The compartmentalization of GSH includes separate redox pools that are distinct from the cytoplasmic pool in terms of the balance between GSH and GSSG forms, their redox potential, and their control of cellular activities (95). In the nucleus, GSH maintains critical protein sulfhydryls that are necessary for DNA repair and expression (141) and functions also as a hydrogen donor in ribonucleotide reductase–catalyzed reduction of ribonucleotides to deoxyribonucleotides and thus plays a contributory role in DNA synthesis (60). GSH is found predominantly in its reduced form, except in the ER, where it exits mainly as oxidized glutathione (GSSG), GSSG being the main source of oxidizing equivalents to provide the adequate environment necessary for favoring disulfide bond formation and the proper folding of nascent proteins (61). In mitochondria, however, GSH is found mainly in reduced form and represents a minor fraction of the total GSH pool (10–15%). Considering the volume of the mitochondrial matrix, the concentration of mitochondrial GSH (mGSH) is similar to that of cytosol (10–14 mM) (44, 48, 95).
GSSG is accumulated inside the cells, and the ratio of GSH to GSSG is a good measure of oxidative stress. The GSH/GSSG redox couple can readily interact with most of the physiologically relevant redox couples, undergoing reversible oxidation or reduction reactions, thereby maintaining the appropriate redox balance in the cell (95). Mitochondria are an excellent example of subcellular organelles whose function is closely linked to maintenance of redox balance. The mitochondria are the primary intracellular site of oxygen consumption and, despite the presence of a wide array of antioxidants and detoxifying enzymes, are the major source of reactive oxygen species (ROS), most of them originating from the mitochondrial respiratory chain. Conversely, the mitochondria are also a target for the damaging effect of oxygen radicals (38, 65, 109). Although normal electron transport in mitochondria involves four-electron reduction of molecular oxygen to water, partial reduction reactions occur even under physiologic conditions, causing release of O2∂− and hydrogen peroxide. In accordance with this, it has been estimated that the steady-state concentration of O2•− in the mitochondrial matrix is five- to tenfold higher than that in the cytosol (15). In addition, toxic or pathologic conditions that lead to an impairment of mitochondrial function can increase the release of ROS.
This review summarizes current knowledge of mGSH control of oxidative stress, mGSH transport, and its role in cellular apoptosis and in different pathologic settings.
Mitochondrial Control of Oxidative Stress
Although mitochondria are exposed to the constant generation of oxidant species, the organelle remains functional because of the existence of an antioxidant defense system to repair oxidative damage generated during normal aerobic metabolism. The main redox buffering systems in mitochondria are the glutathione, glutaredoxin, and thioredoxin systems (Fig. 2).
FIG. 2.
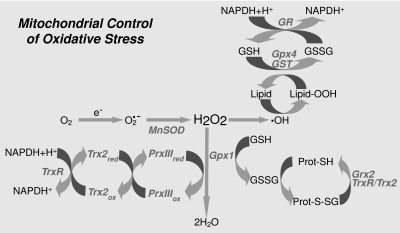
Mitochondrial control of oxidative stress. Scheme depicting the different reactions that take place inside the mitochondria to cope with the oxidative stress derived from the presence of anion superoxide, hydrogen peroxide, and hydroxyl radical. GSH peroxidase (Gpx); GSSG-reductase (GR); GSSG, glutaredoxin (Grx); Mn-dependent superoxide dismutase (MnSOD); thioredoxin-2 (Trx2); Trx-reductase (TrxR); peroxiredoxin III (PrxIII).
As mentioned earlier, the mitochondrial respiratory chain has long been recognized as an effective source of ROS, predominantly O2•−. Within the mitochondrial matrix, Mn-dependent superoxide dismutase (MnSOD) converts O2•− into hydrogen peroxide. If the accumulation of hydrogen peroxide is not limited, it can diffuse to the cytosol or participate in a chain of reactions that generate more reactive free radicals, such as hydroxyl radical, which can oxidize mitochondrial components (proteins, lipids, and DNA). Therefore, mitochondria must ensure a balance between the activity of MnSOD and the GSH redox cycle to dispose of hydrogen peroxide efficiently (38, 95). Because mitochondria lack catalase, the metabolism of hydrogen peroxide is accomplished mainly by GSH, with the participation of either GSH peroxidase or peroxiredoxin, and by the later conversion of GSSG back into GSH by the NADPH-dependent GSSG reductase (GR). Among GSH peroxides (Gpxs) that detoxify hydrogen peroxide, Gpx1 is the major isoform localized mainly in the cytosol, with small fraction also present in the mitochondrial matrix.
mGSH is also the primary defense against oxidative damage to mitochondrial membranes by ensuring the reduction of hydroperoxide groups on phospholipids and other lipid peroxides through the actions of mitochondrial GSH-S-transferases (GSTs), that exhibit modest Se-independent Gpx activity, and of Gpx4, a membrane-associated enzyme that is partly localized to the intermembrane space of the mitochondria (19, 109, 129). Actually, because of its capacity to reduce hydroperoxide groups on phospholipids, cholesteryl esters, and lipoproteins, Gpx4 is considered a critical defense enzyme in protecting membranes against oxidative stress. The critical role of Gpx4 is underscored by the fact that whereas Gpx1-knockout mice are fully viable, Gpx4-knockout animals demonstrate embryonic lethality, and Gpx4+/− cell lines are markedly sensitive to inducers of oxidative stress, including γ-irradiation, tert-butyl-hydroperoxide, and hydrogen peroxide, as compared with cell lines derived from wild-type control littermates (147).
Moreover, GSH can act nonenzymatically by reacting with electrophiles that are generated as a consequence of metabolic processes involving both endogenous compounds and xenobiotics, although this reaction is greatly enhanced in the presence of GSTs. Although several cytosolic GSTs isoforms occur (at least 17 in humans), the only GST present in the mitochondria is the class kappa isoform (57).
The GSH system collaborates also with the glutaredoxin system (Fig. 2). Under oxidative-stress conditions, the level of GSSG increases, which in turn enhances the concentration of protein mixed disulfides. A significant number of proteins can be altered in their function by formation of mixed disulfides. Glutaredoxin (Grx), a GSH-dependent disulfide oxidoreductase, catalyzes dithiol reactions, reducing GSH-protein mixed disulfides in a coupled system with GR and NADPH as an electron donor (58, 72). Two known Grx isoforms are found in mammals, Grx1 and Grx2. Grx1 is of cytosolic expression, and Grx2 localizes to both the mitochondrial matrix and nucleus (90).
Finally, the thioredoxin (Trx) system (Fig. 2), which includes Trx and Trx-reductase (TrxR), contributes to protein thiol maintenance. When Trx is in a reduced state, Trx-(SH)2, the two active-site cysteines form a dithiol group that is able to catalyze the reduction of disulfides in a number of proteins. Oxidized thioredoxin (Trx-S2) can be reduced by NADPH through the catalytic action of the TrxR (113). In addition, the Trx system can interact also with the peroxiredoxins (Prxs), which constitute a novel family of thiol-specific peroxidases that rely on Trx as the hydrogen donor for the reduction of hydrogen peroxides and lipid-hydroperoxides (16). One Prx isoform, PrxIII, is exclusively detected in mitochondria. On reaction with hydrogen peroxide, the redox-sensitive cysteine residue of each subunit of PrxIII homodimer is oxidized to Cys-SOH, which then reacts with neighboring Cys-SH of the other subunit to form an intermolecular disulfide that can be readily reduced by Trx2 (18, 109).
mGSH Transport
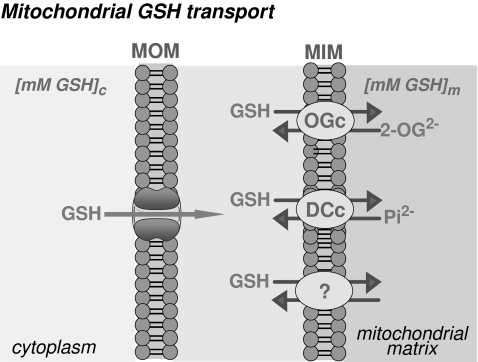
As noted earlier, the concentration of mGSH is in the range of cytosol GSH. However, unlike cytosol, mitochondria do not contain the enzymatic machinery to synthesize GSH from its constituent amino acids (48). GSH can cross easily the mitochondrial outer membrane (MOM) through porin channels. However, given the anionic nature of GSH at physiologic pH, it cannot diffuse across the mitochondrial inner membrane (MIM) into the matrix because of the oxidative phosphorylative–mediated negative membrane potential relative to the cytoplasm. Oxidized GSSG cannot be exported into cytosol, so matrix NADPH reduces GSSG to GSH in the matrix through GR. Consequently, mGSH arises from the cytosol GSH into the mitochondrial matrix by a carrier-mediated transport located in the MIM that overcomes the unfavorable entry against an electrochemical gradient (26, 45, 78, 96) (Fig. 3).
FIG. 3.
Mitochondrial GSH transport. GSH moves easily through the mitochondrial outer membrane (MOM); however, it needs carrier-mediated transport to cross the mitochondrial inner membrane (MIM). The dicarboxylate carrier (DCc) and the 2-oxoglutarate carrier (OGc) have been shown to function as GSH transporters. In addition, the presence of unidentified additional carriers cannot be discarded at present.
