Abstract
In this study, we conducted a thorough analysis of mitochondrial bioenergetic function as well as the biochemical and molecular factors that are deregulated and contribute to compromised adenosine triphosphate (ATP) production in the myocardium during Trypanosoma cruzi infection. We show that ADP-stimulated state 3 respiration and ATP synthesis supported by pyruvate/malate (provides electrons to complex I) and succinate (provides electrons to complex II) substrates were significantly decreased in left ventricular tissue and isolated cardiac mitochondria of infected mice. The decreased mitochondrial ATP synthesis in infected murine hearts was not a result of uncoupling between the electron-transport chain and oxidative phosphorylation and decreased availability of the intermediary metabolites (e.g., NADH). The observed decline in the activities of complex-I, -IV, and -V was not physiologically relevant and did not contribute to compromised respiration and ATP synthesis in infected myocardium. Instead, complex III activity was decreased above the threshold level and contributed to respiratory-chain inefficiency and the resulting decline in mitochondrial ATP synthesis in infected myocardium. The loss in complex III activity occurred as a consequence of cytochrome b depletion. Treatment of infected mice with phenyl-α-tert-butyl nitrone (PBN, antioxidant) was beneficial in preserving the mtDNA-encoded cytochrome b expression, and subsequently resulted in improved complex III activity, mitochondrial respiration, and ATP production in infected myocardium. Overall, we provide novel data on the mechanism(s) involved in cardiac bioenergetic inefficiency during T. cruzi infection. Antioxid. Redox Signal. 12, 27–37.
Introduction
Chagas disease is a major human health problem in Latin America (47). It is estimated that 13 million people are infected and 40 million are exposed to the risk of Trypanosoma cruzi infection in countries in which it is endemic. In ∼30% of the infected individuals, symptomatic chronic chagasic cardiomyopathy developed with a progressive global ventricular dilation, arrhythmia, and contractile dysfunction (24).
The myocardial contractile function is essentially supported by oxidative phosphorylation (33), in which respiratory electron flow in the inner membrane of the mitochondria is coupled to the FoF1 ATP synthase and produces high-energy metabolite ATP (35). Studies with experimental animals infected with T. cruzi and biopsies from human patients have shown that mitochondrial alterations, cellular damage, and oxidized proteins and lipids are dominantly present in chagasic hearts with progressive disease (4, 13, 22, 23, 44). The enzymatic activity of the respiratory complexes was compromised in the myocardium (41) and not in other tissues of infected animals (45). Accordingly, a decline in cardiac energy status was noted in experimental models of T. cruzi infection (38, 41) and in chagasic humans (16), and it was proposed that mitochondrial dysfunction and energy depletion contribute, at least partially, to myocardial contractile dysfunction during Chagas disease. No detailed studies have; however, been conducted to determine whether and how mitochondrial abnormalities affect the rate of ATP synthesis in chagasic myocardium.
In this study, we aimed to determine whether the bioenergetics of cardiac mitochondria is altered in T. cruzi–infected mice, and if such is the case, to identify the subcellular mechanisms that contribute to the dissipation of energy output in infected myocardium. We treated infected mice with phenyl-α-tert-butyl nitrone (PBN, antioxidant) to determine whether oxidative stress affects the mitochondrial respiration and coupled oxidative phosphorylation capacity in chagasic myocardium. PBN reacts with short-lived oxygen and carbon-centered radicals at the nitrone double bond and result in the formation of relatively stable nitroxide that does not propagate the destructive radical chain reaction (11). We chose PBN because inflammatory responses elicited by T. cruzi infection (6) and ROS production by mitochondria (46) can cause oxidative stress, and PBN treatment was found to be beneficial in preventing the myocardial oxidative damage in infected mice (42). The findings in the current study demonstrate that a decline in mitochondrial DNA-encoded cytochrome b (cyt b) expression resulted in compromised complex III activity that led to inefficient electrochemical gradient and decreased ATP synthesis in infected myocardium. Infected mice treated with PBN exhibited improved cardiac mitochondrial respiration and ATP synthesis associated with preservation of mtDNA, cyt b expression, and complex III activity. We discuss the mechanism(s) involved in bioenergetic inefficiency of the heart during T. cruzi infection.
Materials and Methods
Mice and parasites
Six-to-eight-week-old male C3H/HeN mice (Harlan Labs, Indianapolis, IN) were infected with T. cruzi trypomastigotes (SylvioX10/4 strain, 10,000/mouse, IP). Mice were treated with PBN beginning day 0 (50 mg/kg body weight, IP., alternate days) until the end of the study. Mice were killed at day 27 to 35 after infection (acute phase). The UTMB Animal Care and Use Committee had approved all animal experiments performed in this study.
Isolation of mitochondria
All solutions were prepared with highly pure chemicals (Sigma-Aldrich, St. Louis, MO) devoid of divalent cations in HPLC grade H2O. Freshly harvested heart ventricular tissues were washed with ice-cold HMSB medium (10 mM HEPES pH 7.4, 225 mM mannitol, 75 mM sucrose, and 0.2% fatty acid free BSA) and resuspended in HMSB containing 200 U/ml collagenase (1:20 ratio, tissue/buffer). Tissues were homogenized in a dounce homogenizer, incubated for 3 min, and 1 mM EGTA added to stop collagenolysis and prevent mitochondrial Ca2+ uptake (43). Samples were centrifuged sequentially at 480 g and 8,000 g and pelleted mitochondria kept on ice without dilution. To evaluate the contamination of other organelles in isolated mitochondria, the enzymatic activities of glucose-6-phosphatase (G-6-Pase, endoplasmic reticulum marker) (14) and acid phosphatase (APase, peroxisome marker) (21) were measured according to published procedures. Protein content was measured with the Bradford method.
In some experiments, mitochondria were subjected to Percoll density-gradient centrifugation (34). In brief, the mitochondrial pellet (above) was suspended in 15% Percoll, layered on top of 23% and 40% Percoll, and centrifuged at 30,700 g. The mitochondrial fraction at the lower interface was suspended in HMSB buffer (1:20, vol/vol), and centrifuged at 14,000 g. The pellet was washed again, centrifuged at 8,100 g, and used for various assays. All centrifugations were performed for 10 min at 4°C.
