Abstract
Mitochondria can initiate cell death or activate genes that promote cell survival in response to low oxygen. The BCL-2 family of proteins regulate cell death in response to anoxia (0–0.5% O2). By contrast, under hypoxia (0.5–3% O2), mitochondrial oxidative stress activates hypoxia-inducible factors (HIFs) to promote cell survival. In this review, we discuss how mitochondria, BCL-2 proteins, and HIFs are crucial for cellular responses to low oxygen. Antioxid. Redox Signal. 11, 2673–2683.
Introduction
The ability of mammals to maintain oxygen homeostasis is essential for their survival. Because of this, mammals have evolved ways to ensure that optimal oxygen concentrations are delivered to cells and tissues. Physiologic responses to low-oxygen environments include increased production of the hormone erythropoietin to enhance red blood cell mass and hemoglobin concentration in the blood; increased neurotransmitter release from the carotid body to increase breathing; and pulmonary vascular constriction to increase blood flow to the better-oxygenated regions of the lung (8). The physiologic adaptations that allow mammals to survive hypoxia have been well understood for many years.
Much progress also has been made in understanding the molecular mechanisms that allow cells to adapt and survive in low-oxygen environments, but many questions remain. Oxygen plays a role in a variety of cellular processes, including sterol and fatty acid synthesis, and is critical for oxidative phosphorylation (8). Thus, it is not surprising that changes in oxygen availability can have drastic effects on the function of a cell. Several studies indicate that low-oxygen conditions can induce apoptosis (76, 86). This occurs when oxygen levels decrease to at, or below, 0.5% (anoxia). When oxygen levels are 0.5–3% (hypoxia), cells do not undergo apoptosis. Instead, hypoxia activates a variety of cellular events that ultimately lead to cell survival.
Low oxygen triggers signal-transduction pathways involved in both cell death and survival (Fig. 1). Anoxia activates proapoptotic BCL-2 proteins and caspases to initiate apoptosis. The adaptive cellular events that occur in response to hypoxia are mediated largely by the transcription factor hypoxia-inducible factor-1 (HIF-1) (91). HIF-1 induces the expression of multiple antiapoptotic BCL-2 proteins to promote cell survival (2, 13, 81). Interestingly hypoxia increases production of mitochondrial reactive oxygen species (ROS), which serve as signaling molecules to activate HIF-1. In this review, we discuss the mechanisms underlying anoxia-induced apoptosis and hypoxia-induced cell survival.
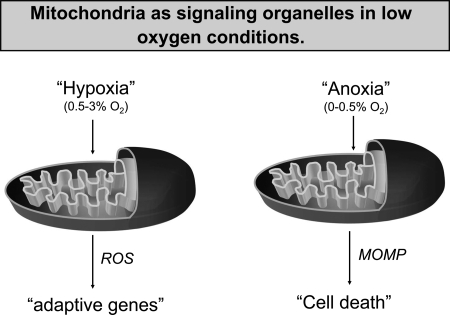
FIG. 1.
Mitochondria act as signaling organelles in low-oxygen conditions. Hypoxia (0.5–3% oxygen) increases mitochondrial ROS that activate transcription of adaptive genes. Anoxia (0–0.5% oxygen) initiates mitochondrial outer membrane permeabilization (MOMP) to activate cell death.
Anoxia Initiates Mitochondria-Dependent Cell Death
Overview of apoptosis
Cells can activate cell death through a process known as apoptosis. Apoptosis is a genetically programmed form of cellular suicide and is essential for normal development and tissue homeostasis (21). Apoptosis, unlike necrosis, removes excess or damaged cells without harming surrounding cells and is an ATP-dependent process (26, 55). Appropriate control of apoptosis is important for normal functioning, and unregulated apoptosis has been linked to a variety of disease states. Excess apoptosis is thought to contribute to neurodegenerative diseases, whereas insufficient apoptosis can lead to diseases such as cancer (9, 59). Apoptosis can be activated by a variety of apoptotic cues, including ligand activation of death receptors, growth-factor withdrawal, oncogenes, and anoxia. Depending on the death stimulus, cells can activate apoptosis through two pathways, the extrinsic or the intrinsic pathway (Fig. 2) (33, 94). Both pathways lead to activation of cysteine-dependent aspartate-directed proteases, termed caspases, which cleave an array of cellular proteins, resulting in cell death.
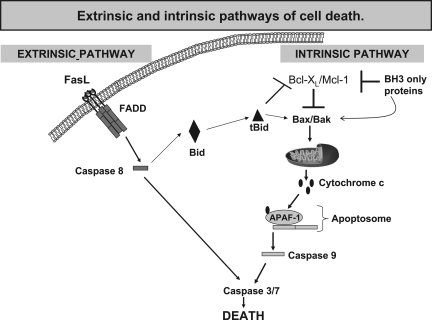
FIG. 2.
Extrinsic and intrinsic pathways of cell death. Initiation of the extrinsic apoptotic pathway through FasL results in activation of capase-8. Activated caspase-8 cleaves and activates executioner caspases-3 and - 7, resulting in cell death. Caspase-8 can also cleave and activate Bid, resulting in initiation of the intrinsic apoptotic pathway. Initiation of the intrinsic apoptotic pathway occurs when BH3-only proteins diminish the prosurvival function of antiapoptotic BCL-2 family members resulting in BAX/BAK-dependent mitochondrial outer-membrane permeabilization (MOMP). MOMP results in cytochrome c release, apoptosome formation, caspase-9 activation, and cell death.
Critical elements of the extrinsic pathway include membrane receptors, adaptor proteins such as TNFR-associated death domain protein (TRADD), and caspase-8. A classic example of apoptosis through this pathway involves tumor necrosis factor (TNF) binding to its receptor, TNFR1 (47). Binding of TNF induces formation of a homotrimeric complex of ligand-bound receptors. This complex then associates with TRADD and caspase-8. This leads to cleavage and activation of caspase-8, resulting in activation of downstream caspases (71). Caspase-8 also cleaves the BH3-only protein BID, which can amplify the apoptotic signal by activating the intrinsic pathway.
The intrinsic pathway, also known as the mitochondrial pathway, is initiated by many stimuli, including anoxia. This pathway involves disruption of the mitochondrial outer membrane and is regulated primarily by the BCL-2 family of proteins (17). Disruption of the mitochondrial outer membrane results in the release of apoptogenic proteins from the intermembrane space of the mitochondria to the cytosol. These factors include cytochrome c, Smac/Diablo, and apoptosis-inducing factor (AIF) (51). In healthy cells, cytochrome c is a protein in the mitochondrial electron-transport chain that functions as a mobile electron carrier. When released into the cytosol, cytochrome c interacts with APAF-1 and pro-caspase-9 to form the apoptosome in an ATP-dependent manner (57, 60). Once formed, the apoptosome activates caspase-9, which subsequently activates downstream executioner caspases, resulting in apoptosis. The release of Smac/Diablo promotes caspase activation by eliminating inhibitory of apoptosis protein (IAP) function (25). AIF contributes to a caspase-independent cell death and is important in developmental apoptosis (95).