Out of several cloned mitochondrial membrane carriers (112), the dicarboxylate and the 2-oxoglutarate have been shown to function as GSH-transporting polypeptides. These two antiport carriers are electroneutral, because they catalyze the exchange of anions so that no net transfer of charge occurs across the MIM (20, 21, 78) (Fig. 3). The reconstitution of recombinant mitochondrial dicarboxylate carrier into proteoliposomes showed a transport activity for GSH with kinetics, substrate specificity, and inhibitor sensitivity similar to those observed in rat kidney proximal tubules (77). Furthermore, Lash et al. (77) showed that overexpression of the rat kidney dicarboxylate carrier in a cell line derived from rat kidney proximal tubules (NRK-52E) increased the mGSH pool size by two- to 10-fold with respect to wild-type NRK-52E cells, thus demonstrating a role for this carrier in the mitochondrial transport of GSH in exchange with inorganic phosphate. Evidence for a role of the 2-oxoglutarate carrier as a GSH transporter was based on substrate specificity and pattern of inhibition in rabbit kidney mitochondria and in a partially purified preparation of mitochondrial transporters from kidney mitoplasts reconstituted in proteoliposomes (20, 21). In addition, the functional expression of the hepatic 2-oxoglutarate carrier in mitochondria from Xenopus laevis, showing the transport of reduced GSH in a phenylsuccinate-sensitive manner (26), further confirmed the suggestion that the 2-oxoglutarate carrier contributes to the transport of GSH in liver mitochondria. The initial rate of 2-oxoglutarate transport in rat liver mitochondria was reduced after depletion of mGSH, suggesting a 2-oxoglutarate/GSH exchange. In contrast to the transport of GSH in kidney mitochondria, which occurs with a single kinetic component (21), the kinetics of GSH in rat liver mitochondria displays a high-affinity and a low-affinity component (25, 96). Moreover, the kinetics of 2-oxoglutarate transport in rat liver mitochondria exhibited a single Michaelis–Menten component with sensitivity to phenylsuccinate and GSH; in addition, a mutual cis competition was found in the mitochondrial transport of 2-oxoglutarate and GSH (26). Intriguingly, the kinetic parameters of the low-affinity component of GSH transport (25, 96) and that of 2-oxoglutarate (26) are similar, both showing a Michaelis constant in the micromolar range and a similar maximal velocity. Although this suggests that the 2-oxoglutarate carrier may be responsible for the low-affinity transport of GSH, alternative carrier(s) may account for the high-affinity transport of GSH in rat liver mitochondria (25, 96) (Fig. 3). In line with this, a recent study showed that incubation of rat liver mitochondria with butylmalonate (a dicarboxylate carrier inhibitor) and phenylsuccinate (a 2-oxoglutarate carrier inhibitor) inhibited GSH uptake by 45–50%, suggesting that these two transporters are only partially responsible for GSH uptake in rat liver mitochondria (153). Moreover, in H4IIE cells, a rat hepatoma cell line, that were stably transfected with the cDNA for the 2-oxoglutarate carrier, exhibited increased uptake of GSH and 2-oxoglutarate and were protected from cytotoxicity induced by hydrogen peroxide, methyl vinyl ketone, or cisplatin, demonstrating the protective function of increased mitochondrial GSH transport and mGSH levels in the liver (153).
Because 2-oxoglutarate is a dicarboxylate, it could share the dicarboxylate carrier for its mitochondrial entry; thus, the competition seen between 2-oxoglutarate and GSH does not discard the participation of dicarboxylate carrier in the transport of GSH in liver mitochondria (26). Although clear evidence exists of the participation of the dicarboxylic carrier in kidney (78), its contribution in the transport of GSH in rat liver seems unlikely for two reasons. First, the transport of 2-oxoglutarate appears to occur preferentially through the 2-oxoglutarate carrier, based on the differential sensitivity to glutamate inhibition between the 2-oxoglutarate and dicarboxylate (20, 21, 77). More important, in contrast to the evidence reported from rabbit kidney, the functional expression in oocytes microinjected with the dicarboxylate cRNA from HepG2 cells or rat liver did not result in significant GSH transport activity (26). The reasons for the differential behavior of the dicarboxylic carrier in kidney and liver are unknown and probably reflect that interorgan or intercellular differences may exist regarding mGSH transport.
The function of membrane proteins, including carriers, can be modulated by the microenvironment of the membrane where they are inserted. Both the cholesterol/phospholipid ratio and the saturation state of fatty acyl groups in phospholipids contribute to membrane fluidity changes. Cholesterol enrichment of cellular or artificial membranes has been shown to decrease membrane fluidity.
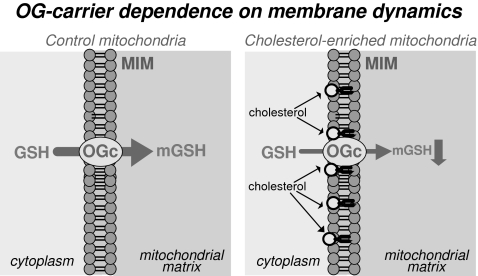
One of the highlights of mGSH transport through the 2-oxoglutarate carrier in the liver is its dependence on appropriate membrane dynamics. For instance, the initial rate of 2-oxoglutarate transport was reduced in mitochondria from alcohol-fed rat livers, because of changes in mitochondrial membrane fluidity induced by alcohol (26). The main determinant for the increase in membrane rigidity in mitochondria after ethanol intake was a higher cholesterol/phospholipids molar ratio (25) (Fig. 4). The fluidization of mitochondria by the fluidizing agent 2-(2-methoxyethoxy)ethyl 8-(cis-2-n-octylcyclopropyl) (A2C) restored the initial transport rate of both GSH and 2-oxoglutarate. Additionally, these changes were reproduced in normal liver mitochondria enriched in cholesterol where the fluidization of cholesterol-enriched mitochondria with A2C restored the order membrane parameter and the mitochondrial 2-oxoglutarate uptake (26). In line with this observation, cholesterol-enriched mitochondria from the human hepatocellular cell line HepG2 displayed an impaired initial rate of GSH uptake at different GSH concentrations, an effect that was reversed on fluidization of mitochondria with A2C, whereas the uptake of ADP through the adenine nucleotide translocator was unaffected (85).
FIG. 4.
2-Oxoglutarate carrier dependence on membrane dynamics. A decrease in membrane fluidity in mitochondria, or an increased cholesterol/phospholipids molar ratio, such as that observed in rat liver mitochondria after chronic alcohol intake, results in impairment of the mitochondrial GSH transport through the 2-oxoglutarate carrier and, therefore, in a decrease in mGSH levels.
Secondary to the impaired mGSH transport after prolonged ethanol intake in rat liver is the depletion of mGSH (Fig. 4), underlying the selective depletion of mGSH in the hepatocytes from alcohol-fed rats (36, 37, 39), which has been confirmed by other investigations (reviewed in 38). In agreement with the impairment of mitochondrial GSH transport by alcohol intake, increasing cytosolic GSH levels with N-acetylcysteine, a GSH precursor, did not increase mGSH in hepatocytes from alcohol-fed rats (45). However, S-adenosyl-methionine, which, in addition to promoting GSH synthesis, was shown to prevent alterations in membrane lipid composition including cholesterol/phospholipids increase (103, 117), normalizes mGSH levels in alcohol-fed rats by preventing alcohol-induced membrane-fluidity loss (24, 45). Thus, alcohol-stimulated cholesterol increase and subsequent deposition in mitochondrial membrane accounts for the impairment of mitochondrial GSH transport in mitochondria, leading to depleted mGSH levels.
The key contribution of mGSH in preserving cell viability was first proposed by Meredith and Reed (98, 99) on the basis of the greatly increased death of hepatocytes when both GSH pools were experimentally depleted compared with cells with losses of cytoplasmic GSH alone. Accordingly, in the next sections, we discuss recent data showing the importance of mGSH in cell regulation and in pathologic states.
mGSH and Apoptosis
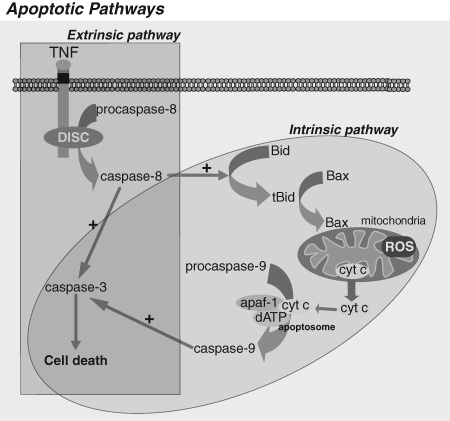
Apoptosis, also known as programmed cell death, describes a particular mode of cell death that is characterized by a series of biochemical events that lead to a variety of morphologic changes, including membrane blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation. Ultimately, apoptotic cells are ingested by neighboring cells and phagocytes, preventing inflammation and tissue damage that might ensue on cell lysis. Apoptosis is induced by two main routes involving either the mitochondria (the intrinsic pathway) or the activation of death receptors (the extrinsic pathway) (Fig. 5). Both pathways converge to induce the activation of caspases, the final executioners of cell death. Caspases are synthesized as proenzymes and require proteolytic processing to be catalytically active. Thus, caspase activation is the key in the apoptotic process. Caspases with long prodomains (i.e., caspase-2, -8, -9, and -10) belong to the group of initiator caspases, and those with short prodomain (i.e., caspase-3, -6, and -7) are the effector caspases. Initiator caspases initiate the apoptotic cascade and lead to the activation of effector caspases (8, 105, 152).
FIG. 5.
Apoptotic pathways. Apoptosis occurs through two main pathways: the extrinsic pathway or death-receptor pathway, and the intrinsic or mitochondrial pathway. Both pathways converge to a final common pathway involving the activation of caspase-3 that culminates in the execution of cell death. In many cell types, the extrinsic and intrinsic pathways are integrated through the cleavage of Bid by caspase-8, consequently conferring on mitochondria a central role in the control of cell death by apoptosis.
In the extrinsic pathway, or receptor-mediated pathway, ligand binding results in the formation of a multiprotein complex called the death-inducing signaling complex (DISC) and the activation of procaspase-8 (8, 105). Caspase-8 can directly activate procaspase-3, an effector caspase mainly responsible for the cleavage of target proteins essential for maintaining cellular viability, leading to apoptosis.
However, in certain cell types, in which the direct activation of caspase 3 by caspase-8 is weak, caspase-8 first cleaves Bid, a Bcl2 family member protein that induces the translocation, oligomerization, and insertion of other Bcl2-family member such as Bax and/or Bak into the MOM (130), resulting in the integration of the extrinsic and intrinsic pathways, the latter also known as the mitochondrial pathway (Fig. 5). This is followed by permeabilization of the MOM and release of several proteins from the mitochondrial intermembrane space, including cytochrome c. Once cytochrome c is released, it binds with Apaf-1 and ATP, recruiting procaspase-9 to create a protein complex known as an apoptosome. The apoptosome cleaves procaspase-9 to its active form, caspase-9, which in turn activates the effector caspase-3, resulting in the execution of apoptosis (79).
Similarly, during the execution of the intrinsic or mitochondrial pathway, death signals converge in the mitochondria at the level of translocation of Bax/Bak into the MOM, triggering the release of cytochrome c to the cytosol, commitment step of the mitochondrial apoptotic cascade (Fig. 5).