Oxygen consumption and respiration
Mitochondrial respiration was assessed by using a Mitocell S200A Micro Respirometry System (Strathkelvin Instruments, Motherwell, U.K.). In brief, a microcathode oxygen electrode was calibrated and, after baseline measurements, mitochondria (200 μg), suspended in 0.5 ml MSP medium (225 mM mannitol, 75 mM sucrose, 20 mM K2HPO4/KH2PO4 buffer, pH 7.4), were added to the mitocell. The substrate-stimulated oxygen consumption (state 4) was measured in the presence of 10 mM pyruvate/2.5 mM malate (pyr/mal), 5 mM succinate/6.25 μM rotenone (suc/rot), or 3 mM ascorbate/0.25 mM N,N,N,N-tetramethyl-p-phenylenediamine (asc/TMPD). The state 3 oxygen consumption was measured after addition of 230 μM ADP. Respirometry software (Strathkelvin) was used to calculate the respiratory control ratio (RCR). The ADP/O ratio (amount of ADP phosphorylated/O atom consumed) determines the mitochondrial capacity to produce ATP and was calculated by measuring the decrease in O2 concentration during the rapid burst of state 3 respiration, by using “total” oxygen as defined by Lemasters (18).
To measure myocardial oxygen consumption, we harvested the heart from anesthetized mice and perfused with Kreb's solution to remove blood. Left ventricle tissue was dissected longitudinally, incubated in Kreb's solution at 37°C, 5% CO2 for 2 h, and oxygen consumption measured as described earlier. To assess in vivo mitochondrial respiration, tissue slices were incubated in air-saturated Kreb's solution containing 5 mM succinate/6.25 μM rotenone (20).
Mitochondrial ATP production
Freshly isolated mitochondria (15–20 μg protein/μl) were diluted 15-fold (just before use) in reaction buffer (15 mM K2HPO4/KH2PO4 buffer, pH 7.2, 250 mM sucrose, 2 mM MgAc2, 0.5 mM EDTA and 0.5 g/L human serum albumin). In black-bottomed 96-well plates, 140 μl reaction buffer (2.5 μg/ml luciferase, 20-μg/ml d-luciferin and 20 μl substrate) and 20 μl mitochondria (25–50 μg protein) were added. After incubation for 2–3 min to deplete endogenous ATP, 20 μl ADP solution (6 mM ADP and 20 μM P1, P5-di(adenosine)-pentaphosphate) was added, and the change in luminiscence recorded by using a Promega Veritas Microplate Luminometer (standard curve, 10 pM to 10 μM ATP) (48). The use of highly pure ADP consisting of less than 0.04 μM ATP (Apollo Scientific) was essential to keep the background below 1% of the sample values.
Enzymatic and biochemical assays
Freshly isolated mitochondria (15 μg protein) were used in all assays. Complex I (NADH: ubiquinone reductase) activity was monitored by oxidation of NADH (200 μM) in the presence of 80 μM 2,3-dimethoxy-5-methyl-6-decyl-1, 4-benzoquinone (DB) as electron acceptor (±6.5 μM rotenone, ɛ340 nm: 6.1 mM/cm). Complex III (ubiquinol: cytochrome c reductase) activity was quantitated by the oxidation of 60 μM of reduced DB, by using 50 μM cytochrome c (cyt c) as an electron donor (±1 μM antimycin, ɛ550nm: 19.1 mM/cm). To assess the NADH level, isolated mitochondria were incubated at 55°C for 10 min in 0.2 M NaOH (1/10, wt/vol), neutralized by using 0.1 M HCl and centrifuged at 12,000 g for 10 min. The concentration of NADH in supernatants was determined by the rate of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reduction by alcohol dehydrogenase (yeast type II, Sigma) in the presence of phenazine ethosulfate at 570 nm (19).
Titration curves
Mitochondria were incubated for 5 min with varying concentrations of complex I (0–100 nM rotenone) and complex III (0–100 nM antimycin) inhibitors. Mitochondria were then energized with pyr/mal or succinate substrates, and the effect of inhibitors on the rate of ADP-stimulated state 3 respiration (by oxigraphy), ATP production (by luciferase-bioluminescence assay) and complex activities (by spectrophotometry) were monitored as described earlier. Threshold rates for the state 3 respiration and ATP production rate were derived from titration curves. Each point of the threshold curve represents the respiratory rate or ATP production rate as a function of the respiratory complex activity.
Cytochrome b expression
Cytochrome b DNA level was determined by Southern blotting. In brief, heart tissues (50 mg) were subjected to proteinase K lysis, treated with RNase A to remove contaminating RNA and total DNA isolated by phenol/chloroform extraction/ethanol precipitation. Total DNA (3 μg), after digestion with EcoRI, was resolved on agarose gel, transferred to membranes, and hybridized with [α32P]-labeled probes for cyt b or 18S ribosomal RNA (41). Images were acquired by using a Storm 860 phosphorimager (Amersham, Piscataway, NJ). To validate the Southern results, total DNA (50 ng) was used as a template, and real-time PCR was performed by using 12.5 μl of Power SYBR-Green PCR Master Mix (ABI) and 0.5 μl of 20 μM specific primer pairs (cyt b: forward, 5′-gttcgcagtcatagccacag-3′; reverse, 5′-ggcggaatattaggcttcgt-3′; GAPDH: forward, 5′- tggcaaagtggagattgttg-3′; reverse, 5′-ttcagctctgggatgacctt-3′) on a ABI7000 Prism system (Applied Biosystems, Foster City, CA). The threshold cycle (Ct) values were normalized to GAPDH, and the relative cyt b DNA level was calculated by using the formula 2−ΔCt where ΔCt represents the Ct (target)−Ct (control) (12).
To assess cyt b mRNA levels with Northern blotting, total RNA from heart tissues (50 mg) was isolated with the guanidinium thiocyanate-phenol-chloroform method (8) and treated with DNase (Ambion, Austin, TX) to remove contaminating DNA. Samples (2.5 μg total RNA) were denatured in 50% formamide/2 M formaldehyde, resolved on 1% agarose gel containing 2 M formaldehyde in 4-morpholinepropanesulfonic acid (MOPS) buffer. Membranes were hybridized with [α32P]-labeled cyt b or 18S ribosomal cDNA probes and images captured. To validate Northern results by real-time RT-PCR, first-strand cDNA was synthesized from DNase-treated total RNA (2 μg) by using 2.5 units of MuMLV reverse transcriptase (New England Biolabs, Ipswich, MA) and 1 μM poly(dT)18 oligonucleotide. The cDNA (2 μl) was used as a template with cyt b- or GAPDH-specific primers, and a PCR reaction performed on an ABI7000 Prism system (as described earlier). In some instances, RT-PCR was performed by using a thermocycler (Perkin-Elmer, Waltham, MA) and individual amplicons resolved on 1.5% agarose gel.