The BCL-2 family of proteins
The BCL-2 family of proteins are master regulators of the intrinsic apoptotic pathway. The BCL-2 family of proteins are divided into three subclasses defined by function and conserved sequence within the BCL-2 homology (BH) domains (18). These family members can have either pro- or antiapoptotic function. The antiapoptotic members include BCL-2, BCL-XL, BCL-w, MCL-1, and A1 and have sequence conservation in all four BH domains. Overexpression of any of these proteins protects cells from apoptosis triggered by a variety of cytotoxic stimuli (19). The proapoptotic members are further divided into two classes, the multidomain members, which possess homology in BH1-3 and include BAX, BAK, and BOK, and those that contain sequence homology only in the BH3 domain (Fig. 3). BAX and BAK are ubiquitously expressed, whereas BOK is more cell-type specific. Once activated, BAX and BAK initiate apoptosis through the intrinsic pathway by causing mitochondrial outer membrane permeabilization (MOMP) (1, 22). Thus, cells deficient in BAX and BAK do not undergo MOMP and are resistant to apoptosis (102). The BH3-only members are the third subset of BCL-2 proteins and include BID, BIM, PUMA, BAD, NOXA, BIK, BMF, and HRK. The BH3 domain itself is significant in that it is necessary for binding to antiapoptotic proteins. The α-helical conformation of the BH3 domain inserts into a hydrophobic groove of antiapoptotic proteins, thereby negating their activity and promoting apoptosis (79, 80).
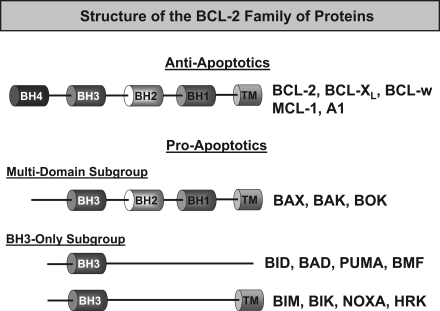
FIG. 3.
Structure of the BCL-2 family of proteins. Antiapoptotic BCL-2 proteins, which include BCL-2, BCL-XL, BCL-w, MCL-1, and A1, contain BH1 to 4 domains. The proapoptotic members are further divided into two groups: those that contain only one BH3 domain (BH3-only proteins) and others containing multiple BH3 domains. The BH3-only proteins include BAD, BID, BIK, BMF, BIM, HRK, NOXA, and PUMA, and act as sensors of apoptosis. Members of the multidomain subgroup (BAK, BAX, and BOK) act as executioners of apoptosis.
It is well established that the BH3-only proteins and the antiapoptotics are upstream regulators of BAX and BAK, yet the exact mechanism by which these proteins induce apoptosis is not completely understood. Currently, two models explain how these proteins regulate BAX and BAK activity. The indirect-activation model postulates that, in healthy cells, antiapoptotics associate with BAX and BAK and repress their activity. With a death stimulus, BH3-only proteins interact with the antiapoptotics, thereby displacing them from BAX and BAK, resulting in MOMP (105). A second model, the direct-activation model, proposes that antiapoptotic family members prevent cell death by disrupting signaling upstream of BAX and BAK, mainly by binding to the activator BH3-only proteins (BID, BIM, and PUMA) and negating their function (15, 16, 52, 56). With a death stimulus, a subset of BH3-only proteins, termed sensitizers, can bind antiapoptotic proteins, thereby displacing activators and allowing them to induce apoptosis (56).
Anoxia activates the intrinsic apoptotic pathway
During anoxia, when oxygen levels decrease to at or below 0.5% oxygen, evidence suggests that most cells will commit to apoptosis (76, 86). Because apoptosis is an ATP-dependent process and cells can not generate ATP through oxidative phosphorylation in an anoxic environment, glycolytic ATP is required for anoxia-induced apoptosis. If cells are deprived of glucose and oxygen, cells will die through necrosis. Interestingly, cells remain viable in hypoxia. The initial studies demonstrating that anoxia induces the intrinsic apoptotic pathway show that severe oxygen deprivation causes a decrease in the mitochondrial membrane potential. This was followed by the release of cytochrome c from the mitochondria into the cytosol and activation of caspase-9 (68). Treatment with the pan-caspase inhibitor zVAD did not prevent cytochrome c release, indicating that cytochrome c is released independent of caspase activity. Furthermore, overexpression of the antiapoptotic protein BCL-XL prevented cytochrome c release, caspase-9 activation, and cell death. Studies show also that BCL-2 is able to prevent anoxia-induced cell death (78). The antiapoptotic proteins BAX and BAK have also been implicated in anoxia-induced apoptosis. In response to anoxia, BAX has been shown to translocate from the cytosol to the mitochondria (84). Additionally, fibroblasts lacking BAX and BAK are resistant to anoxia-induced apoptosis (76). Genetic loss of caspase-9 or APAF-1, which are required proteins for apoptosome formation, also protects cells from anoxia-induced apoptosis (93). The extrinsic pathway can feed into the intrinsic pathway through BID and amplify the death response. However, BID-null fibroblasts die in anoxia (7). Collectively, these data demonstrate that anoxia initiates apoptosis through the intrinsic apoptotic pathway.
Multiple proteins diminish the prosurvival function of antiapoptotic BCL-2 proteins to initiate anoxia-induced cell death
A key step in initiating the intrinsic apoptotic pathway is to prevent the prosurvival function of antiapoptotic BCL-2 proteins. Negation of their activity is largely mediated by the BH3-only proteins. Whereas MCL-1 is degraded by the proteasome during anoxia, BCL-XL and BCL-2 protein levels remain stable, and inhibition of their function likely occurs through BH3-only binding. The BH3-only protein BAD was shown selectively to bind BCL-XL, BCL-2, and BCL-w, thereby inhibiting the prosurvival function of these antiapoptotic proteins (52, 56). However, Bad-null cells still undergo cell death in response to anoxia (7). BIM, BID, and PUMA inhibit the prosurvival function of all the antiapoptotic proteins, yet the individual loss of any of these proteins does not provide protection against anoxia. It is likely that a combination of BH3-only proteins, or a novel BH3-only protein, prevents the prosurvival activity of BCL-XL, BCL-2, and BCL-w (7).