Thus, mitochondria are a central organelle in the execution of apoptosis, in both the extrinsic and the intrinsic pathways, and hence the regulation of release of cytochrome c from the mitochondrial intermembrane space is decisive during this process (59). GSH depletion has been connected to apoptosis either by predisposing the cells to cell death or by triggering the modulation of mitochondrial permeabilization and the activation of effector caspases (4, 27, 54, 106).
As alluded to earlier, because mGSH plays an essential role in the mitochondrial defense against constant ROS generation, the depletion of mGSH can pose a critical threat to the cell by sensitizing mitochondrial components and lipids to oxidative modifications by ROS and other reactive species that could compromise the vital mitochondrial function.
The role of mitochondria in ROS production and apoptosis and necrosis in mammalian cells is well established and has been reviewed extensively (10, 67, 95, 109). Accordingly, protection from oxidative stress by administration of antioxidants has been found to increase cell viability in multiple experimental model systems (32).
Because the concentration of GSH (GSH + GSSG) is so much higher in comparison to any other system (intracellular concentration of thioredoxin is 100- to 1,000-fold lower than glutathione), the GSH/GSSG pool within the cell dominates the intracellular redox environment and composes the principal redox system of the cell (67). Thus, depletion of mGSH below a certain threshold will endanger detoxification processes and allow accumulation of hydrogen peroxide and lipid hydroperoxides.
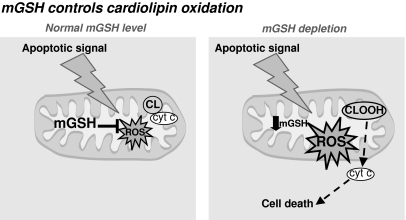
One of the molecules that can be affected by oxidative damage if mGSH levels are compromised is cardiolipin (Fig. 6). Cardiolipin, an anionic phospholipid found only in mitochondria, plays a key role in mitochondrial physiology and cell-death regulation. Because of its unique structure among phospholipids, cardiolipin confers fluidity and stability to the mitochondrial membrane. Cardiolipin is present almost exclusively in the MIM but can be also found at low levels in the MOM. Cytochrome c is normally bound to the MIM by association with cardiolipin, and it has been postulated that cardiolipin loss or peroxidation lessens the binding of cytochrome c to the MIM, favoring the permeabilization of isolated liver mitochondria and release of cytochrome c (66, 111).
FIG. 6.
mGSH control of cardiolipin oxidation. Under normal conditions, mGSH is able to cope with the stress derived from many apoptotic stimuli. However, depletion of mGSH below a certain threshold will compromise ROS detoxification, leading to its accumulation, resulting ultimately in cardiolipin oxidation.
An additional view on the role of cardiolipin in cytochrome c release comes from studies using synthetic liposomes or reconstituted membranes, demonstrating that cardiolipin is required for Bax-mediated pore formation by cooperating with Bax to form large-scale openings independent of the formation of large (>100 kDa) Bax aggregates (76).
Moreover, we previously showed that selective depletion of mGSH after incubation with 3-hydroxy-4-pentenoate (HP) sensitizes hepatocytes to tumor-necrosis factor-α (TNF-α) without interfering with NF-κB activation. By using this strategy, mGSH becomes depleted by 70–80% while sparing the cytosol pool of GSH (41). Moreover, we observed that acidic sphingomyelinase (ASMase)–induced ceramide generation plays a key role in TNF-induced hepatocellular death and liver injury through stimulation of mitochondrial ROS (mROS) (41, 94). In further analyzing the role of mGSH on the sensitization of hepatocytes to TNF, we observed that MOM permeabilization, cytochrome c release, and procaspase-3 activation take place only after mGSH depletion (93). These observations suggest that the pre-mitochondrial TNF signaling, including tBid generation and mitochondrial Bax activation, are independent of the mGSH status, yet their predicted consequences on mitochondria (MOM permeabilization) and downstream events (cytochrome c release and apoptosome assembly) are controlled by the levels of mGSH. This outcome implies that Bax insertion and oligomerization in MOM, which has been considered a critical determinant of MOM permeabilization, is not sufficient for TNF-induced hepatocellular death, requiring additional factors that potentiate or facilitate the ability of Bax to induce MOM permeabilization. The most salient observation in this study was that the formation of oxidized cardiolipin by TNF occurs only in mGSH-depleted hepatocytes, despite the presence of other mitochondrial antioxidant defenses such as PrxIII and Trx2, which are unaffected on mGSH depletion. This oxidized cardiolipin potentiated the ability of Bax to induce the permeabilization in MOM-like liposomes.
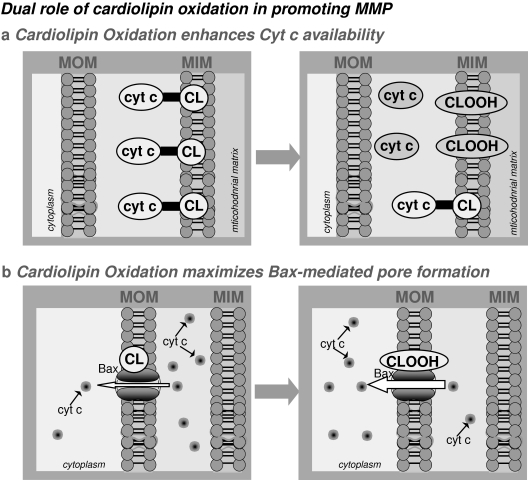
Thus, our findings not only indicate that mGSH levels control the oxidation state of cardiolipin, but they also describe a dual role for oxidized cardiolipin in TNF-induced MOM permeabilization and cell death (Fig. 7): the regulation of the availability of free cytochrome c and the optimization of the Bax-lipid pore formation due to structure stress and membrane remodelling (93). Additionally, these events were preceded by mitochondrial ROS overgeneration that predominantly oxidized cardiolipin, changes that were not observed in acidic ASMase−/− hepatocytes. Consequently, ASMase is a critical player in TNF-induced mitochondrial ROS and subsequent hepatocellular death (41, 94). The ROS overgeneration observed only after TNF challenge in mGSH-depleted cells is critical in the regulation of cell death, because it occurs very quickly (15–30 min after TNF), whereas NF-κB–dependent induction of survival genes take extra time (hours). In other words, the oxidative changes occurring in mGSH-depleted hepatocytes precede the upregulation of the NF-κB survival program, committing hepatocytes to TNF-mediated cell death.
FIG. 7.
Dual role of cardiolipin oxidation in promoting mitochondrial membrane permeabilization (MMP). Oxidized cardiolipin exerts a dual role in TNF-induced MOM permeabilization and cell death: (a) contributes to the availability of free cytochrome c; and (b) maximizes the Bax-lipid pore formation, without affecting Bax translocation and oligomerization to the MOM.
Thus, strategies to restore mGSH depletion or to prevent the impairment of its mitochondrial transport may be of therapeutic significance in the treatment of numerous pathologies, as discussed in the following section.
mGSH in Pathologic Settings
Hypoxia and reperfusion injury
Hypoxia occurs in tissues when the availability of O2 is insufficient for the cellular demand, as happens in physiologic (embryonic development, exercising muscle) and pathologic conditions (stroke, tissue ischemia, inflammation, solid-tumor formation) (6, 116, 126, 132, 143). Molecular oxygen is required for the maintenance of aerobic metabolism, being an essential electron acceptor in many cellular reactions, especially in the mitochondrial respiratory chain. Although hypoxia was previously shown to stimulate a burst of ROS of mitochondrial and extramitochondrial origin (e.g., xanthine oxidase), recent evidence demonstrated the critical role of ROS production from the mitochondrial electron-transport chain (65), in particular through superoxide release to the intermembrane space by complex III (13, 49). Under normal redox status, cells adapt and survive because of the existence of specific sensors and the activation of a number of genes stimulated by O2 deprivation. However, when the hypoxic insult is strong and sustained, or the mitochondrial antioxidant defense is compromised, or both, cell viability may be at risk. In this sense, mGSH depletion was shown to sensitize primary hepatocytes to O2-induced ROS generation, an effect prevented by rotenone plus TTFA, which abolishes mitochondrial ROS generation, or on GSH replenishment by GSH ester (86). These results suggest that mGSH restoration may be an effective strategy in protecting perivenous hepatocytes from chronic alcohol intake because of mGSH depletion and exposure to hypoxia and TNF (24, 86).
Among the genes stimulated by O2 deprivation, one of the best characterized is the hypoxia-inducible factor (HIF), a heterodimeric transcription factor [consisting of an O2-regulated α subunit (HIFα) and a constitutively expressed β subunit (HIFβ)] that upregulates genes involved in angiogenesis, proliferation, and glycolysis (6, 116, 126, 132, 143). In normoxic cells, the hydroxylation of proline residues in the oxygen domain within HIF by HIF prolyl hydroxylases (PHDs) enables the binding to the von Hippel–Lindau tumor-suppressor protein (VHL), leading to the ubiquitinization and subsequent proteasome-dependent degradation of HIF-1α subunits (68, 70). However, when O2 decreases, PHDs become inhibited, and HIF-α subunits are subsequently stabilized. Recent studies provided clear-cut evidence for a role of mitochondrial reactive oxygen species (mROS) in HIF-1α activation by using genetic and biochemical approaches (13, 49, 65, 86). Blocking superoxide anion production by suppressing the Rieske iron-sulfur protein of complex III impairs HIF-1α induction by hypoxia, and moreover, hydrogen peroxide or agents that produce mROS activate HIF-1α during normoxia (65), indicating a central role for ROS in HIF-1α stabilization.
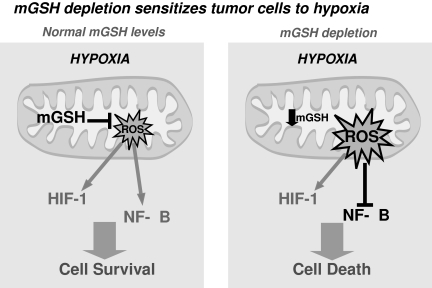
HIF-1α stabilization and NF-κB activation may also have a role in promoting the survival of cancer cells, angiogenesis, neovascularization, glycolytic ATP generation, and tumor invasion. Therefore, hypoxia-induced mROS may promote cancer development and progression. However, overgeneration of mROS, occurring after mGSH depletion or by blocking mitochondrial respiration (84, 114), may sensitize tumor cells by inhibiting the NF-κB survival pathway (Fig. 8). Because hypoxia is expected to affect predominantly cells from solid tumors, more than cells from healthy tissues, the combination of mGSH depletion, or strategies that increase mROS, and hypoxia may be an interesting approach in cancer therapy that deserves further study.