The cyt b protein level was assessed with Western blotting (13). In brief, isolated mitochondria (15 μg) or the complex III purified from the blue native gel (50 μg loading) (29) were resolved on a 10–15% gradient SDS-polyacrylamide gel, and proteins were transferred to PVDF membrane (BioRad). Membranes were incubated with a rabbit polyclonal antibody against cyt b (1:200 dilution, Santa Cruz), followed by HRP-conjugated anti-rabbit IgG (1:2,000 dilution). Signal was developed by using ECL Western Blotting Detection Reagents (Amersham) and quantitated with densitometry.
Data analysis
Data (mean ± SD) were derived from at least triplicate observations per sample (n ≥ 9 animals/group). Results were analyzed for significant differences by using ANOVA and Student's t test. The level of significance was accepted at p < 0.05.
Results
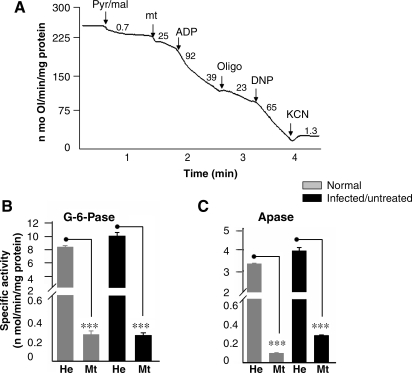
Our first objective was to isolate intact mitochondria with high purity. We used differential centrifugation and Percoll gradient methods to isolate cardiac mitochondria and evaluated the state 4 and state 3 oxygen consumption as an indicator of mitochondrial integrity. Shown in Fig. 1A is a typical measurement of mitochondrial oxygen consumption by oxigraphy. The rate of pyr/mal (complex I substrates) and succinate (complex II substrate)-stimulated state 4 respiration was significantly higher in Percoll-purified normal cardiac mitochondria as compared with that noted in mitochondria isolated by differential centrifugation (Table 1). No significant difference was observed in ADP-stimulated respiration in substrate-energized cardiac mitochondria, isolated by either of the two methods (Table 1). These results suggest that mitochondria isolated by differential centrifugation were coupled and maintained a high RCR (state 3/state 4 > 4). The measurement of possible contaminants in mitochondria isolated with differential centrifugation indicated that 6% or less of the glucose-6-phosphatase (ER marker) (Fig. 1B) and acid phosphatase (peroxisome marker) activities (Fig. 1C) were present in mitochondrial preparations when compared with that detected in the heart homogenates. We detected no statistically significant difference in citrate synthase activity in different cardiac mitochondrial preparations from normal, infected, and infected/PBN-treated mice (data not shown). Together, these data showed that the differential centrifugation method was efficient for isolation of >94% pure highly coupled mitochondria in different extractions from all experimental groups. All further studies, therefore, were conducted with mitochondria isolated by differential centrifugation, and additional purification by Percoll method was not used.
FIG. 1.
Isolation of mitochondria of high purity. C3H/HeN mice were infected with T. cruzi. Animals were killed during the acute infection phase and cardiac mitochondria were isolated as described in Materials and Methods. (A) A typical polarographic measurement with cardiac mitochondria by using a Mitocell S200A Respirometry system. Arrows, The time point of addition of substrate (pyr/mal), mitochondria (mt), ADP (stimulates state 3 respiration), oligomycin (oligo, inhibits ATP synthesis and ADP-stimulated state 3 respiration), dinitrophenol (DNP, uncoupler), and KCN (complex IV inhibitor, blocks respiration). The rate of oxygen consumption was calculated as nonomoles of O/min/mg protein. The activities of glucose-6-phosphase (G-6-Pase) (B) and acid phosphatase (APase) (C) were monitored in isolated mitochondria and heart homogenates (n = 9 per group; ***p < 0.001).
Table 1.
Mitochondria Isolated by Differential Centrifugation Are Coupled
| |
|
Respiration (nmol atom O/min/mg protein) |
|
|
|---|---|---|---|---|
| Substrate | Method | State 4 (−ADP) | State 3 (+ADP) | RCR value |
| Pyruvate + malate | DF | 20.11 ± 1.76 | 93.72 ± 8.01 | 4.68 ± 0.49 |
| Percoll | 40.58 ± 9.45 | 92.58 ± 12.84 | 2.35 ± 0.47 | |
| Succinate + rotenone | DF | 16.05 ± 3.89 | 86.08 ± 8.39 | 5.58 ± 1.22 |
| Percoll | 41.26 ± 4.56 | 85.58 ± 5.64 | 2.09 ± 0.24 | |
Cardiac mitochondria from 8-week-old normal mice were isolated by differential centrifugation (DF). Half of mitochondrial fraction was further purified by the discontinuous Percoll gradient method. Mitochondria were incubated with the indicated substrates, and respiration rates were determined with oxigraphy. Respiratory control ratio (RCR) was calculated as the ratios of state 3 rate to state 4 rate. The data (mean ± SD) are representative of three independent experiments (n = 3 per experiment/group).
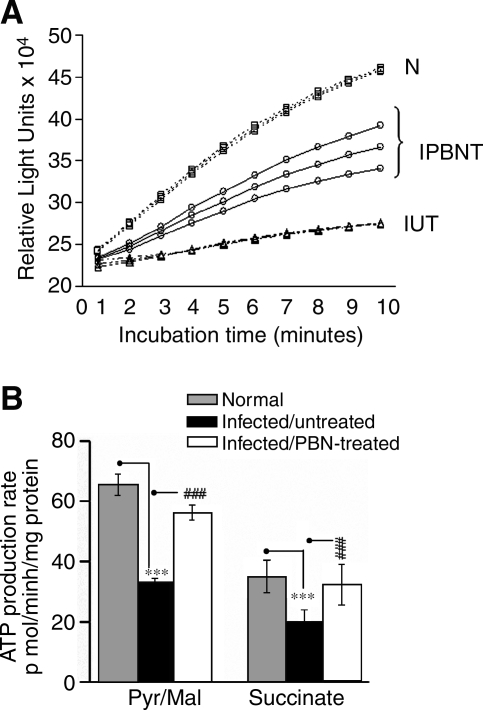
The myocardial ATP content is compromised in infected mice (42). To determine whether such is the case because of decreased mitochondrial oxidative phosphorylation efficiency, we measured ATP production by using a luciferin/luciferase-based bioluminescence method. The change in luminescence per unit of time (Fig. 2A) was extrapolated to calculate the ATP production (Fig. 2B). The pyr/mal- and succinate-supported ATP production was decreased by 50% and 44%, respectively, in infected myocardial mitochondria (Fig. 2B, Table 2). The ATP production rate was substantially inhibited by myxothiazol (complex III inhibitor, 87% decline or more) and oligomycin (complex V inhibitor, 75% decline), and completely abolished by complex IV inhibitor (KCN) or mitochondrial uncoupler (DNP). Rotenone (complex I inhibitor) resulted in an 88% or more inhibition of ATP synthesis in pyr/mal-respiring mitochondria, whereas malonate (complex II inhibitor) resulted in a >93% inhibition of the succinate-supported ATP production (Table 2). The inhibition studies confirmed that the observed changes in ATP production in infected myocardium (Fig. 2) are mitochondria specific and dependent on the coupling of respiratory chain and oxidative phosphorylation. The pyr/mal and succinate-supported ATP synthesis in cardiac mitochondria of infected/PBN-treated mice was decreased by only 8–14% as compared with the 44–50% loss detected in infected/untreated mice (Fig. 2). These data suggest that PBN treatment of infected mice was effective in improving the mitochondrial function.