MCL-1 protein levels decrease in response to anoxia, and this is dependent on the proteasomal pathway. Loss of MCL-1 as the result of DNA damage, adenoviral infection, and growth-factor withdrawal has been shown to cause apoptotic cell death (66, 105). Although MCL-1 protein levels decrease, MCL-1 mRNA levels increase during anoxia (7). MCL-1 degradation does not occur during hypoxia, which also does not cause cell death. MCL-1 protein levels decrease in Bax−/−/Bak−/− cells cultured in anoxia. This indicates that the loss of MCL-1 occurs upstream of BAX and BAK activation. In response to DNA damage, NOXA has been shown to bind to MCL-1, causing its degradation through the proteasomal pathway (20). However, MCL-1 degradation has been shown in Noxa-null fibroblasts in response to anoxia (7). Additionally, Noxa-null cells die in response to anoxia. However, contradictory data report that anoxia increases both mRNA and protein expression of NOXA and that suppression of NOXA reduces anoxic cell death in an osteosarcoma cell line (44). It is likely that anoxia activates different BH3-only proteins in different cells that bind and diminish the prosurvival activity of antiapoptotics to initiate BAX/BAK-mediated cell death (Fig. 4).
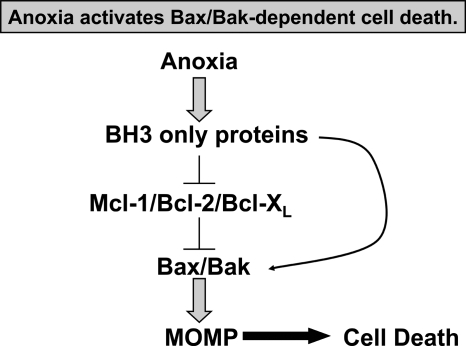
FIG. 4.
Anoxia activates BAX/BAK-dependent cell death. BH3-only proteins are activated during anoxia and inhibit prosurvival BCL-2 proteins. Inhibition of prosurvival proteins results in BAX and BAK activation, mitochondrial outer membrane permeabilization (MOMP), and cell death.
The confusing role of BNIP3 in cell death
BCL-2/E1B 19-kDa interacting protein (BNIP3) is regulated by HIF-1 during anoxia. BNIP3 has two hypoxia-response elements (HREs) in its promoter region, and HIF-1 binds to HRE2. Conditions that stabilize HIF-1α, such as 0.1% oxygen, CoCl2, and deferoxamine mesylate, increase BNIP3 expression. BNIP3 is a unique proapoptotic BCL-2 protein that heterodimerizes with BCL-2 and BCL-XL at mitochondrial and nonmitochondrial sites and neutralizes their function (83, 99). Initially, BCL-2 overexpression delays BNIP3 activity, but this is eventually overcome. Unusually, the transmembrane domain, and not the BH3 domain, has proven to be essential for the proapoptotic function of BNIP3. Furthermore, the BNIP3 BH3 domain does not cause cytochrome c release (56).
A clear role for BNIP3 during anoxia has proven difficult to determine. BNIP3-induced cell death has characteristics that are both consistent and inconsistent with apoptosis. Transfection of BNIP3 into MCF7 or Rat-1 fibroblasts resulted in apoptosis as defined by DNA fragmentation, yet cell death occurred much slower when compared with more-potent inducers such as Bid (48 h vs. 12 h). Bax−/−/Bak−/− MEFs are resistant to BNIP3 overexpression, which is consistent with apoptosis. Additionally, mitochondrial dysfunction has been associated with BNIP3-induced cell death, including membrane depolarization, permeability transition pore opening, and increased ROS production. Whether cytochrome c is released remains an area of controversy. The role of caspases in BNIP3-induced cell death also is controversial. Some studies show that the pan-caspase inhibitor Z-VAD-FMK failed to protect cells, whereas other studies show complete protection (37, 99). Studies have shown little evidence of caspase-3 cleavage and caspase-3−/− MEFs were not protected from BNIP3 (99). Additionally, Apaf1−/− and caspase-9−/− MEFs were sensitive to BNIP3-induced cell death (99). Despite upregulation of BNIP3 during anoxia and the role of BNIP3 in cell death, it is unlikely that BNIP3 mediates cell death in response to anoxia, because knockdown of BNIP3 did not protect MEFs, HeLa, or RKO cells from anoxia-induced apoptosis (76). Recent studies have shown that BNIP3 can induce autophagy as a survival mechanism, yet it has been reported that BNIP3 is not involved in autophagy during anoxia (77). Conversely, Tracy et al. (98) showed that, in the absence of the RB tumor suppressor, BNIP3 induces autophagic cell death during anoxia. The exact role of BNIP3 during anoxia is not clear.
The role of p53 in anoxia-induced apoptosis
Several studies suggest that p53 mediates anoxia-induced apoptosis [reviewed in (38)]. The transcription factor p53 is stabilized by anoxia (32, 58) and controls the expression of apoptotic genes such as Puma, Noxa, Bid, and Bax (70, 72, 74, 88, 106). However, oxygen deprivation results in p53 interaction with the transcriptional repressor mSin3A, indicating that p53 transcriptional activity may not be functional during anoxia (49). p53 also is thought to control apoptosis by a transcription-independent mechanism. It has been shown to bind directly to antiapoptotic proteins and to diminish their prosurvival function. For example, co-transfection experiments identify BCL-XL and BCL-2 as binding partners of p53 (69). The specific contact regions between BCL-XL and p53 were mapped to codons 239 to 248 of the DNA-binding domain of p53. Whereas wild-type p53 was able to induce cytochrome c release from isolated mitochondria, mutations in the DNA-binding domain of p53 prevented this phenotype. The addition of BCL-XL is able to prevent cytochrome c release. The p53–BCL-XL interaction is hypothesized to free BCL-XL from BAK, allowing MOMP. Thus, p53 acts similar to BH3 proteins.
Initial studies showed that p53 is required for anoxia-induced apoptosis in oncogene-transformed cells (50). In contrast, anoxia-induced apoptosis has been shown to be independent of p53 but dependent on BAX/BAK in oncogene-transformed cells (6). One possibility to reconcile these opposite studies is that anoxia-induced apoptosis in transformed cells occurs only when coupled with acidosis (89). p53 has been shown to be upregulated in certain cell lines when coupled with acidosis (75). Thus, p53-mediated anoxic cell death is likely restricted to oncogenically transformed cells when coupled with acidosis.