FIG. 8.
mGSH depletion sensitizes tumor cells to hypoxia. HIF-1α stabilization and NF-κB activation participate in promoting survival of cancer cells under hypoxic conditions. However, overgeneration of mitochondrial ROS, as obtained after mGSH depletion, may sensitize tumor cells by inhibiting the NF-κB survival pathway, despite HIF stabilization.
In addition, hypoxia-induced mROS have been shown to enhance the DNA binding of NF-κB through a redox-dependent mechanism (17). The canonic pathway of NF-κB activation by a wide variety of stimuli involves the phosphorylation of the inhibitory subunit IκB at specific serine residues that targets it subsequent degradation by the proteasome (5, 140), allowing NF-κB transactivating subunits to translocate to the nucleus and to bind specific sites in the promoter/enhancer region of target genes. However, an additional pathway of NF-κB activation without IκB-α proteolytic degradation (62) is triggered by hypoxia (73) because of the phosphorylation of IκB-α at tyrosine residues. Interestingly, this alternative NF-κB–activation pathway has been characterized in hypoxic cells by a c-Src–mediated mechanism (31, 84) that was prevented in mutants expressing a redox-insensitive c-Src (84). Similarly, tyrosine phosphorylation of IκB-α due to c-Src activation by mROS has been observed in hypoxia-related clinical settings, such as ischemia/reperfusion (I/R) liver injury (83, 154), in which NF-κB transcription of protective proteins in parenchymal cells emerges as a key mechanism regulating the final outcome (83). Therefore, ROS generation after (I/R) is responsible for the induction of Src-phosphorylation, NF-κB activation, and transcription of κB-dependent protective genes, such as MnSOD, in hepatocytes. However, extended ischemia induces a massive production of ROS and GSSG generation, which antagonizes NF-κB gene induction, similarly to that previously observed in hepatocytes after TNF exposure (87), consistent with the notion that NF-κB activation requires a balanced level of GSSG (30). The predominant bulk of ROS are generated during the reperfusion phase after ischemia, associated with increasing levels of TNF in serum and liver. Given that TNF is an important inducer of mROS, the role of mGSH may be key in I/R injury. TNF was shown to play a central role in I/R damage (124). However, the molecular signaling of TNF in I/R is not well established. In addition to the protein–protein interactions underlying the signaling of TNF on its binding to its receptor TNFR1, TNF also is known to give rise to sphingolipid intermediates, such as ceramide and ganglioside GD3, which have been shown to interact with mitochondria and generate ROS (42, 43). In line with this, prevention of ceramide production may be an effective strategy to protect the liver against I/R-induced injury (82). Thus, ROS generation may play a dual role in I/R injury. Although preventing ROS overgeneration by antioxidants may be of therapeutic value, the early counteraction of ROS generation during ischemic preconditioning was shown to cancel the protective mechanisms triggered by preconditioning, including MnSOD overexpression (120, 125).
Conversely, ischemia-mediated NF-κB–dependent induction of inflammatory proteins by Kupffer cells, resident macrophages in the liver, occurs mainly through a redox-independent canonic pathway of NF-κB activation during I/R (83) triggered by ligands such as TNF or LPS, consistent with the protection observed in knockout models that block this receptor-mediated signaling (89, 137) or after Kupffer cell inactivation (63, 82). Therefore, mitochondrial redox status and particularly mGSH may determine liver function after prolonged ischemia, and reduction of mitochondrial oxidative stress would be a valid therapeutic strategy to improve the outcome of livers exposed to I/R.
Liver diseases
The liver is one of the organs with the highest content of GSH, and alterations in its regulation can contribute to several pathologies. In particular, hepatic mitochondria are recognized as a major source of ROS, which in turn can regulate vital liver functions and disease pathogenesis (95).
Studies in animal models of prolonged ethanol feeding showed functional alterations in the oxidative phosphorylation, whereas patients with alcoholic steatohepatitis (ASH) displayed mitochondria with morphologic and functional aberrations (12, 123, 138). As mentioned earlier, one of the clues to the progression of ASH relates to the alcohol-mediated susceptibility of hepatocytes to cell death by reactive oxygen species (ROS) and inflammatory cytokines (38). TNF has been considered a key ASH mediator, with ASMase-mediated ceramide generation playing a critical role (41, 93). Furthermore, alcohol feeding has been shown to sensitize hepatocytes to TNF because of the limitation of mGSH through impaired transport of GSH to the mitochondria, as a result of the altered membrane-order parameter caused by mitochondrial cholesterol increase (26, 38, 85). Selective pharmacologic depletion of mGSH sensitizes hepatocytes to TNF-mediated cell death, which reproduces the observations found with alcohol feeding (24, 34). This selective mGSH depletion after alcohol intoxication was confirmed by others (144, 150, 151).
ASH and nonalcoholic steatohepatitis (NASH) are two of the most common forms of liver disease worldwide and represent an advanced stage in the spectrum of fatty liver diseases. Although the primary etiology of ASH and NASH is different, these two diseases show almost identical histology, characterized by steatosis (macrovesicular more than microvesicular), mixed lobular inflammation with scattered polymorphonuclear leukocytes as well as mononuclear cells, and hepatocellular cell death and ballooning due to sensitivity to oxidative stress and fibrosis. Besides the accumulation of lipids in the cytoplasm of hepatocytes, mostly in the form of free fatty acids (FFA) and triglycerides (TG), cytokine overexpression, particularly TNF and the membrane receptors, was shown to contribute to steatohepatitis and subsequent progression to hepatocellular apoptosis in both ASH and NASH (33, 148). Thus, the understanding of the mechanisms that determine the susceptibility of steatotic hepatocytes to inflammatory cytokines (e.g., TNF/Fas) is of relevance to develop novel therapeutic strategies for the treatment ASH and NASH. In a recent study, by using genetic and nutritional models of NASH, we observed that free cholesterol accumulation in mitochondria, but not TG or FFA loading, sensitizes hepatocytes to TNF, contributing to the transition from steatosis to steatohepatitis through mGSH depletion (92). In addition, the treatment of obese mice with atorvastatin prevented the free-cholesterol accumulation in mitochondria and the subsequent mGSH depletion, thus abolishing the susceptibility to cytokine-induced liver damage (92). Thus, because free-cholesterol deposition influences the mitochondrial transport of GSH by modulation of membrane physical properties, the transport of cholesterol to mitochondria may be of significance in pathologic states, such as in ASH and NASH, and merits further study (34).
In line with this, liver cirrhosis also is characterized by mitochondrial dysfunction, and similar findings on mGSH regulation have been reported (74, 75). For instance, secondary biliary cirrhosis in rats induced by bile-duct ligation caused the depletion of mGSH with respect to sham-operated controls; interestingly, this depletion preceded the decrease of GSH levels in liver homogenates (75). Although the mechanism involved was not further investigated, mitochondria from bile duct–ligated rats exhibited altered lipid composition with a two- to threefold increase in the cholesterol/phospholipid ratio in the mitochondrial inner membrane (74), thus suggesting the possibility of impaired transport of GSH into mitochondria.
Another clinical setting in which mGSH levels are of critical importance is in drug toxicity (97, 98). Acetaminophen, or N-acetyl-p-aminophenol, is a widely used analgesic considered to be safe when taken at therapeutic doses; however, it causes a potentially fatal, hepatic centrilobular necrosis when taken in overdose. Acetaminophen is metabolically activated by cytochrome P450 enzymes to a very reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which is detoxified by conjugation with GSH. Thus, acetaminophen overdose causes a severe depletion of GSH levels. The current clinical treatment, or antidote, for acetaminophen overdose is N-acetylcysteine, a GSH precursor that can replenish cellular GSH levels (101, 107). NAPQI initiates its toxicity by first attacking mitochondria (14, 107, 139), followed by mGSH depletion (142). Although acetaminophen can decrease GSH in both cytosol and mitochondria, the kinetics of GSH depletion in hepatocytes exposed to acetaminophen showed that the depletion of mGSH preceded that of cytosol GSH (142), indicating the relevance of this pool of GSH in the hepatotoxicity of acetaminophen. In addition, and similar to what happens with many other drugs, acetaminophen cytotoxicity is mediated through mitochondrial membrane permeabilization (47), and agents that block it, such as cyclosporin A, protect hepatocytes against acetaminophen-induced cell death (53). Hence, these observations highlight the importance of mGSH in drug toxicity.
Neurologic disorders
Despite the evidence for the important role of mGSH in nonneural cells, surprisingly little attention has been given to the properties and functions of this antioxidant pool in cells from the central nervous system (134). Cerebellar granule neurons in culture exhibit marked functional deterioration and die in response to complete loss of both the mitochondrial and cytoplasmic GSH, but not with cytoplasmic GSH loss alone (146). Additionally, although astrocytes with mGSH depletion did not show changes in viability compared with nondepleted mGSH astrocytes, they were much more susceptible to nitric oxide exposure (104).
It was reported that an early and selective loss of mGSH in mitochondria isolated from rat brain in a model of stroke by occlusion of the middle cerebral artery (2, 133) resulted in enhanced brain injury and repletion of mGSH with GSH-ester resulted in reduced infarct volume by >60% (1).
The brain is particularly vulnerable to oxidative damage because of its high oxygen use, content of oxidizable polyunsaturated fatty acids, and presence of redox-active metals (Cu, Fe). Oxidative stress increases with age, and therefore, it can be considered an important causative factor in several neurodegenerative disorders, such as Alzheimer disease (AD) (141). The brains of Alzheimer disease patients show a significant extent of oxidative damage associated with marked accumulation of amyloid-β peptide (Aβ), the main constituent of senile plaques in the brain (50, 136). Current evidence indicates that targeting of Aβ to intracellular sites, most notably mitochondria, causes oxidative stress, mitochondrial dysfunction, and cell death (80, 91). Aβ may cause mitochondrial ROS production, but also it is becoming clear that oxidative damage occurs early in the AD brain, even before the onset of significant plaque pathology (81). ROS may activate signaling pathways that alter the amyloid precursor protein or tau processing (88), and oxidant-induced inactivation of critical molecules, such as Pin1, may also play a contributing role (135). Together, these results suggest that oxidative damage and subsequent mitochondrial dysfunction probably contribute causally to AD-related pathology.