FIG. 2.
Isolated cardiac mitochondria were energized with pyruvate/malate (pyr/mal) or succinate substrates, and the rate of ATP production was determined with a luciferin/luciferase-based assay. (A) Luminescence curves demonstrating the ATP-production rate in pyruvate/malate–respiring cardiac mitochondria from normal (N), infected/untreated (IUT), and infected/PBN-treated (IPBNT) mice. (B) The data from luminescence curves were extrapolated to calculate the ATP production (n = 9 per group). The level of significance is shown by ***p < 0.001 (normal vs. infected mice) and ###p < 0.001 (infected/PBN-treated vs. infected mice).
Table 2.
Mitochondrial ATP-production Rate in the Myocardium of T. cruzi-infected Mice ± PBN
| |
|
Mitochondrial ATP synthesis rate (pmol/mg protein/min) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Substrate | Animals | None | 6.5 μM Rot | 2.5 mM Mal | 10 μM Myx | 10 μM Ant | 1 mM KCN | 12 μM Oligo | 0.2 mM DNP |
| Pyr/Mal | Normal | 65.36 ± 3.65 | 6.17 ± 0.67 | ND | 6.50 ± 1.67 | 2.00 ± 0.33 | 0.72 ± 0.17 | 10.00 ± 1.67 | 2.00 ± 0.50 |
| IUT | 32.83 ± 1.82a | 3.83 ± 0.67 | ND | 4.33 ± 1.33 | 4.00 ± 0.83 | 0.67 ± 0.17 | 7.00 ± 0.67 | 1.00 ± 0.50 | |
| IPBNT | 56.19 ± 2.4b | 3.67 ± 2.83 | ND | 4.50 ± 0.17 | 2.67 ± 0.17 | 1.00 ± 0.12 | 8.50 ± 3.67 | 1.83 ± 1.50 | |
| Succinate | Normal | 34.98 ± 5.37 | ND | 2.50 ± 0.50 | 1.17 ± 0.50 | 5.00 ± 0.33 | 1.50 ± 0.67 | 10.33 ± 2.00 | −0.17 ± 0.17 |
| IUT | 19.74 ± 4.21a | ND | 1.33 ± 0.50 | 0.50 ± 0.50 | 3.67 ± 0.50 | 0.50 ± 0.33 | 4.50 ± 0.33 | −0.83 ± 0.67 | |
| IPBNT | 32.27 ± 6.79b | ND | 1.50 ± 0.50 | 1.17 ± 0.50 | 8.50 ± 0.83 | 0.83 ± 0.33 | 8.00 ± 1.00 | 0.08 ± 0.33 | |
Cardiac mitochondria from normal, infected/untreated (IUT) and infected/PBN-treated (IPBNT) mice were isolated as described in Materials and Methods. Mitochondria were incubated at room temperature with the indicated substrates (with or without inhibitors), and the ATP-production rate was determined by luciferin/luciferase-based luminescence assay. The data (mean ± SD) are representative of three independent experiments (n = 3 per experiment/group). Pyr/Mal, pyruvate/malate. Inhibitors: Rot, rotenone (CI); Mal, malonate (CII); Myx, myxothiazol (CIII); Ant, antimycin A (CIII); KCN, (CIV); Oligo, oligomycin (CV); DNP, dinitrophenol (uncoupler). The level of significance is shown by ap < 0.001 (normal vs. infected/untreated (IUT) mice) and bp < 0.001 (infected/untreated vs. infected/PBN-treated (IPBNT) mice). ND, not determined.
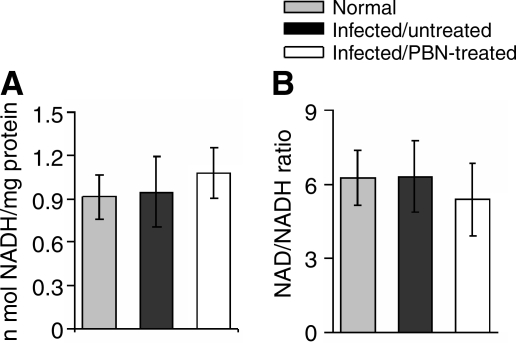
To identify the factors that may contribute to decreased ATP production in infected heart, we first determined the level of intermediate metabolites that provide electron energy for ATP synthesis in isolated cardiac mitochondria. We found no statistically significant difference in the mitochondrial NADH level (Fig. 3A), NAD+/NADH ratio (Fig. 3B) and succinate metabolism (data not shown) in normal, infected. and infected/PBN-treated mice. suggesting that the availability of an electron donor for ATP synthesis is not compromised in infected myocardium.
FIG. 3.
Bar graphs showing the NADH level (A) and NAD+/NADH ratio (B) in isolated cardiac mitochondria from normal, infected/untreated and infected/PBN-treated mice (n = 9 per group).