Hypoxia Promotes Cell Survival Through HIFs
Structure and regulation of HIFs
HIF-1 was originally identified by its binding to the hypoxia-response element (HRE) of the erythropoietin gene after hypoxic exposure (101). HIF-1 is a heterodimeric transcription factor consisting of an α (HIF-1α) and a β subunit [HIF-1β/aryl hydrocarbon receptor nuclear translocator (ARNT)]. The stability of the HIF-1α subunit is sensitive to oxygen concentrations, whereas the HIF-1β subunit is constitutively stable (43). Both components are members of the Per/ARNT/Sim (PAS) family of proteins (101). Since its original identification, two additional HIF family members have been identified: HIF-2α and HIF-3α (73, 101, 104). Whereas HIF-1α is ubiquitously expressed, HIF-2α and HIF-3α are localized to specific cell types (34, 67, 103). HIF-2α is biologically similar to HIF-1α and binds to HIF-1β (103, 104). HIF-3α has many splice variants, one of which may negatively regulate hypoxia-inducible gene expression (63, 64, 67).
HIF-1α is hydroxylated at prolines 402 and 564 within the oxygen-dependent degradation (ODD) domain, and this is dependent on oxygen availability (39, 41). A family of proline hydroxylation enzymes, termed PHDs, catalyze this reaction (4, 27). Hydroxylation of HIF-1α requires iron in ferrous form (Fe2+), oxygen, and 2-oxoglutarate as cofactors. The hydroxylated prolines are recognized by and serve as binding sites for the von Hippel-Lindau (VHL) ubiquitin E3 ligase complex, which targets the protein for degradation by the 26S proteosome (39–41). HIF activity also is regulated in the nucleus by factor-inhibiting HIF-1 (FIH-1) in an oxygen-dependent manner. FIH was originally identified by Semenza and colleagues (61) as a novel HIF-binding partner. FIH is a 2-oxoglutarate–dependent dioxygenase and functions similarly to the prolyl hydroxylases in that it uses molecular oxygen to modify its substrates. In normal-oxygen conditions, FIH-1 inactivates HIF by hydroxylating asparagine residues in the carboxy-terminus transactivation domain (C-TAD) of HIF, thus preventing binding between HIF and its coactivator, CBP/p300 (53, 54). Therefore, FIH regulates the DNA binding and transcriptional activity of HIF. During hypoxia, proline hydroxylation of HIF-α is inhibited, preventing its recognition by VHL-mediated proteosomal degradation. Thus, HIF-α rapidly accumulates and binds to HIF-1β to activate transcription. Low oxygen availability also inhibits FIH activity. In the absence of FIH activity, HIF binds to CBP/p300, allowing the transcription of target genes.
Many studies have focused on the precise mechanism for HIF regulation during hypoxia. More than 90% of oxygen intake is consumed by the mitochondria for oxidative phosphorylation. Therefore, the mitochondria have been implicated as potential oxygen sensors that can regulate a variety of hypoxic responses, including the regulation of HIF activity. The initial experiments designed to test the role of the mitochondria in HIF activation used respiration-incompetent ρ0 cells. A subset of the respiratory-chain protein subunits are encoded by the nuclear genome, yet 13 of these subunits are encoded by mitochondrial DNA (mtDNA) (12). Ethidium bromide (EtBr) preferentially inhibits mitochondrial DNA synthesis by targeting the mitochondrial DNA polymerase (97). Culturing cells in relatively low levels of EtBr is a method used to generate cells that are deficient in mtDNA. These cells are termed ρ0 cells and do not have a functional electron-transport chain. ρ0 cells, which fail to respire, are unable to activate HIF-1α in hypoxic conditions (10, 11). Interestingly, HIF-1α is stabilized in ρ0 cells exposed to anoxia. This indicates that HIF-1α is regulated by different mechanisms in hypoxia versus anoxia (90). Additionally, this provides evidence that the proteins of the electron-transport chain are required for hypoxic stabilization of HIF-1α. Anoxic stabilization of HIF is likely caused by direct inhibition of the PHDs due to the absence of oxygen.
Two models have been proposed as possible mechanisms by which the mitochondria activate HIF during hypoxia. The conversion of oxygen to water at complex IV for the generation of ATP is a major sink for oxygen in a cell (87). This, combined with the requirement of oxygen as a substrate for PHD activity, is the basis for the first model (Fig. 5). This model hypothesizes that during hypoxia, much of the oxygen in the cell is directed to the mitochondria, resulting in an intracellular oxygen gradient. This would result in the sequestration of oxygen away from the PHDs, therefore limiting their ability to hydroxylate HIF-1α (23, 36). However, data that contradict this model shows that HIF is stabilized during hypoxia in respiration-incompetent cells that are not ρ0 cells, are able to stabilize HIF-1α during hypoxia (5).
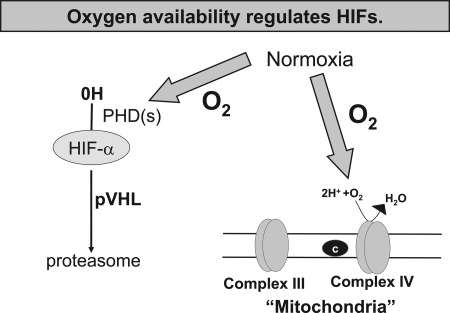
FIG. 5.
Oxygen availability regulates HIFs. Oxygen is required as a substrate for hydroxylation of the HIF-α subunit. Oxygen also is consumed by mitochondrial complex IV. Thus, in certain conditions, an increase in oxygen consumption by complex IV results in reduced availability of oxygen for hydroxylation of the HIF-α subunit.
The second model proposes that mitochondria-generated ROS increase during hypoxia to prevent HIF-1α protein degradation (Fig. 6) (reviewed in ref. 48). Interestingly, cytosolic ROS increases during hypoxia (10). Cells deficient in the mitochondrial electron-transport chain proteins cytochrome c or Rieske iron-sulfur protein (RISP) do not show an increase in ROS in response to hypoxia, and these cells are unable to stabilize HIF-1α (5, 35, 65). Treatment with the mitochondria-targeted antioxidant MitoQ prevents HIF activation (84), but recent studies indicate that MitoQ also affects respiration (85). The strongest evidence supporting the ROS model involves the use of cells deficient in cytochrome b, an electron-transport chain protein encoded by mitochondrial DNA (3). Cells deficient in cytochrome b are able to generate ROS but are respiratory deficient. These cells are able to stabilize HIF-1α protein during hypoxia. However, depleting the RISP by using RNAi in cytochrome b cells attenuates both ROS generation and HIF-1α protein stabilization during hypoxia. Furthermore, MitoQ prevents HIF-1α protein stabilization in the respiratory-deficient cytochrome b–null cells. These results indicate that ROS generated from complex III activate HIFs (Fig. 7).
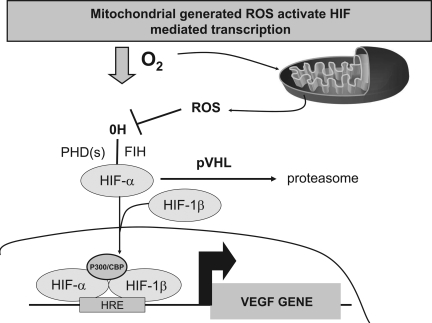
FIG. 6.