In addition, epidemiologic observations identified hypercholesterolemia in midlife as a major risk factor for AD (3, 145). The epidemiologic evidence linking cholesterol to AD was further supported by in vitro studies (69, 110), showing that membrane cholesterol promotes the amyloidogenic processing of the amyloid precursor protein (APP) to generate Aβ. In a recent study in our laboratory with genetic mouse models of brain cholesterol accumulation, such as Tg-SREBP-2 (35), we expanded the role of cholesterol and mGSH in AD, indicating that, in addition to fostering Aβ-production, mitochondrial cholesterol loading modulates Aβ neurotoxicity through selective mGSH depletion. Although total GSH depletion and altered GSH redox cycle have been described in AD (9, 121), two lines of evidence indicate that the mGSH depletion specifically accounted for the increased susceptibility to Aβ. First, the fact that the pool of mGSH rather than cytosol GSH determines the sensitivity of brain mitochondria to Aβ-mediated oxidative stress and release of intermembrane apoptotic proteins is dependent on the levels of mGSH. Second, treatment with GSH ethyl ester, which recovers the depleted pool of mGSH, prevents the enhanced neuroinflammation and neuronal damage observed in Tg-SREBP-2 mice after Aβ intracerebroventricular infusion (35). Collectively, these results imply that efforts should be directed specifically to replenish mGSH to slow disease progression, by using, for instance, strategies that bypass the block of GSH transport into mitochondria imposed by cholesterol deposition.
Because many neurologic disorders are characterized by oxidative stress and mitochondrial dysfunction, the possible participation of mGSH in clinical settings other than AD cannot be discarded. For instance, Parkinson's disease (PD) is characterized by a mitochondrial dysfunction, and established animal models of PD are based on prolonged infusion of inhibitors of complex I of the mitochondrial electron-transport chain, such as rotenone (131) or MPTP (40). These animals have a parkinsonian phenotype distinguished by progressive rigidity, bradykinesia, and tremor, and pathologically by nigral degeneration with presence of Lewy bodies, cytoplasmic inclusions immunoreactive for α-synuclein and ubiquitin. Interestingly, one of the earliest biochemical derangements observed in patients with PD is loss of total GSH levels (115), which may contribute to progressive cell death. In addition, a reduction in both cellular and mitochondrial GSH levels results in increased oxidative stress and a decrease in mitochondrial function through a selective decrease in complex I activity, probably through an NO-mediated mechanism (22). Brain GSH elevation by means of GSH-ethyl ester protected against dopamine loss in a long-term model of mitochondrial impairment used to mimic PD in rat (149). Related to the mitochondrial injury observed in PD patients, total GSH depletion has been shown to precede the decreases in both mitochondrial complex I activity and dopamine levels (7), suggesting a role of mGSH in this pathology. Therefore, strategies aimed to boost mGSH levels in brain may result in clinical benefit or neuroprotection or both in animal models or in human diseases.
Diabetes
The mGSH pool also seems to be an important player in diabetes. The majority of diabetes patients have type-2 diabetes (90%), or non–insulin-dependent diabetes. Thus, decreased uptake of glucose into muscles and adipose tissue leads to chronic hyperglycemia that can result in tissue damage. The rate of the ROS production depends on the metabolic status of the cell; for instance, during hyperglycemia, the increased substrate availability for mitochondrial oxidation generates a high mitochondrial inner-membrane potential, leading to inhibition of further electron transfer, predominantly at complex III, increasing the risk for reduction of molecular oxygen into the superoxide (11). Hyperglycemia has been shown to increase the enzymatic conversion of glucose to sorbitol by sorbitol dehydrogenase, with concomitant decreases in NADPH and GSH. This depletion in reducing equivalents results in augmented sensitivity to oxidative stress (122). A good example of the effect of hyperglycemia on mitochondrial stress is diabetic retinopathy (71), in which retinal mitochondria display a twofold increase in superoxide levels in diabetic mice compared with nondiabetic mice. In the same retina, hyperglycemia decreased mGSH levels by 40%, and it increased mitochondrial membrane permeability (swelling) by more than twofold. Similarly, oxidative stress due to excessive ROS and depleted mGSH can give rise to cardiomyocyte apoptotic cell death in diabetic hearts, leading to heart disease (46, 127), underscoring the importance of mGSH loss in diabetes-mediated cardiac apoptotic death.
Aging
The biologic basis of aging is unknown, and numerous theories reflect some of the current directions in biologic aging research. Among these, the “shortening of the telomeres” and the “free radical” theories have attracted special attention recently.
The free radical or oxidative stress theory of aging, first proposed by Harman (55) in 1956, states that the age-related loss of physiological function is due to the progressive accumulation of oxidative damage, which ultimately determines the life span of an organism. Shortly after the discovery of the mitochondrial genome (mtDNA), Harman modified his original theory to incorporate the contribution/role of mitochondria in oxidative stress and proposed the mitochondrial theory of aging (56). In subsequent years, the mitochondrial theory of aging was further refined and developed by Miquel and colleagues (100), who suggested that the accumulation of somatic mutations in the mtDNA induced by oxidative stress is the major contributor of aging and age-related degenerative diseases. Currently, the mitochondrial theory of aging implies the overgeneration of ROS derived from the mitochondrial respiratory chain, which damage macromolecules, especially mtDNA. As a result, an accumulation of mtDNA mutations leads to production of defective mitochondrial respiration, further increasing ROS generation and oxidative damage. This so-called “vicious cycle” of ROS generation and concomitant oxidative damage is proposed as the ultimate determinant of mammalian life span (64). Accordingly, studies in mice have indicated that during aging, the glutathione redox state (GSH/GSSG ratio) in tissues and mitochondria shifts progressively toward oxidation (28, 118, 119), and a significant decrease in mGSH levels in brain, skeletal muscle, and liver of senescence-accelerated mice (displaying 48% shorter average life span than control mice) was reported (119). In addition, another study pointed to a striking relation between mtDNA damage and glutathione redox state in mitochondria (28). It is known that mtDNA is more susceptible to oxidative damage because of the lack of protective histone-like proteins in the mitochondria, its open circular structure, and their limited repair capacity. In accordance, mtDNA displays an increased susceptibility to the radiation-induced loss of integrity compared with nuclear DNA, an effect that was potentiated by GSH depletion in mitochondria (102).
Given that the aging process is associated with a gradual prooxidizing shift in the glutathione redox state, more studies aimed to address the specific role of mGSH and GSH-replenishing agents are needed in this area.
Other pathologies
Lungs are among the major sources of GSH storage (6.1–17.5 nmol/mg lung) and have higher levels of γ-glutamylcysteine synthetase than other tissues. Alterations in the levels of reduced GSH in the lung-lining fluid have been shown in various inflammatory conditions. GSH deficiency has been largely correlated with pulmonary diseases, including chronic pulmonary disease, acute respiratory distress syndrome, neonatal lung damage, and asthma (23). A clinical setting in which mGSH levels are of utmost importance is hyperoxia or administration of supplemental oxygen; despite being an important clinical therapy, it can cause significant lung damage. In line with this, prematurely born human infants, which exhibit underdeveloped lungs, frequently require supportive therapies that use elevated oxygen concentrations that put them at risk for developing pulmonary oxygen toxicity. This risk is made even greater by the immaturity of the cellular antioxidant defenses. Although the exact mechanisms of oxygen toxicity are still not fully defined, cellular damage is probably mediated by increased production of chemically ROS in the mitochondria. Thus, in conditions that increase mitochondrial production of ROS, such as exposure to high concentrations of oxygen, therapies based on enhancing mGSH concentrations could be highly beneficial (108).
Moreover, in models of chemical toxicity, nephrotoxicity and renal cell apoptosis induced by cisplatin have been associated with mitochondrial dysfunction and decreases in mGSH and NADPH levels, as well as in loss of mitochondrial membrane potential and cardiolipin oxidation, leading to cell death by apoptosis, as evidenced by the increased caspase-3 activity (128).
Concluding Remarks
Through the control of the mitochondrial oxidative stress, mGSH is as a critical factor in the control of cell survival/death. The significance of mGSH in this regard has been illustrated by the increasing number of pathologic settings in which its depletion results in increased cellular damage and hence cell death. Thus, modulation of mGSH levels can influence disease progression, and therapies to enhance mGSH levels could be of medical significance in the treatment of numerous human diseases. However, although our knowledge of its regulation at the level of transport across the mitochondrial inner membrane has increased substantially in recent years, further characterization of its transport system is needed to ensure cell-type–specific modulation of mGSH levels to regulate disease pathogenesis. Whereas the use of cell-permeable GSH prodrugs may be a critical strategy to regulate diseases characterized by mGSH depletion, such as steatohepatitis and cholestasis, in liver malignancies, a preferred alternative will be preferentially to deplete mGSH to sensitize liver cancer cells against hypoxia.
Abbreviations Used
- Aβ
amyloid-β peptide
- ASH
alcoholic steatohepatitis
- ASMase
acidic sphingomyelinase
- GPx
GSH peroxidase
- GR
GSSG-reductase
- Grx
glutaredoxin
- GSH
glutathione
- GSSG
oxidized GSH
- GST
GSH-S-transferase
- HP
3-hydroxy-4-pentenoate
- mGSH
mitochondrial GSH
- MIM
mitochondrial inner membrane
- MnSOD
Mn-dependent superoxide dismutase
- MOM
mitochondrial outer membrane
- mtDNA
mitochondrial genome
- NASH
nonalcoholic steatohepatitis
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor alpha
- Trx
thioredoxin
- TrxR
Trx-reductase
Acknowledgments
The work was supported by CIBEREHD and grants PI070193 and PI060395 (Instituto de Salud Carlos III); by grants SAF2006-06780, SAF2008-02199, and SAF2008-04974 (Plan Nacional de I + D), and the Mutua Madrileña, Spain; and by grant P50-AA-11999 (Research Center for Liver and Pancreatic Diseases, U.S. National Institute on Alcohol Abuse and Alcoholism).