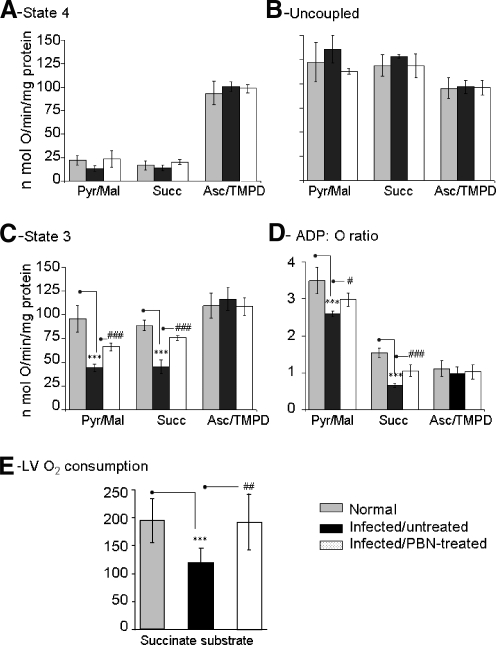
Alterations in respiratory complex activities, noted in the chagasic hearts (41, 45), may affect electron-transport efficiency and coupled oxidative phosphorylation. We measured oxygen consumption in isolated mitochondria and heart tissue to evaluate the respiratory efficiency in infected mice. The state 4 respiration supported by pyr/mal, succinate, and ascorbate/TMPD substrates that provide electrons to complex I, II, and IV, respectively (Fig. 4A), and maximal oxygen consumption in presence of dinitrophenol (uncoupler, Fig. 4B) was not statistically different in isolated cardiac mitochondria of the three groups. These results indicate that mitochondrial oxygen uptake and substrate-dependent respiration were not compromised in infected myocardium. The RCR values of > 4.0 (Table 3) suggested that coupled mitochondria were isolated from infected myocardium. Yet, the ADP-stimulated state 3 respiration (indicates proton gradient for ATP synthesis) and ADP/O ratio (rate of ADP phosphorylation per O atom consumed) were substantially decreased in substrate-respiring cardiac mitochondria of infected mice (Fig. 4C and D). The deficiency of state 3 respiration and ADP phosphorylation was validated in heart tissue. Left ventricle (LV) sections from normal and infected mice were incubated with pyr/mal or succinate to energize mitochondria, and the rate of respiration was determined (Fig. 4E, data not shown). The substrate-dependent, endogenous ADP-stimulated, respiration in LV of infected mice was 38% lower than that of normal controls, and remained depressed with exogenous ADP addition. These data suggest that the electrochemical gradient required for ATP synthesis was compromised in infected myocardium. The observation that pyr/mal- and succinate-stimulated, but not ascorbate/TMPD-stimulated, state 3 respiration was decreased in infected cardiac mitochondria indicated that electron transport at complex IV and downstream (to complex V) was intact, and instead, the deficiencies of complex I or complex III or both contributed to decreased state 3 rate and ATP synthesis in infected myocardium.
FIG. 4.
Respiratory properties of cardiac mitochondria from T. cruzi–infected mice (±PBN). (A) State 4 respiration in isolated cardiac mitochondria of normal, infected, and infected/PBN-treated mice when pyruvate/malate (pyr/mal), succinate (succ), or ascorbate/TMPD (asc/TMPD) were used as substrates. (B) DNP-induced uncoupled (Unc) respiration rate. (C) The rate of state 3 respiration was determined after addition of ADP. (D) The ADP/O ratio (amount of ADP phosphorylated to O atom consumed) was calculated by measuring the decrease in O2 concentration during state 3 respiration when 230 μM ADP was added. (E) The hearts were harvested, perfused, and LV sections used for ex vivo determination of substrate-dependent, state 3 respiration stimulated by endogenous ADP (*,#p < 0.05; **,##p < 0.01; ***p < 0.001; n = 9 mice/group).
Table 3.
Respiratory Parameters of Heart Mitochondria from Mice Infected with T. cruzi ± PBN
| |
|
Rate of respiration (nmol atom O/min/mg protein) |
|
|
||
|---|---|---|---|---|---|---|
| Substrate | Animals | State 4 (−ADP) | State 3 (+ADP) | DNP-stimulated | RCR value (state 3/state 4) | ADP/O |
| Pyruvate+ malate | Normal | 21.89 ± 5.11 | 95.95 ± 14.01 | 121.92 ± 20.3 | 4.54 ± 1.32 | 3.36 ± 0.40 |
| T. cruzi infected | 13.30 ± 2.83 | 44.42 ± 3.94a | 135.33 ± 14.31 | 4.11 ± 0.4 | 2.69 ± 0.17b | |
| Infected/PBN-treated | 23.58 ± 8.98 | 66.32 ± 4.15c | 112.57 ± 2.56 | 3.62 ± 0.53 | 2.92 ± 0.37 | |
| Succinate+ rotenone | Normal | 16.43 ± 4.96 | 88.85 ± 5.14 | 118.63 ± 10.96 | 5.55 ± 1.49 | 1.54 ± 0.125 |
| T. cruzi-infected | 14.13 ± 2.26 | 45.34 ± 7.3a | 127.81 ± 1.66 | 4.44 ± 1.51 | 0.66 ± 0.05a | |
| Infected/PBN-treated | 19.95 ± 3.99 | 76.04 ± 2.66c | 118.05 ± 12.3 | 5.53 ± 0.87 | 1.05 ± 0.20c | |
| Ascorbate+ TMPD | Normal | 93.36 ± 12.64 | 109.53 ± 13.15 | 94.98 ± 10.59 | 1.19 ± 0.05 | 1.12 ± 0.21 |
| T. cruzi-infected | 100.58 ± 5.1 | 116.55 ± 12.08 | 96.74 ± 6.88 | 1.16 ± 0.15 | 0.98 ± 0.18 | |
| Infected/PBN-treated | 98.24 ± 4.15 | 105.92 ± 9.42 | 95.64 ± 7.58 | 1.12 ± 0.09 | 1.03 ± 0.2 | |
Cardiac mitochondria were isolated as described in Material and Methods. Mitochondria were incubated with the indicated substrates, and the respiration rate was determined by oxygraphy. ADP/O ratio represents the nmol ADP phosphorylated per ng atom of oxygen consumed. DNP, dinitrophenol; NA, not applicable; RCR, respiratory control ration. The data (mean ± SD) are representative of three independent experiments (n three mice per experiment/group). bp < 0.05; ap < 0.001 (normal vs. infected mice), cp < 0.001 (infected/PBN treated mice vs. infected mice).
In PBN-treated/infected mice, the substrate stimulated state 3 respiration and the ADP/O ratio of isolated cardiac mitochondria were significantly improved (Fig. 4C and D). Likewise, the substrate-dependent respiration was normalized in the LV of PBN-treated/infected mice as compared with infected/untreated mice, both when endogenous ADP was used (Fig. 4E) and with exogenous ADP addition (data not shown). These results imply the role of ROS in respiratory inefficiency and compromised ATP production in infected myocardium.