Mitochondria-generated ROS activate HIF-mediated transcription. Mitochondrial ROS inhibit the hydroxylation of HIF through a yet-unidentified mechanism. In the absence of hydroxylation, HIF-α is not recognized by VHL; thus, it is not targeted for degradation by the proteosome. HIF-α heterodimerizes with HIF-1β and, in the nucleus, binds to hypoxic response elements (HREs), resulting in the transcription of genes such as VEGF.
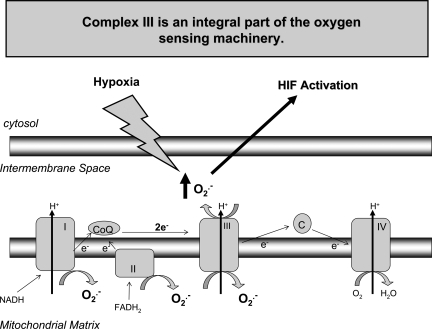
FIG. 7.
Complex III is an integral part of the oxygen-sensing machinery. Hypoxia stimulates the release of superoxide from complex III into the mitochondrial intermembrane space. Superoxide converts to hydrogen peroxide and enters the cytosol to activate HIFs.
HIF and cell survival
The BCL-2 proteins integrate signals of survival and death. The decision to commit to one fate or the other is dependent largely on the balance between pro- and antiapoptotic BCL-2 proteins. HIF has been shown to increase the expression of antiapoptotic BCL-2 family members and to decrease the expression of proapoptotic family members. For example, HIF-1α has been shown to increase the expression of the antiapoptotic protein MCL-1 in HepG2 cells (81). Analysis of the MCL-1 promoter sequence revealed a putative HIF-1 binding site. HIF-1 was able to bind to this promoter sequence and induce transcription. Furthermore, overexpression of MCL-1 protected HepG2 cells from apoptosis. HIF-1α also was shown to regulate BCL-XL (13). This was demonstrated in a prostate cancer PC-3 cell line with constitutively high HIF-1α levels. Knockdown of HIF-1α in this cell line was correlated with a dramatic decrease in BCL-XL expression. This occurred in parallel with sensitization to apoptosis and caspase-3 activation. It was further shown that HIF-1α directly regulates BCL-XL transcription by binding to an HRE in the BCL-XL promoter (−865 to −847). HIF-1α expression has been correlated with BCL-2 expression, yet direct regulation is not known (2). Thus, HIF activation plays an important role in triggering cellular protection through the regulation of antiapoptotic proteins.
HIF also was shown to decrease expression of proapoptotic BCL-2 proteins. Hypoxia caused a decrease in mRNA and protein levels of BAD and BID in human colon cancer cells (28). Whereas HIF-1α was dispensable for the downregulation of BAD, it was required for the downregulation of BID. HIF was able to bind to the BID promoter in region −8484 to −8475. Furthermore, hypoxia caused a proteosome-independent decrease in BAX expression. The loss of BAX expression was the result of reduced translational efficiency but was not correlated to HIF activity. Both BID and BAX downregulation was confirmed in tumors in vivo, and forced expression of BID in hypoxic cells resulted in increased sensitivity to apoptosis (28). Collectively, these studies demonstrate that HIF can directly regulate the activity of both pro- and antiapoptotic family members to tip the balance toward cell survival.
In addition to the BCL-2 family of proteins, HIF also has been implicated in the regulation of other proteins involved in apoptosis. Survivin is a structurally unique protein of the IAP family. Both HIF-1α and survivin were found to be overexpressed in non–small cell lung cancer, and their expression increased in A549 cells cultured in hypoxia (14). Inhibition of HIF-1α by RNAi decreased survivin expression. Another member of this family, IAP-2, has been shown to be upregulated during hypoxia but independent of HIF activity (24). Thus, hypoxia activates cell-survival pathways through activation of HIF.
A newly identified regulator of HIF-1 and survival is DJ-1 (CAP1/RS/PARK7). Loss of DJ-1 leads to cell death in neurodegenerative disease, whereas gain of function results in cell survival in cancer. Loss-of-function mutations in the human DJ-1 gene have been associated with early onset of Parkinson disease (46). DJ-1 is overexpressed in lung cancer and positively correlates with activated AKT (45). Loss- and gain-of-function experiments revealed that DJ-1 promotes cell survival by inhibiting PTEN, thereby enhancing AKT phosphorylation. Activated AKT can prevent BAX/BAK-dependent release of cytochrome c (62). Overexpression of DJ-1 also prevents oxidative stress–induced cell death (96). Recently, Mak and colleagues (100) demonstrated that DJ-1 expression is critical for AKT and mTOR activation during hypoxia (100). This sustains HIF-1α protein stability. In contrast, the loss of DJ-1 impairs transcription of HIF-1 target genes. Mouse embryonic fibroblasts or cancer cells deficient in DJ-1 are highly sensitized to anoxia-induced apoptosis. DJ-1–deficient dopaminergic neurons also show enhanced sensitivity to oxygen and glucose deprivation (82). Collectively, these results indicate that DJ-1 is an important regulator of survival in low-oxygen conditions.
Both anoxia and hypoxia regulate tumorigenesis
Accumulation of oncogene expression coupled with loss of tumor suppressors promotes deregulated proliferation, resulting in the formation of a small tumor. Low-oxygen microenvironments are a hallmark of solid tumors. This is due in part to deregulated growth, which overrides the ability of the vasculature to supply sufficient oxygen (38). In addition, tumor blood vessels are structurally and functionally impaired compared with normal tissue vasculature. New blood vessels recruited to tumors are disorganized and have a variety of abnormalities, including leakiness (42). As a result, tumors have both anoxic and hypoxic regions. Cell death is prominent in areas farthest from the tumor vasculature, where anoxia causes apoptosis. Anoxia-induced apoptosis serves as a safeguard against tumor development. Anoxia also provides a selective pressure for tumor growth (31). Anoxia will select for tumor cells that have mutations in p53 or overexpress antiapoptotic BCL-2 proteins, because these oncogenically transformed cells are resistant to anoxia-induced cell death (31). Thus, anoxia provides a selective pressure in tumors for the expansion of cells that have lost apoptotic potential.
For a small tumor to grow, it requires adaptation to hypoxia and development of new blood vessels in anoxic regions. HIFs directly control the transcription of genes involved in glycolytic metabolism, angiogenesis, and metastasis (92). Multiple studies have shown that loss of HIFs prevents tumor formation. Because mitochondrial ROS regulate HIFs, antioxidants should prevent tumorigenesis in an HIF-dependent manner. Dang and colleagues (30) demonstrated that the antioxidant N-acetylcysteine prevents tumor growth, which can be rescued by a constitutively active HIF-1 (30).