References
- 1.Anderson MF. Nilsson M. Eriksson PS. Sims NR. Glutathione monoethyl ester provides neuroprotection in a rat model of stroke. Neurosci Lett. 2004;354:163–165. doi: 10.1016/j.neulet.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MF. Nilsson M. Sims NR. Glutathione monoethylester prevents mitochondrial glutathione depletion during focal cerebral ischemia. Neurochem Int. 2004;44:153–159. doi: 10.1016/s0197-0186(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 3.Anstey KJ. Lipnicki DM. Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong JS. Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–1265. doi: 10.1096/fj.02-0097fje. [DOI] [PubMed] [Google Scholar]
- 5.Baeuerle PA. Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1998;242:540–548. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 6.Bertout JA. Patel SA. Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharath S. Andersen JK. Glutathione depletion in a midbrain-derived immortalized dopaminergic cell line results in limited tyrosine nitration of mitochondrial complex I subunits: implications for Parkinson's disease. Antioxid Redox Signal. 2005;7:900–910. doi: 10.1089/ars.2005.7.900. [DOI] [PubMed] [Google Scholar]
- 8.Boldin MP. Goncharov TM. Goltsev YV. Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 9.Boyd-Kimball D. Sultana R. Abdul HM. Butterfield DA. Gamma-glutamylcysteine ethyl ester-induced up-regulation of glutathione protects neurons against Abeta(1-42)-mediated oxidative stress and neurotoxicity: implications for Alzheimer's disease. J Neurosci Res. 2005;79:700–706. doi: 10.1002/jnr.20394. [DOI] [PubMed] [Google Scholar]
- 10.Brookes PS. Yoon Y. Robotham JL. Anders MW. Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 11.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 12.Bruguera M. Bertran A. Bombi JA. Rodes J. Giant mitochondria in hepatocytes: a diagnostic hint for alcoholic liver disease. Gastroenterology. 1977;73:1383–1387. [PubMed] [Google Scholar]
- 13.Brunelle JK. Bell EL. Quesada NM. Vercauteren K. Tiranti V. Zeviani M. Scarpulla RC. Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Burcham PC. Harman AW. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem. 1991;266:5049–5054. [PubMed] [Google Scholar]
- 15.Cadenas E. Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 16.Chae HZ. Kang SW. Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol. 1999;300:219–226. doi: 10.1016/s0076-6879(99)00128-7. [DOI] [PubMed] [Google Scholar]
- 17.Chandel NS. Trzyna WC. McClintock DS. Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 18.Chang TS. Cho CS. Park S. Yu S. Kang SW. Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J Biol Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 19.Chen J. Schenker S. Henderson GI. 4-Hydroxynonenal detoxification by mitochondrial glutathione S-transferase is compromised by short-term ethanol consumption in rats. Alcohol Clin Exp Res. 2002;26:1252–1258. doi: 10.1097/01.ALC.0000024081.89523.81. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z. Lash LH. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J Pharmacol Exp Ther. 1998;285:608–618. [PubMed] [Google Scholar]
- 21.Chen Z. Putt DA. Lash LH. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Arch Biochem Biophys. 2000;373:193–202. doi: 10.1006/abbi.1999.1527. [DOI] [PubMed] [Google Scholar]
- 22.Chinta SJ. Andersen JK. Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: implications for Parkinson's disease. Free Radic Biol Med. 2006;41:1442–1448. doi: 10.1016/j.freeradbiomed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Circu ML. Aw TY. Glutathione and apoptosis. Free Radic Res. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colell A. García-Ruiz C. Miranda M. Ardite E. Marí M. Morales A. Corrales F. Kaplowitz N. Fernández-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 25.Colell A. García-Ruiz C. Morales A. Ballesta A. Ookhtens M. Rodés J. Kaplowitz N. Fernández-Checa JC. Transport of reduced glutathione in hepatic mitochondria and mitoplasts from ethanol-treated rats: effect of membrane physical properties and S-adenosyl-l-methionine. Hepatology. 1997;26:699–708. doi: 10.1002/hep.510260323. [DOI] [PubMed] [Google Scholar]
- 26.Coll O. Colell A. García-Ruiz C. Kaplowitz N. Fernández-Checa JC. Sensitivity of the 2-oxoglutarate carrier to alcohol intake contributes to mitochondrial glutathione depletion. Hepatology. 2003;38:692–702. doi: 10.1053/jhep.2003.50351. [DOI] [PubMed] [Google Scholar]
- 27.Coppola S. Ghibelli L. GSH extrusion and the mitochondrial pathway of apoptotic signalling. Biochem Soc Trans. 2000;28:56–61. doi: 10.1042/bst0280056. [DOI] [PubMed] [Google Scholar]
- 28.de la Asuncion JG. Millan A. Pla R. Bruseghini L. Esteras A. Pallardo FV. Sastre J. Viña J. Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J. 1996;10:333–338. doi: 10.1096/fasebj.10.2.8641567. [DOI] [PubMed] [Google Scholar]
- 29.DeLeve L. Kaplowitz N. Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther. 1991;52:287–305. doi: 10.1016/0163-7258(91)90029-l. [DOI] [PubMed] [Google Scholar]
- 30.Dröge W. Schulze-Osthoff K. Mihm S. Galter D. Schenk H. Eck HP. Roth S. Gmünder H. Functions of glutathione and glutathione disulfide in immunology and immunopathology. FASEB J. 1994;8:1131–1138. [PubMed] [Google Scholar]
- 31.Fan C. Li Q. Ross D. Engelhardt JF. Tyrosine phosphorylation of I kappa B alpha activates NF kappa B through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J Biol Chem. 2003;278:2072–2080. doi: 10.1074/jbc.M206718200. [DOI] [PubMed] [Google Scholar]
- 32.Fariss MW. Chan CB. Patel M. Van Houten B. Orrenius S. Role of mitochondria in toxic oxidative stress. Mol Interv. 2005;5:94–111. doi: 10.1124/mi.5.2.7. [DOI] [PubMed] [Google Scholar]
- 33.Feldstein AE. Canbay A. Angulo P. Taniai M. Burgart LJ. Lindor KD. Gores GJ. Hepatocyte apoptosis and Fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 34.Fernández A. Colell A. Garcia-Ruiz C. Fernandez-Checa JC. Cholesterol and sphingolipids in alcohol-induced liver injury. J Gastroenterol Hepatol. 2008;23(suppl 1):S9–S15. doi: 10.1111/j.1440-1746.2007.05280.x. [DOI] [PubMed] [Google Scholar]
- 35.Fernández A. Llacuna L. Fernandez-Checa JC. Colell A. Mitochondrial cholesterol loading exacerbates amyloid beta peptide-induced inflammation and neurotoxicity. J Neurosci. 2009;20:6394–6405. doi: 10.1523/JNEUROSCI.4909-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández-Checa JC. García-Ruiz C. Ookhtens M. Kaplowitz N. Impaired uptake of glutathione by hepatic mitochondria from chronic ethanol-fed rats: tracer kinetic studies in vitro and in vivo and susceptibility to oxidant stress. J Clin Invest. 1991;87:397–405. doi: 10.1172/JCI115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernández-Checa JC. Kaplowitz N. García-Ruiz C. Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis. 1998;18:389–401. doi: 10.1055/s-2007-1007172. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Checa JC. Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol. 2005;204:263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Fernández-Checa JC. Ookhtens M. Kaplowitz N. Effect of chronic ethanol feeding on rat hepatocytic glutathione: compartmentation, efflux, and response to incubation with ethanol. J Clin Invest. 1987;80:57–62. doi: 10.1172/JCI113063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fornai F. Schlüter OM. Lenzi P. Gesi M. Ruffoli R. Ferrucci M. Lazzeri G. Busceti CL. Pontarelli F. Battaglia G. Pellegrini A. Nicoletti F. Ruggieri S. Paparelli A. Südhof TC. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci U S A. 2005;102:3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García-Ruiz C. Colell A. Marí M. Morales A. Calvo M. Enrich C. Fernández-Checa JC. Defective TNF-alpha-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J Clin Invest. 2003;111:197–208. doi: 10.1172/JCI16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García-Ruiz C. Colell A. Marí M. Morales A. Fernández-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species: role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 43.García-Ruiz C. Colell A. París R. Fernández-Checa JC. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 2000;14:847–858. doi: 10.1096/fasebj.14.7.847. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Ruiz C. Morales A. Ballesta A. Rodes J. Kaplowitz N. Fernandez-Checa JC. Effect of chronic ethanol feeding on glutathione and functional integrity of mitochondria in periportal and perivenous rat hepatocytes. J Clin Invest. 1994;94:193–201. doi: 10.1172/JCI117306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García-Ruiz C. Morales A. Colell A. Rodés J. Yi JR. Kaplowitz N. Fernández-Checa JC. Evidence that the rat hepatic mitochondrial carrier is distinct from the sinusoidal and canalicular transporters for reduced glutathione: expression studies in Xenopus laevis oocytes. J Biol Chem. 1995;270:15946–15949. doi: 10.1074/jbc.270.27.15946. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh S. Pulinilkunnil T. Yuen G. Kewalramani G. An D. Qi D. Abrahani A. Rodrigues B. Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am J Physiol Heart Circ Physiol. 2005;289:H768–H776. doi: 10.1152/ajpheart.00038.2005. [DOI] [PubMed] [Google Scholar]
- 47.Green DR. Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 48.Griffith OW. Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985;82:4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guzy RD. Hoyos B. Robin E. Chen H. Liu L. Mansfield KD. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Haass C. Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 51.Hammond CL. Lee TK. Ballatori N. Novel roles for glutathione in gene expression, cell death, and membrane transport of organic solutes. J Hepatol. 2001;34:946–954. doi: 10.1016/s0168-8278(01)00037-x. [DOI] [PubMed] [Google Scholar]
- 52.Han D. Hanawa N. Saberi B. Kaplowitz N. Mechanisms of liver injury, III: role of glutathione redox status in liver injury. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1–G7. doi: 10.1152/ajpgi.00001.2006. [DOI] [PubMed] [Google Scholar]