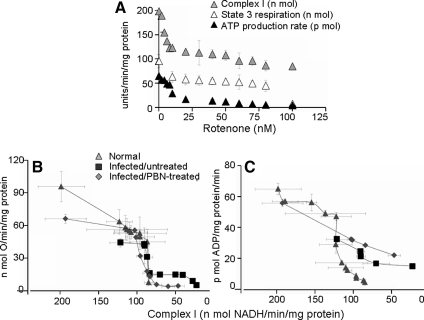
To delineate whether complex I or III or both are the site of electron-transport chain inefficiency, we carried out titration studies in presence of specific inhibitors. The titration of complex I inhibition with rotenone (0–100 nM) and its effect on ATP synthesis are shown in Fig. 5. In normal heart mitochondria, we observed rotenone concentration–dependent proportional decline in complex I activity and state 3 and ATP synthesis rates (Fig. 5A). The state 3 and ATP synthesis rates dropped by 58% and 22%, respectively, when complex I inhibition of 43% (from 198.4 to 113.1 nmol NADH oxidized/min/mg protein) was achieved with 30 nM rotenone (Fig. 5A). In infected myocardium, we found a 39%, 54% and 50% decline in mitochondrial complex I activity, state 3 respiration (44.4 ± 3.9 vs. 95.9 ± 14.0 nmol O/min/mg protein; p < 0.001) and ATP synthesis (32.8 ± 1.8 vs. 65.4 ± 3.6 pmol ATP/min/mg protein; p < 0.001), respectively, when compared with normal controls (Fig. 5B and C). Rotenone-induced further inhibition of complex I in cardiac mitochondria of infected mice corresponded to a similar pattern of respiration change, as was noted in normal controls (Fig. 5B). ATP synthesis in infected myocardial mitochondria was less sensitive to further inhibition of complex I activity (Fig. 5C). PBN treatment normalized the complex I activity in infected mice (Fig. 5B and C). However, the state 3 respiration (Fig. 5B), ATP production (Fig. 5C), and ADP/O ratio (Table 3) were only partially improved. The diminished state 3 and ATP synthesis rates and ADP/O ratio despite normalization of complex I activity by PBN treatment suggests that factors additional to complex I inhibition contributed to compromised respiration and ATP synthesis in infected mice.
FIG. 5.
Titration of complex I inhibition and its effect on respiration and ATP-production rate in cardiac mitochondria of T. cruzi–infected mice. Freshly isolated cardiac mitochondria were incubated with rotenone (0–100 nM) and energized with pyr/mal substrates. (A) Effects of rotenone on complex I activity, state 3 respiration, and ATP-production rate in normal heart mitochondria. Threshold curves show the effect of rotenone-induced changes in complex I–specific activity on the state 3 (B) and ATP-production (C) rates in isolated cardiac mitochondria from normal, infected/untreated, and infected/PBN-treated mice. Data (mean ± SD) are representative of three independent experiments (n = 9 mice per group).
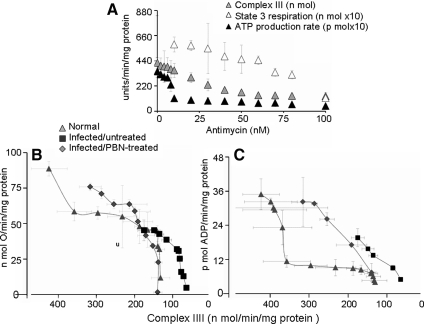
Next, we titrated the complex III inhibition and its effect on respiration and ATP synthesis. Treatment with antimycin (0–100 nM) resulted in a proportional decline in complex III activity, state 3 rate, and ATP production in succinate-respiring normal cardiac mitochondria (Fig. 6A). Threshold curves showed that complex III inhibition of 67% (from 424.1 ± 47.1 to 140. 9 ± 12.6 nmol cyt c oxidized/min/mg protein) was required before a 49% decline in state 3 rate (from 88.9 ± 5.1 to 45.7 ± 12.4 nmol O/min/mg protein) occurred (Fig. 6B). The ATP production rate was more sensitive to complex III inhibition. A 16% loss in complex III activity (from 424.1 ± 47.2 to 357.9 ± 45.0 nmol cyt c oxidized/min/mg protein) resulted in a 67% decline in ATP synthesis rate (from 35.0 ± 5.4 to 11.4 ± 2.0 pmol ATP/min/mg protein) in normal cardiac mitochondria (Fig. 6C). In infected myocardium, complex III activity was decreased by 59%. This loss in complex III activity in infected myocardial mitochondria corresponded to a 49% decline in respiration (45.3 ± 7.3 vs. 88.8 ± 5.1 nmol O/min/mg protein) and 44% decline in ATP production (19.7 ± 4.2 vs. 35.0 ± 5.4 pmol ATP/min/mg protein; p < 0.001) than normal controls (Fig. 6B and C; Tables 2 and 3). Antimycin-induced further inhibition of complex III resulted in a sharp decline in the state 3 and ATP production rates in infected myocardium (Fig. 6B and C). PBN treatment of infected mice recovered 58% loss of complex III activity (Fig. 6B and C) (42) that was associated with normalized state 3 respiration (Fig. 6B) and a substantial increase in ATP synthesis (Fig. 6C) and ADP/O ratio (Table 3). The sensitivity of the ATP synthesis rate to complex III inhibition in infected mice and the improvement in respiration and ATP production with preservation of complex III activity in PBN-treated/infected mice suggest that compromised respiratory efficiency and ATP synthesis are an outcome of complex III inhibition in chagasic myocardium.
FIG. 6.
Titration of complex III inhibition and its effect on respiration and ATP-production rate in cardiac mitochondria of T. cruzi–infected mice. Freshly isolated cardiac mitochondria were incubated with antimycin (0–100 nM) and energized with succinate. (A) Effects of antimycin on CIII activity, state 3, and ATP-production rate in normal heart mitochondria. Threshold curves show the effect of antimycin-induced changes in complex III activity on the state 3 (B) and ATP-production (C) rates in cardiac mitochondria from normal, infected/untreated, and infected/PBN-treated mice. Data (mean ± SD) are representative of three independent experiments (n = 9 mice per group).