Well-oxygenated tumors have a better prognosis than do tumors in low-oxygenated regions, independent of treatment. This indicates that low-oxygen microenvironments may not be a by-product of tumorgenicity but may actually contribute to tumor progression. The best evidence comes from a recent study in which ectopically expressed myoglobin (Mb) in A549 human lung carcinoma cells was used to keep cells from becoming hypoxic (29). Mb is a cytosolic hemoprotein present in skeletal and heart muscle. This protein binds oxygen reversibly and facilitates oxygen transport from the blood to the mitochondria or acts as an oxygen reservoir during hypoxic conditions. Mb expression in A549 cells prevented the hypoxic response in vitro. Mb expression also delayed tumor engraftment and reduced tumor growth after xenotransplantation into mice. Mb-expressing tumors showed little or no hypoxia, minimal HIF-1α levels, and a homogeneously low vessel density. Mb-mediated tumor oxygenation promoted differentiation of cancer cells and suppressed metastasis. Constitutively active HIF-1α rescued tumor growth in A549 cells expressing Mb. As a control, point mutations in Mb that prevented oxygen binding allowed tumor growth and metastasis. This study provided evidence that hypoxia is not just a side effect, but actually a driving force for tumor growth.
In summary, it is clear that mitochondria are decision makers for cell death and cell survival during anoxia and hypoxia, respectively. Anoxia (0–0.5% oxygen) induces apoptosis through the intrinsic apoptotic pathway. Hypoxia (0.5% to 3% oxygen) promotes cell survival through mitochondrial ROS-dependent activation of HIFs and their target genes. The signaling events that occur in response to anoxia and hypoxia are significant to human disease in which anoxia selects for apoptosis-resistant cells in tumors and hypoxia allows tumor growth. A firm understanding of these events will allow better therapeutic strategies in the treatment of cancer.
Glossary
Abbreviations Used
- BCL
B-cell lymphoma
- HIF
hypoxia-inducible factor
- HRE
hypoxia response element
- MOMP
mitochondrial outer membrane permeabilization
- mtDNA
mitochondrial DNA
- PHD
prolyl hydroxylase domain
- ROS
reactive oxygen species
- VHL
von Hippel–Lindau
Acknowledgments
This work was supported by NIH grant R01CA123067-03 as well as the LUNGevity Foundation and a Consortium of Independent Lung Health Organizations convened by Respiratory Health Association of Metropolitan Chicago to NSC.
References
- 1.Antonsson B. Montessuit S. Lauper S. Eskes R. Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- 2.Ardyanto TD. Osaki M. Tokuyasu N. Nagahama Y. Ito H. CoCl2-induced HIF-1alpha expression correlates with proliferation and apoptosis in MKN-1 cells: a possible role for the PI3K/Akt pathway. Int J Oncol. 2006;29:549–555. [PubMed] [Google Scholar]
- 3.Bell EL. Klimova TA. Eisenbart J. Moraes CT. Murphy MP. Budinger GR. Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruick RK. McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 5.Brunelle JK. Bell EL. Quesada NM. Vercauteren K. Tiranti V. Zeviani M. Scarpulla RC. Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Brunelle JK. Santore MT. Budinger GR. Tang Y. Barrett TA. Zong WX. Kandel E. Keith B. Simon MC. Thompson CB. Hay N. Chandel NS. c-Myc sensitization to oxygen deprivation-induced cell death is dependent on Bax/Bak, but is independent of p53 and hypoxia-inducible factor-1. J Biol Chem. 2004;279:4305–4312. doi: 10.1074/jbc.M312241200. [DOI] [PubMed] [Google Scholar]
- 7.Brunelle JK. Shroff EH. Perlman H. Strasser A. Moraes CT. Flavell RA. Danial NN. Keith B. Thompson CB. Chandel NS. Loss of Mcl-1 protein and inhibition of electron transport chain together induce anoxic cell death. Mol Cell Biol. 2007;27:1222–1235. doi: 10.1128/MCB.01535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunn HF. Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 9.Cannon RO., 3rd Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin Chem. 1998;44:1809–1819. [PubMed] [Google Scholar]
- 10.Chandel NS. Maltepe E. Goldwasser E. Mathieu CE. Simon MC. Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandel NS. McClintock DS. Feliciano CE. Wood TM. Melendez JA. Rodriguez AM. Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 12.Chandel NS. Schumacker PT. Cells depleted of mitochondrial DNA (rho0) yield insight into physiological mechanisms. FEBS Lett. 1999;454:173–176. doi: 10.1016/s0014-5793(99)00783-8. [DOI] [PubMed] [Google Scholar]
- 13.Chen N. Chen X. Huang R. Zeng H. Gong J. Meng W. Lu Y. Zhao F. Wang L. Zhou Q. BCL-xL is a target gene regulated by hypoxia-inducible factor-1-alpha. J Biol Chem. 2009;284:10004–10012. doi: 10.1074/jbc.M805997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YQ. Zhao CL. Li W. Effect of hypoxia-inducible factor-1alpha on transcription of survivin in non-small cell lung cancer. J Exp Clin Cancer Res. 2009;28:29. doi: 10.1186/1756-9966-28-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng EH. Levine B. Boise LH. Thompson CB. Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-XL. Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 16.Cheng EH. Wei MC. Weiler S. Flavell RA. Mak TW. Lindsten T. Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 17.Chipuk JE. Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cory S. Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 19.Cory S. Huang DC. Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 20.Czabotar PE. Lee EF. van Delft MF. Day CL. Smith BJ. Huang DC. Fairlie WD. Hinds MG. Colman PM. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci U S A. 2007;104:6217–6222. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danial NN. Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 22.Desagher S. Osen-Sand A. Nichols A. Eskes R. Montessuit S. Lauper S. Maundrell K. Antonsson B. Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doege K. Heine S. Jensen I. Jelkmann W. Metzen E. Inhibition of mitochondrial respiration elevates oxygen concentration but leaves regulation of hypoxia-inducible factor (HIF) intact. Blood. 2005;106:2311–2317. doi: 10.1182/blood-2005-03-1138. [DOI] [PubMed] [Google Scholar]