- 53.Haouzi D. Cohen I. Vieira HL. Poncet D. Boya P. Castedo M. Vadrot N. Belzacq AS. Fau D. Brenner C. Feldmann G. Kroemer G. Mitochondrial permeability transition as a novel principle of hepatorenal toxicity in vivo. Apoptosis. 2002;7:395–405. doi: 10.1023/a:1020026923038. [DOI] [PubMed] [Google Scholar]
- 54.Haouzi D. Lekehal M. Tinel M. Vadrot N. Caussanel L. Lettéron P. Moreau A. Feldmann G. Fau D. Pessayre D. Prolonged, but not acute, glutathione depletion promotes Fas-mediated mitochondrial permeability transition and apoptosis in mice. Hepatology. 2001;33:1181–1188. doi: 10.1053/jhep.2001.24235. [DOI] [PubMed] [Google Scholar]
- 55.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 56.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 57.Hayes JD. Flanagan JU. Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 58.Herrero E. Ros J. Glutaredoxins and oxidative stress defense in yeast. Methods Enzymol. 2002;348:136–146. doi: 10.1016/s0076-6879(02)48633-8. [DOI] [PubMed] [Google Scholar]
- 59.Hockenbery DM. Giedt CD. O'Neill JW. Manion MK. Banker DE. Mitochondria and apoptosis: new therapeutic targets. Adv Cancer Res. 2002;85:203–242. doi: 10.1016/s0065-230x(02)85007-2. [DOI] [PubMed] [Google Scholar]
- 60.Holmgren A. The function of thioredoxin and glutathione in deoxyribonucleic acid synthesis. Biochem Soc Trans. 1977;5:611–612. doi: 10.1042/bst0050611. [DOI] [PubMed] [Google Scholar]
- 61.Hwang C. Sinskey AJ. Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 62.Imbert V. Rupec RA. Livolsi A. Pahl HL. Traenckner EB. Mueller-Dieckmann C. Farahifar D. Rossi B. Auberger P. Baeuerle PA. Peyron JF. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 63.Jang JH. Moritz W. Graf R. Clavien PA. Preconditioning with death ligands FasL and TNF-alpha protects the cirrhotic mouse liver against ischaemic injury. Gut. 2008;57:492–499. doi: 10.1136/gut.2007.137703. [DOI] [PubMed] [Google Scholar]
- 64.Jang YC. Remmen VH. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol. 2009;44:256–260. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Kaelin WG., Jr ROS: really involved in oxygen sensing. Cell Metab. 2005;1:357–358. doi: 10.1016/j.cmet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Kagan VE. Tyurin VA. Jiang J. Tyurina YY. Ritov VB. Amoscato AA. Osipov AN. Belikova NA. Kapralov AA. Kini V. Vlasova II. Zhao Q. Zou M. Di P. Svistunenko DA. Kurnikov IV. Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 67.Kakkar P. Singh BK. Mitochondria: a hub of redox activities and cellular distress control. Mol Cell Biochem. 2007;305:235–253. doi: 10.1007/s11010-007-9520-8. [DOI] [PubMed] [Google Scholar]
- 68.Kallio PJ. Wilson WJ. O'Brien S. Makino Y. Poellinger L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J Biol Chem. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- 69.Kalvodova L. Kahya N. Schwille P. Ehehalt R. Verkade P. Drechsel D. Simons K. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem. 2005;280:36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- 70.Kamura T. Sato S. Iwai K. Czyzyk-Krzeska M. Conaway RC. Conaway JW. Activation of HIF1α ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A. 2000;97:10430–10435. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanwar M. Chan PS. Kern TS. Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 72.Koehler CM. Beverly K. Leverich EP. Redox pathways in the mitochondrion. Antioxid Redox Signal. 2006;8:813–822. doi: 10.1089/ars.2006.8.813. [DOI] [PubMed] [Google Scholar]
- 73.Koong AC. Chen EY. Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994;54:1425–1430. [PubMed] [Google Scholar]
- 74.Krähenbühl S. Stucki J. Reichen J. Reduced activity of the electron transport chain in liver mitochondria isolated from rats with secondary biliary cirrhosis. Hepatology. 1992;15:1160–1166. doi: 10.1002/hep.1840150630. [DOI] [PubMed] [Google Scholar]
- 75.Krähenbühl S. Talos C. Lauterburg BH. Reichen J. Reduced antioxidative capacity in liver mitochondria from bile duct ligated rats. Hepatology. 1995;22:607–612. doi: 10.1002/hep.1840220234. [DOI] [PubMed] [Google Scholar]
- 76.Kuwana T. Mackey MR. Perkins G. Ellisman MH. Latterich M. Schneiter R. Green DR. Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 77.Lash LH. Putt DA. Matherly LH. Protection of NRK-52E cells, a rat renal proximal tubular cell line, from chemical-induced apoptosis by overexpression of a mitochondrial glutathione transporter. J Pharmacol Exp Ther. 2002;303:476–486. doi: 10.1124/jpet.102.040220. [DOI] [PubMed] [Google Scholar]
- 78.Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem Biol Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li P. Nijhawan D. Budihardjo I. Srinivasula SM. Ahmad M. Alnemri ES. Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 80.Lin MT. Beal MF. Alzheimer's APP mangles mitochondria. Nat Med. 2006;12:1241–1243. doi: 10.1038/nm1106-1241. [DOI] [PubMed] [Google Scholar]
- 81.Lin MT. Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 82.Llacuna L. Marí M. Garcia-Ruiz C. Fernandez-Checa JC. Morales A. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology. 2006;44:561–572. doi: 10.1002/hep.21285. [DOI] [PubMed] [Google Scholar]
- 83.Llacuna L. Marí M. Lluis JM. García-Ruiz C. Fernández-Checa JC. Morales A. Reactive oxygen species mediate liver injury through parenchymal NF-κB inactivation in prolonged ischemia/reperfusion. Am J Pathol. 2009;174:1776–1785. doi: 10.2353/ajpath.2009.080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lluis JM. Buricchi F. Chiarugi P. Morales A. Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signalling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Res. 2007;67:7368–7377. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- 85.Lluis JM. Colell A. García-Ruiz C. Kaplowitz N. Fernnandez-Checa JC. Acetaldehyde impairs mitochondrial glutathione transport in HepG2 cells through endoplasmic reticulum stress. Gastroenterology. 2003;124:708–724. doi: 10.1053/gast.2003.50089. [DOI] [PubMed] [Google Scholar]
- 86.Lluis JM. Morales A. Blasco C. Colell A. Mari M. Garcia-Ruiz C. Fernandez-Checa JC. Critical role of mitochondrial glutathione in the survival of hepatocytes during hypoxia. J Biol Chem. 2005;280:3224–3232. doi: 10.1074/jbc.M408244200. [DOI] [PubMed] [Google Scholar]
- 87.Lou H. Kaplowitz N. Glutathione depletion down-regulates tumor necrosis factor alpha-induced NF-kappaB activity via IkappaB kinase-dependent and -independent mechanisms. J Biol Chem. 2007;282:29470–29481. doi: 10.1074/jbc.M706145200. [DOI] [PubMed] [Google Scholar]
- 88.Lovell MA. Xiong S. Xie C. Davies P. Markesbery WR. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J Alzheimers Dis. 2004;6:659–671. doi: 10.3233/jad-2004-6610. [DOI] [PubMed] [Google Scholar]
- 89.Luedde T. Assmus U. Wüstefeld T. Meyer zu Vilsendorf A. Roskams T. Schmidt-Supprian M. Rajewsky K. Brenner DA. Manns MP. Pasparakis M. Trautwein C. Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis, but protects from ischemia/reperfusion injury. J Clin Invest. 2005;115:849–859. doi: 10.1172/JCI23493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lundberg M. Johansson C. Chandra J. Enoksson M. Jacobsson G. Ljung J. Johansson M. Holmgren A. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. J Biol Chem. 2001;276:26269–26275. doi: 10.1074/jbc.M011605200. [DOI] [PubMed] [Google Scholar]
- 91.Manczak M. Anekonda TS. Henson E. Park BS. Quinn J. Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 92.Marí M. Caballero F. Colell A. Morales A. Caballeria J. Fernandez A. Enrich C. Fernandez-Checa JC. García-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Marí M. Colell A. Morales A. Caballero F. Moles A. Fernández A. Terrones O. Basañez G. Antonsson B. García-Ruiz C. Fernández-Checa JC. Mechanism of mitochondrial glutathione-dependent hepatocellular susceptibility to TNF despite NF-kappaB activation. Gastroenterology. 2008;134:1507–1520. doi: 10.1053/j.gastro.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 94.Marí M. Colell A. Morales A. Pañeda C. Varela-Nieto I. García-Ruiz C. Fernández-Checa JC. Acidic sphingomyelinase downregulates the liver-specific methionine adenosyltransferase 1A, contributing to tumor necrosis factor-induced lethal hepatitis. J Clin Invest. 2004;113:895–904. doi: 10.1172/JCI19852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marí M. Colell A. Morales A. Von Montfort C. García-Ruiz C. Fernández-Checa JC. Redox control of liver function in health and disease. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2634. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mårtensson J. Lai JC. Meister A. High-affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proc Natl Acad Sci U S A. 1990;87:7185–7189. doi: 10.1073/pnas.87.18.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meister A. Mitochondrial changes associated with glutathione deficiency. Biochim Biophys Acta. 1995;1271:35–42. doi: 10.1016/0925-4439(95)00007-q. [DOI] [PubMed] [Google Scholar]
- 98.Meredith MJ. Reed DJ. Depletion in vitro of mitochondrial glutathione in rat hepatocytes and enhancement of lipid peroxidation by Adriamycin and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) Biochem Pharmacol. 1983;32:1383–1388. doi: 10.1016/0006-2952(83)90451-3. [DOI] [PubMed] [Google Scholar]
- 99.Meredith MJ. Reed DJ. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem. 1982;257:3747–3753. [PubMed] [Google Scholar]
- 100.Miquel J. Economos AC. Fleming J. Johnson JE., Jr Mitochondrial role in cell aging. Exp Gerontol. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 101.Mitchell JR. Jollow DJ. Potter WZ. Gillette JR. Brodie BB. Acetaminophen-induced hepatic necrosis, IV: protective role of glutathione. J Pharmacol Exp Ther. 1977;187:211–217. [PubMed] [Google Scholar]
- 102.Morales A. Miranda M. Sánchez-Reyes A. Biete A. Fernández-Checa JC. Oxidative damage of mitochondrial and nuclear DNA induced by ionizing radiation in human hepatoblastoma cells. Int J Radiat Oncol Biol Phys. 1998;42:191–203. doi: 10.1016/s0360-3016(98)00185-0. [DOI] [PubMed] [Google Scholar]