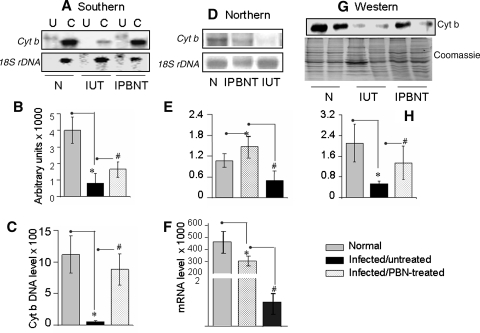
Considering the beneficial effects of PBN (Fig. 6) and our recent observations documenting that complex III is the site for increased electron leakage to molecular oxygen (46), we considered that ROS contributed to mitochondrial loss of complex III activity in infected myocardium. The mtDNA, highly susceptible to oxidative damage encodes the cyt b subunit of the complex III. Southern blotting (Fig. 7A and B) and real-time PCR (Fig. 7C) showed that cyt b DNA was significantly decreased in infected murine hearts as compared with normal controls. The cyt b mRNA level was determined with Northern blotting (Fig. 7D and E) and traditional (not shown) and real-time (Fig. 7F) RT-PCR. All three techniques showed about an 80% decline in cyt b mRNA levels in infected murine hearts. Subsequently, Western blot analysis showed the cyt b protein level decreased by 74% in cardiac mitochondria of infected mice as compared with normal controls (Fig. 7G and H).
FIG. 7.
Expression of cytochrome b is altered in the myocardium of T. cruzi–infected mice. Total DNA and total RNA were isolated from myocardial tissue of normal (N), infected/untreated (IUT), and infected/PBN-treated (IPBNT) mice. (A, B) Southern blotting. Total genomic DNA (3 μg; U, uncut; C, EcoRI digested) was resolved on agarose gel and hybridized with a [32P]-labeled sequence for cyt b or 18S rRNA (A). Densitometric analysis of cyt b DNA signal, normalized with 18S rDNA signal (B). (C) A real-time PCR was performed to measure the cyt b DNA level, normalized to GAPDH. (D, E) Northern blot analysis. Total RNA (2.5 μg) was resolved on gel and hybridized with [32P]-labeled cDNA probes for cyt b or 18S rRNA (D). Densitometric analysis of cyt b mRNA signal, normalized with 18S rRNA signal (E). (F) A real-time RT-PCR was performed to measure the cyt b mRNA level, normalized to GAPDH cDNA. Data in C and F represent mean 2−ΔCt values obtained from two independent experiments (n = 6). The standard deviation for all the data points was <10% (p < 0.05). (G, H) Mitochondrial lysates (15 μg protein) were resolved by SDS-PAGE, and Western blot analysis was performed by using an anti-cyt b antibody (G). Membranes were stained with Coomassie R250 to confirm equal loading of the samples. Densitometric analysis of cyt b protein is shown in (H). Data in B, E, and H represent mean ± SD (n = 9 mice per group; *,#p < 0.001).
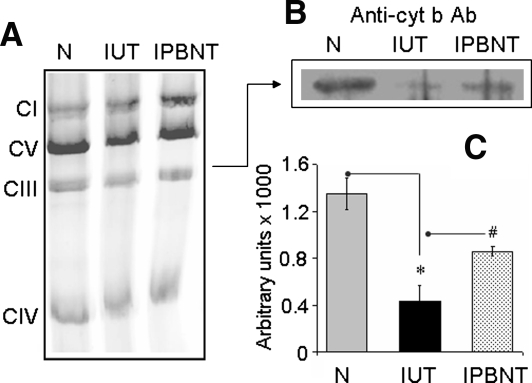
To investigate whether decreased the cyt b level affected complex III in infected heart, we resolved the respiratory complexes on blue native gel, and performed catalytic staining and Western blot analysis to evaluate complex III assembly, activity, and cyt b incorporation. Our data showed that the assembly of complex III and other respiratory complexes occurred at a similar level in cardiac mitochondria of infected and normal mice (Fig. 8A). However, catalytic activity (40% decline, also validated by spectrophotometric observations) and cyt b level (61% decline; Fig. 8B and C) were decreased in the CIII complex of infected myocardium. PBN treatment of infected mice improved the cyt b DNA, mRNA, and protein levels (Fig. 7). Subsequently, the complex III cyt b level (Fig. 8) and catalytic activity were restored in PBN-treated/infected mice. Together, these results suggest that decreased expression of mtDNA-encoded cyt b contributed to complex III inhibition in cardiac mitochondria of T. cruzi–infected mice, and this defect was alleviated by PBN treatment.
FIG. 8.
Assembly of cytochrome b–depleted complex III in infected myocardium. (A) Isolated mitochondria (50 μg protein) from normal (N), infected/untreated (IUT), and infected/PBN-treated (IPBNT) mice were subjected to blue-native gel electrophoresis, and respiratory complexes were resolved. (B) Complex III from the blue native gel was subjected to second-dimension SDS-PAGE to resolve the subunit components, and Western blotting was performed by using an anti-cyt b antibody. (C) Densitometric analysis of cyt b protein, normalized to complex III level (*,#p < 0.001).
Discussion
We used a murine model of T. cruzi infection in this study that exhibits characteristics of human chagasic disease. Experimentally infected mice elicit reproducible and comparable acute disease associated with parasitemia (tissues parasites). The presence of myocarditis and focal necrosis elicits the acute disease in mice (13, 45) similar to acute infection in humans (2). Tissue damage and fibrosis associated with diffused inflammation become prominent during the chronic phase (13). Symptomatic defects include cardiomegaly, diffused myocarditis, self-perpetuating myofibril destruction, and a decline in LV ejection fraction (9, 26, 36), a picture reminiscent of the chronic form of Chagas disease observed in humans (24, 25).
Moderate-to-severe electrocardiographic and echocardiographic alterations evident in rodent hearts (3, 7) are associated with electrical conduction disturbances, diastolic dysfunction, lower O2 consumption, and anaerobic threshold (26). We and others have shown that myocardial energetics and ATP content were compromised in infected rodents (38, 42). Our observations in this study demonstrating that the oxygen consumption, state 3 rate, ATP production, and ADP-to-O ratio were decreased in LV and isolated cardiac mitochondria of infected mice provide the first molecular evidence for mitochondrial decline in oxidative phosphorylation in chagasic hearts.