- 24.Dong Z. Venkatachalam MA. Wang J. Patel Y. Saikumar P. Semenza GL. Force T. Nishiyama J. Up-regulation of apoptosis inhibitory protein IAP-2 by hypoxia. Hif-1-independent mechanisms. J Biol Chem. 2001;276:18702–18709. doi: 10.1074/jbc.M011774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du C. Fang M. Li Y. Li L. Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 26.Eguchi Y. Shimizu S. Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 27.Epstein AC. Gleadle JM. McNeill LA. Hewitson KS. O'Rourke J. Mole DR. Mukherji M. Metzen E. Wilson MI. Dhanda A. Tian YM. Masson N. Hamilton DL. Jaakkola P. Barstead R. Hodgkin J. Maxwell PH. Pugh CW. Schofield CJ. Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 28.Erler JT. Cawthorne CJ. Williams KJ. Koritzinsky M. Wouters BG. Wilson C. Miller C. Demonacos C. Stratford IJ. Dive C. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004;24:2875–2889. doi: 10.1128/MCB.24.7.2875-2889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galluzzo M. Pennacchietti S. Rosano S. Comoglio PM. Michieli P. Prevention of hypoxia by myoglobin expression in human tumor cells promotes differentiation and inhibits metastasis. J Clin Invest. 2009;119:865–875. doi: 10.1172/JCI36579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao P. Zhang H. Dinavahi R. Li F. Xiang Y. Raman V. Bhujwalla ZM. Felsher DW. Cheng L. Pevsner J. Lee LA. Semenza GL. Dang CV. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graeber TG. Osmanian C. Jacks T. Housman DE. Koch CJ. Lowe SW. Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 32.Graeber TG. Peterson JF. Tsai M. Monica K. Fornace AJ., Jr Giaccia AJ. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol. 1994;14:6264–6277. doi: 10.1128/mcb.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 34.Gu YZ. Moran SM. Hogenesch JB. Wartman L. Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- 35.Guzy RD. Hoyos B. Robin E. Chen H. Liu L. Mansfield KD. Simon MC. Hammerling U. Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Hagen T. Taylor CT. Lam F. Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 37.Hamacher-Brady A. Brady NR. Logue SE. Sayen MR. Jinno M. Kirshenbaum LA. Gottlieb RA. Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 38.Harris AL. Hypoxia: a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 39.Ivan M. Kondo K. Yang H. Kim W. Valiando J. Ohh M. Salic A. Asara JM. Lane WS. Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–4548. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 40.Iwai K. Yamanaka K. Kamura T. Minato N. Conaway RC. Conaway JW. Klausner RD. Pause A. Identification of the von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci U S A. 1999;96:12436–12441. doi: 10.1073/pnas.96.22.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaakkola P. Mole DR. Tian YM. Wilson MI. Gielbert J. Gaskell SJ. Kriegsheim A. Hebestreit HF. Mukherji M. Schofield CJ. Maxwell PH. Pugh CW. Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 42.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 43.Jiang BH. Semenza GL. Bauer C. Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 44.Kim JY. Ahn HJ. Ryu JH. Suk K. Park JH. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J Exp Med. 2004;199:113–124. doi: 10.1084/jem.20030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim RH. Peters M. Jang Y. Shi W. Pintilie M. Fletcher GC. DeLuca C. Liepa J. Zhou L. Snow B. Binari RC. Manoukian AS. Bray MR. Liu FF. Tsao MS. Mak TW. DJ-1: a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Kim RH. Smith PD. Aleyasin H. Hayley S. Mount MP. Pownall S. Wakeham A. You-Ten AJ. Kalia SK. Horne P. Westaway D. Lozano AM. Anisman H. Park DS. Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kischkel FC. Hellbardt S. Behrmann I. Germer M. Pawlita M. Krammer PH. Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klimova T. Chandel NS. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008;15:660–666. doi: 10.1038/sj.cdd.4402307. [DOI] [PubMed] [Google Scholar]
- 49.Koumenis C. Alarcon R. Hammond E. Sutphin P. Hoffman W. Murphy M. Derr J. Taya Y. Lowe SW. Kastan M. Giaccia A. Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol Cell Biol. 2001;21:1297–1310. doi: 10.1128/MCB.21.4.1297-1310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koumenis C. Giaccia A. Transformed cells require continuous activity of RNA polymerase II to resist oncogene-induced apoptosis. Mol Cell Biol. 1997;17:7306–7316. doi: 10.1128/mcb.17.12.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kroemer G. Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 52.Kuwana T. Bouchier-Hayes L. Chipuk JE. Bonzon C. Sullivan BA. Green DR. Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Lando D. Peet DJ. Gorman JJ. Whelan DA. Whitelaw ML. Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lando D. Peet DJ. Whelan DA. Gorman JJ. Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 55.Leist M. Single B. Castoldi AF. Kuhnle S. Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Letai A. Bassik MC. Walensky LD. Sorcinelli MD. Weiler S. Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 57.Li P. Nijhawan D. Budihardjo I. Srinivasula SM. Ahmad M. Alnemri ES. Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 58.Li YZ. Lu DY. Tan WQ. Wang JX. Li PF. p53 initiates apoptosis by transcriptionally targeting the antiapoptotic protein ARC. Mol Cell Biol. 2008;28:564–574. doi: 10.1128/MCB.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu B. Gao HM. Wang JY. Jeohn GH. Cooper CL. Hong JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci. 2002;962:318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 60.Liu X. Kim CN. Yang J. Jemmerson R. Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 61.Mahon PC. Hirota K. Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:75–86. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Majewski N. Nogueira V. Bhaskar P. Coy PE. Skeen JE. Gottlob K. Chandel NS. Thompson CB. Robey RB. Hay N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Makino Y. Cao R. Svensson K. Bertilsson G. Asman M. Tanaka H. Cao Y. Berkenstam A. Poellinger L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 64.Makino Y. Kanopka A. Wilson WJ. Tanaka H. Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J Biol Chem. 2002;277:32405–32428. doi: 10.1074/jbc.C200328200. [DOI] [PubMed] [Google Scholar]
- 65.Mansfield KD. Guzy RD. Pan Y. Young RM. Cash TP. Schumacker PT. Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maurer U. Charvet C. Wagman AS. Dejardin E. Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Maynard MA. Qi H. Chung J. Lee EH. Kondo Y. Hara S. Conaway RC. Conaway JW. Ohh M. Multiple splice variants of the human HIF-3 alpha locus are targets of the von Hippel-Lindau E3 ubiquitin ligase complex. J Biol Chem. 2003;278:11032–11040. doi: 10.1074/jbc.M208681200. [DOI] [PubMed] [Google Scholar]