- 103.Muriel P. Mourelle M. Characterization of membrane fraction lipid composition and function of cirrhotic rat liver: role of S-adenosyl-l-methionine. J Hepatol. 1992;14:16–21. doi: 10.1016/0168-8278(92)90125-9. [DOI] [PubMed] [Google Scholar]
- 104.Muyderman H. Nilsson M. Sims NR. Highly selective and prolonged depletion of mitochondrial glutathione in astrocytes markedly increases sensitivity to peroxynitrite. J Neurosci. 2004;24:8019–8208. doi: 10.1523/JNEUROSCI.1103-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muzio M. Chinnaiyan AM. Kischkel FC. O'Rourke K. Shevchenko A. Ni J. Scaffidi C. Bretz JD. Zhang M. Gentz R. Mann M. Krammer PH. Peter ME. Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death–inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 106.Nagai H. Matsumaru K. Feng G. Kaplowitz N. Reduced glutathione depletion causes necrosis and sensitization to tumor necrosis factor-alpha-induced apoptosis in cultured mouse hepatocytes. Hepatology. 2002;36:55–64. doi: 10.1053/jhep.2002.33995. [DOI] [PubMed] [Google Scholar]
- 107.Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- 108.O'Donovan DJ. Fernandes CJ. Mitochondrial glutathione and oxidative stress: implications for pulmonary oxygen toxicity in premature infants. Mol Genet Metab. 2000;71:352–358. doi: 10.1006/mgme.2000.3063. [DOI] [PubMed] [Google Scholar]
- 109.Orrenius S. Gogvadze V. Zhivotovsky B. Mitochondrial oxidative stress: implications for cells death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 110.Osenkowski P. Ye W. Wang R. Wolfe MS. Selkoe DJ. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem. 2008;283:22529–22540. doi: 10.1074/jbc.M801925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ott M. Robertson JD. Gogvadze V. Zhivotovsky B. Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Palmieri F. Mitochondrial carrier proteins. FEBS Lett. 1994;346:48–54. doi: 10.1016/0014-5793(94)00329-7. [DOI] [PubMed] [Google Scholar]
- 113.Pedrajas JR. Kosmidou E. Miranda-Vizuete A. Gustafsson JA. Wright AP. Spyrou G. Identification and functional characterization of a novel mitochondrial thioredoxin system in Saccharomyces cerevisiae. J Biol Chem. 1999;274:6366–6373. doi: 10.1074/jbc.274.10.6366. [DOI] [PubMed] [Google Scholar]
- 114.Pelicano H. Feng L. Zhou Y. Carew JS. Hileman EO. Plunkett W. Keating MJ. Huang P. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem. 2003;278:37832–37839. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- 115.Perry TL. Yong VW. Idiopathic Parkinson's disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci Lett. 1986;67:269–274. doi: 10.1016/0304-3940(86)90320-4. [DOI] [PubMed] [Google Scholar]
- 116.Pugh CW. Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 117.Rafique S. Guardascione M. Osman E. Burroughs AK. Owen JS. Reversal of extrahepatic membrane cholesterol deposition in patients with chronic liver diseases by S-adenosyl-l-methionine. Clin Sci (Lond) 1992;83:353–356. doi: 10.1042/cs0830353. [DOI] [PubMed] [Google Scholar]
- 118.Rebrin I. Kamzalov S. Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic Biol Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rebrin I. Sohal RS. Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp Gerontol. 2004;39:1513–1519. doi: 10.1016/j.exger.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rehman H. Connor HD. Ramshesh VK. Theruvath TP. Mason RP. Wright GL. Lemasters JJ. Zhong Z. Ischemic preconditioning prevents free radical production and mitochondrial depolarization in small-for-size rat liver grafts. Transplantation. 2008;85:1322–1331. doi: 10.1097/TP.0b013e31816de302. [DOI] [PubMed] [Google Scholar]
- 121.Resende R. Moreira PI. Proença T. Deshpande A. Busciglio J. Pereira C. Oliveira CR. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 122.Rolo AP. Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 123.Rottenberg H. Robertson DE. Rubin E. The effect of temperature and chronic ethanol feeding on the proton electrochemical potential and phosphate potential in rat liver mitochondria. Biochim Biophys Acta. 1985;809:1–10. doi: 10.1016/0005-2728(85)90160-4. [DOI] [PubMed] [Google Scholar]
- 124.Rüdiger HA. Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202–210. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 125.Rüdiger HA. Graf R. Clavien PA. Sub-lethal oxidative stress triggers the protective effects of ischemic preconditioning in the mouse liver. J Hepatol. 2003;39:972–977. doi: 10.1016/s0168-8278(03)00415-x. [DOI] [PubMed] [Google Scholar]
- 126.Safran M. Kaelin WG., Jr HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–783. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Santos DL. Palmeira CM. Seiça R. Dias J. Mesquita J. Moreno AJ. Santos MS. Diabetes and mitochondrial oxidative stress: a study using heart mitochondria from the diabetic Goto-Kakizaki rat. Mol Cell Biochem. 2003;246:163–170. [PubMed] [Google Scholar]
- 128.Santos NA. Catão CS. Martins NM. Curti C. Bianchi ML. Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 2007;81:495–504. doi: 10.1007/s00204-006-0173-2. [DOI] [PubMed] [Google Scholar]
- 129.Savaskan NE. Ufer C. Kühn H. Borchert A. Molecular biology of glutathione peroxidase 4: from genomic structure to developmental expression and neural function. Biol Chem. 2007;388:1007–1017. doi: 10.1515/BC.2007.126. [DOI] [PubMed] [Google Scholar]
- 130.Schmitz I. Walczak H. Krammer PH. Peter ME. Differences between CD95 type I and II cells detected with the CD95 ligand. Cell Death Differ. 1999;6:821–822. doi: 10.1038/sj.cdd.4400569. [DOI] [PubMed] [Google Scholar]
- 131.Sherer TB. Betarbet R. Testa CM. Seo BB. Richardson JR. Kim JH. Miller GW. Yagi T. Matsuno-Yagi A. Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Simon MC. Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sims NR. Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–526. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 134.Sims NR. Nilsson M. Muyderman H. Mitochondrial glutathione: a modulator of brain cell death. J Bioenerg Biomembr. 2004;36:329–333. doi: 10.1023/B:JOBB.0000041763.63958.e7. [DOI] [PubMed] [Google Scholar]
- 135.Sultana R. Boyd-Kimball D. Poon HF. Cai J. Pierce WM. Klein JB. Markesbery WR. Zhou XZ. Lu KP. Butterfield DA. Oxidative modification and down-regulation of Pin1 in Alzheimer's disease hippocampus: a redox proteomics analysis. Neurobiol Aging. 2006;27:918–925. doi: 10.1016/j.neurobiolaging.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 136.Tanzi RE. Moir RD. Wagne SL. Clearance of Alzheimer's Ab peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 137.Teoh N. Field J. Sutton J. Farrell G. Dual role of tumor necrosis factor-alpha in hepatic ischemia-reperfusion injury: studies in tumor necrosis factor-alpha gene knockout mice. Hepatology. 2004;39:412–421. doi: 10.1002/hep.20035. [DOI] [PubMed] [Google Scholar]
- 138.Thayer WS. Effects of ethanol on proteins of mitochondrial membranes. Ann N Y Acad Sci. 1987;492:193–206. doi: 10.1111/j.1749-6632.1987.tb48668.x. [DOI] [PubMed] [Google Scholar]
- 139.Tirmenstein MA. Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- 140.Traenckner EB. Pahl HL. Henkel T. Schmidt KN. Wilk S. Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Valko M. Leibfritz D. Moncol J. Cronin MT. Mazur M. Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 142.Vendemiale G. Grattagliano I. Altomare E. Turturro N. Guerrieri F. Effect of acetaminophen administration on hepatic glutathione compartmentation and mitochondrial energy metabolism in the rat. Biochem Pharmacol. 1996;52:1147–1154. doi: 10.1016/0006-2952(96)00414-5. [DOI] [PubMed] [Google Scholar]
- 143.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 144.Wheeler MD. Nakagami M. Bradford BU. Uesugi T. Mason RP. Connor HD. Dikalova A. Kadiiska M. Thurman RG. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J Biol Chem. 2001;276:36664–36672. doi: 10.1074/jbc.M105352200. [DOI] [PubMed] [Google Scholar]
- 145.Wolozin B. Kellman W. Ruosseau P. Celesia GG. Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 146.Wüllner U. Seyfried J. Groscurth P. Beinroth S. Winter S. Gleichmann M. Heneka M. Löschmann P. Schulz JB. Weller M. Klockgether T. Glutathione depletion and neuronal cell death: the role of reactive oxygen intermediates and mitochondrial function. Brain Res. 1999;826:53–62. doi: 10.1016/s0006-8993(99)01228-7. [DOI] [PubMed] [Google Scholar]
- 147.Yant LJ. Ran Q. Rao L. Van Remmen H. Shibatani T. Belter JG. Motta L. Richardson A. Prolla TA. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 148.Yin M. Wheeler MD. Kono H. Bradford BU. Gallucci RM. Luster MI. Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 149.Zeevalk GD. Manzino L. Sonsalla PK. Bernard LP. Characterization of intracellular elevation of glutathione (GSH) with glutathione monoethyl ester and GSH in brain and neuronal cultures: relevance to Parkinson's disease. Exp Neurol. 2007;203:512–520. doi: 10.1016/j.expneurol.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao P. Kalhorn TF. Slattery JT. Selective mitochondrial glutathione depletion by ethanol enhances acetaminophen toxicity in rat liver. Hepatology. 2002;36:326–335. doi: 10.1053/jhep.2002.34943. [DOI] [PubMed] [Google Scholar]
- 151.Zhao P. Slattery JT. Effects of ethanol dose and ethanol withdrawal on rat liver mitochondrial glutathione: implication of potentiated acetaminophen toxicity in alcoholics. Drug Metab Dispos. 2002;30:1413–1417. doi: 10.1124/dmd.30.12.1413. [DOI] [PubMed] [Google Scholar]
- 152.Zhivotovsky B. Caspases: the enzymes of death. Essays Biochem. 2003;39:25–40. doi: 10.1042/bse0390025. [DOI] [PubMed] [Google Scholar]
- 153.Zhong Q. Putt DA. Xu F. Lash LH. Hepatic mitochondrial transport of glutathione: studies in isolated rat liver mitochondria and H4IIE rat hepatoma cells. Arch Biochem Biophys. 2008;474:119–127. doi: 10.1016/j.abb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zwaka R. Zhang Y. Zhou W. Halldorson J. Engelhardt JE. Ischemia/reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor κB independently of IkB degradation. Hepatology. 1998;28:1022–1030. doi: 10.1002/hep.510280417. [DOI] [PubMed] [Google Scholar]