- 68.McClintock DS. Santore MT. Lee VY. Brunelle J. Budinger GR. Zong WX. Thompson CB. Hay N. Chandel NS. Bcl-2 family members and functional electron transport chain regulate oxygen deprivation-induced cell death. Mol Cell Biol. 2002;22:94–104. doi: 10.1128/MCB.22.1.94-104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mihara M. Erster S. Zaika A. Petrenko O. Chittenden T. Pancoska P. Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 70.Miyashita T. Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 71.Muzio M. Chinnaiyan AM. Kischkel FC. O'Rourke K. Shevchenko A. Ni J. Scaffidi C. Bretz JD. Zhang M. Gentz R. Mann M. Krammer PH. Peter ME. Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 72.Nakano K. Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 73.O'Rourke JF. Tian YM. Ratcliffe PJ. Pugh CW. Oxygen-regulated and transactivating domains in endothelial PAS protein 1: comparison with hypoxia-inducible factor-1alpha. J Biol Chem. 1999;274:2060–2071. doi: 10.1074/jbc.274.4.2060. [DOI] [PubMed] [Google Scholar]
- 74.Oda E. Ohki R. Murasawa H. Nemoto J. Shibue T. Yamashita T. Tokino T. Taniguchi T. Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 75.Pan Y. Oprysko PR. Asham AM. Koch CJ. Simon MC. p53 cannot be induced by hypoxia alone but responds to the hypoxic microenvironment. Oncogene. 2004;23:4975–4983. doi: 10.1038/sj.onc.1207657. [DOI] [PubMed] [Google Scholar]
- 76.Papandreou I. Krishna C. Kaper F. Cai D. Giaccia AJ. Denko NC. Anoxia is necessary for tumor cell toxicity caused by a low-oxygen environment. Cancer Res. 2005;65:3171–3178. doi: 10.1158/0008-5472.CAN-04-3395. [DOI] [PubMed] [Google Scholar]
- 77.Papandreou I. Lim AL. Laderoute K. Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ. 2008;15:1572–1581. doi: 10.1038/cdd.2008.84. [DOI] [PubMed] [Google Scholar]
- 78.Parsadanian AS. Cheng Y. Keller-Peck CR. Holtzman DM. Snider WD. Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons. J Neurosci. 1998;18:1009–1019. doi: 10.1523/JNEUROSCI.18-03-01009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petros AM. Nettesheim DG. Wang Y. Olejniczak ET. Meadows RP. Mack J. Swift K. Matayoshi ED. Zhang H. Thompson CB. Fesik SW. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 2000;9:2528–2534. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petros AM. Olejniczak ET. Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 81.Piret JP. Minet E. Cosse JP. Ninane N. Debacq C. Raes M. Michiels C. Hypoxia-inducible factor-1-dependent overexpression of myeloid cell factor-1 protects hypoxic cells against tert-butyl hydroperoxide-induced apoptosis. J Biol Chem. 2005;280:9336–9344. doi: 10.1074/jbc.M411858200. [DOI] [PubMed] [Google Scholar]
- 82.Pisani A. Martella G. Tscherter A. Costa C. Mercuri NB. Bernardi G. Shen J. Calabresi P. Enhanced sensitivity of DJ-1-deficient dopaminergic neurons to energy metabolism impairment: role of Na+/K+ ATPase. Neurobiol Dis. 2006;23:54–60. doi: 10.1016/j.nbd.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Ray R. Chen G. Vande Velde C. Cizeau J. Park JH. Reed JC. Gietz RD. Greenberg AH. BNIP3 heterodimerizes with Bcl-2/Bcl-X(L) and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. J Biol Chem. 2000;275:1439–1448. doi: 10.1074/jbc.275.2.1439. [DOI] [PubMed] [Google Scholar]
- 84.Saikumar P. Dong Z. Patel Y. Hall K. Hopfer U. Weinberg JM. Venkatachalam MA. Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene. 1998;17:3401–3415. doi: 10.1038/sj.onc.1202590. [DOI] [PubMed] [Google Scholar]
- 85.Sanjuan-Pla A. Cervera AM. Apostolova N. Garcia-Bou R. Victor VM. Murphy MP. McCreath KJ. A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1alpha. FEBS Lett. 2005;579:2669–2674. doi: 10.1016/j.febslet.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 86.Santore MT. McClintock DS. Lee VY. Budinger GR. Chandel NS. Anoxia-induced apoptosis occurs through a mitochondria-dependent pathway in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L727–L734. doi: 10.1152/ajplung.00281.2001. [DOI] [PubMed] [Google Scholar]
- 87.Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- 88.Sax JK. Fei P. Murphy ME. Bernhard E. Korsmeyer SJ. El-Deiry WS. BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol. 2002;4:842–849. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- 89.Schmaltz C. Hardenbergh PH. Wells A. Fisher DE. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol Cell Biol. 1998;18:2845–2854. doi: 10.1128/mcb.18.5.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schroedl C. McClintock DS. Budinger GR. Chandel NS. Hypoxic but not anoxic stabilization of HIF-1alpha requires mitochondrial reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2002;283:L922–L931. doi: 10.1152/ajplung.00014.2002. [DOI] [PubMed] [Google Scholar]
- 91.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 92.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 93.Soengas MS. Alarcon RM. Yoshida H. Giaccia AJ. Hakem R. Mak TW. Lowe SW. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- 94.Strasser A. Harris AW. Huang DC. Krammer PH. Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Susin SA. Lorenzo HK. Zamzami N. Marzo I. Snow BE. Brothers GM. Mangion J. Jacotot E. Costantini P. Loeffler M. Larochette N. Goodlett DR. Aebersold R. Siderovski DP. Penninger JM. Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 96.Taira T. Saito Y. Niki T. Iguchi-Ariga SM. Takahashi K. Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tarrago-Litvak L. Viratelle O. Darriet D. Dalibart R. Graves PV. Litvak S. The inhibition of mitochondrial DNA polymerase gamma from animal cells by intercalating drugs. Nucleic Acids Res. 1978;5:2197–2210. doi: 10.1093/nar/5.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tracy K. Dibling BC. Spike BT. Knabb JR. Schumacker P. Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007;27:6229–6242. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vande Velde C. Cizeau J. Dubik D. Alimonti J. Brown T. Israels S. Hakem R. Greenberg AH. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20:5454–5468. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vasseur S. Afzal S. Tardivel-Lacombe J. Park DS. Iovanna JL. Mak TW. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci U S A. 2009;106:1111–1116. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang GL. Jiang BH. Rue EA. Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wei MC. Zong WX. Cheng EH. Lindsten T. Panoutsakopoulou V. Ross AJ. Roth KA. MacGregor GR. Thompson CB. Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wiesener MS. Jurgensen JS. Rosenberger C. Scholze CK. Horstrup JH. Warnecke C. Mandriota S. Bechmann I. Frei UA. Pugh CW. Ratcliffe PJ. Bachmann S. Maxwell PH. Eckardt KU. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 104.Wiesener MS. Turley H. Allen WE. Willam C. Eckardt KU. Talks KL. Wood SM. Gatter KC. Harris AL. Pugh CW. Ratcliffe PJ. Maxwell PH. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92:2260–2268. [PubMed] [Google Scholar]
- 105.Willis SN. Chen L. Dewson G. Wei A. Naik E. Fletcher JI. Adams JM. Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu J. Zhang L. Hwang PM. Kinzler KW. Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]