What might contribute to the observed decline in mitochondrial ATP production in infected mice? Parasite-induced cellular injuries can result in isolation of damaged uncoupled mitochondria. In coupled mitochondria, complexes of the electron transport chain (ETC) create a transmembrane electrochemical gradient (Δψ). Complex V (ATP synthase) uses the potential energy stored in Δψ to phosphorylate ADP. The Δψ can collapse to result in the free diffusion of molecules between the matrix and the cytosol (17). Reports of electron microscopic analysis of biopsies from experimental animals have indicated that a degree of mitochondrial structural damage occurs in infected myocardium (13), thus suggesting that mitochondria in infected heart may just be uncoupled. However, a high RCR value (>4.0; Table 3) indicated that the electron-transport chain and oxidative phosphorylation were coupled in mitochondria of infected mice. Another plausible mechanism of decreased ATP production and ATP content is the unavailability of intermediary metabolites (e.g., NADH for complex I or FADH2 for complex II) for maintaining the electrochemical gradient across the respiratory chain. Pyruvate/malate substrates provide NADH to the complex I that pumps electrons to CoQ. From CoQ, electrons are transferred to O2 at the complex IV with coupled ATP synthesis in the presence of ADP (15). Succinate dehydrogenase (SDH) provides FADH2 to complex II, from which electrons flow through complex III and complex IV. The mitochondrial NADH level and NAD+/NADH ratio were not decreased in infected myocardium (Fig. 3), and the impaired state 3 respiration of infected cardiac mitochondria was not relieved when exogenous NADH was added. Likewise, we observed no change in SDH activity in infected myocardium (41). These observations suggest that an impaired ATP production in infected myocardium was not due to a reduced availability of intermediary metabolites (NADH, FADH2) that provide electron energy for mitochondrial ATP synthesis.
The perturbations in electron-transport chain efficiency and complex V activity can also result in decreased bioenergetics in the heart. Reduced ATP synthesis, as measured by a declining state 3 rate, has been demonstrated in isolated cardiac mitochondria from failing animal (31) and human hearts (32). Others have attributed the disease pathology in cardiac failure during ischemia/reperfusion (37) and in neurologic and age-related diseases [e.g., Alzheimer's (1), Parkinson's, and Huntington's (30)] to a loss in respiratory-complex activities and postulated that a lack of ATP production ensues. However, skepticism exists concerning a direct correlation between decline in complex activities and ATP synthesis in a disease condition. This is because mitochondrial respiratory complexes harbor more capacity than is required to maintain electron transport and ATP synthesis for normal cardiac function (27, 28). Thus, a decline in a complex activity, if not sufficient to exhaust the reserve capacity, would not be physiologically relevant and would have no adverse effects on electron transport and ATP-production capacity. Our finding that rotenone inhibition of complex I by 39% had no effect on ATP production in normal cardiac mitochondria (Fig. 5), suggests that a similar percentage loss of complex I activity in infected myocardium (41, 42) was physiologically irrelevant and did not contribute to decreased ATP synthesis in infected myocardium. Likewise, a small, but statistically significant decline in complex IV and complex V activities in chagasic heart (41, 42) was not physiologically relevant. This was evident by the observation that infected myocardial mitochondria respiring TMPD/ascorbate substrates (donate electrons to complex IV) exhibited no decline in state 3 respiration and ADP-to-O ratio (Fig. 4D; Table 3). Others have reported that the kinetics of ATP synthesis/ATP hydrolysis by complex V was not altered in rats acutely infected by T. cruzi (39). Thus, the capacity of complex IV to transfer electrons and the ability of complex V to receive electrochemical gradient energy and synthesize ATP were not affected in cardiac mitochondria of infected mice.
Instead, the ATP production rate was highly sensitive to the complex III inhibition (Fig. 6). The titration assay showed that antimycin-induced inhibition of complex III by 16% resulted in 67% loss in ATP production in normal cardiac mitochondria. In infected mice, a 59% inhibition of complex III activity was observed, and it corresponded to a 44% loss in mitochondrial ATP production (Fig. 6; Table 2). PBN treatment resulted in a substantial improvement in the mitochondrial activity of complex III, state 3 respiration, ATP production, and ADP/O ratios in infected myocardium. Others noted the biochemical threshold value of respiratory complexes decreases with aging (40). We surmise that mitochondrial perturbations of complex III affected ATP production in infected myocardium, and these disturbances will likely be exacerbated during the chronic-disease phase when patients are older and may develop age-related respiratory inefficiency.
Cytochrome b, together with cyt c1 and the iron-sulfur protein (ISP), forms the catalytic core of the CIII complex (5). The Q cycle receives electrons from complex I and complex II, and cytochrome b acts as a bridge for electron transfer from Q cycle to complex IV (10). The mtDNA encoded cyt b was decreased in infected myocardium (Figs. 7 and 8), whereas expression levels for other components of the complex III were not altered in infected heart (43). These observations allow us to propose that in the absence of cytochrome b, complex III would not efficiently receive the electrons coming from complex I (when mitochondria are respiring pyr/mal) and complex II (when mitochondria are respiring succinate) and pump them to the complex IV. The inefficient transfer of electrons from complex III to complex IV would affect the coupled ATP synthesis.
In summary, we demonstrated that the myocardial bioenergetics decline during T. cruzi infection occurs because of inefficient electron transport for ATP synthesis in mitochondria. A decline in cyt b expression caused a loss in complex III activity and constituted a mechanism for decreased mitochondrial state 3 respiration and ATP production in the myocardium of infected mice. Treatment with PBN improved the cyt b expression, complex III activity, mitochondrial respiration, and ATP production in the myocardium of T. cruzi–infected mice.
Abbreviations Used
- APase
acid phosphatase
- Asc/TMPD
ascorbate/N,N,N,N-tetramethyl-p-phenylenediamine
- complex I
NADH ubiquinone oxidoreductase
- complex II
succinate decylubiquinone 2,6-dichlorophenolindophenol reductase
- complex III
ubiquinol cytochrome c oxidoreductase
- complex IV
cytochrome c oxidase
- CS
citrate synthase
- cyt b
cytochrome b
- cyt c
cytochrome c
- DB
2,3- dimethoxy-5-methyl-6-decyl-1, 4-benzoquinone
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- G-6-Pase
glucose-6-phosphatase
- PBN
phenyl-α-tert-butyl nitrone
- pyr/mal
pyruvate/malate
- RCR
respiratory control ratio
- ROS
reactive oxygen species
- suc/rot
succinate/rotenone
- T. cruzi
Trypanosoma cruzi
Acknowledgments
This work was supported by a grant (AI054578) from the National Institute of Allergy and Infectious Diseases/National Institutes of Health to N.J.G.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aleardi AM. Benard G. Augereau O. Malgat M. Talbot JC. Mazat JP. Letellier T. Dachary–Prigent J. Solaini GC. Rossignol R. Gradual alteration of mitochondrial structure and function by beta-amyloids: importance of membrane viscosity changes, energy deprivation, reactive oxygen species production, and cytochrome c release. J Bioenerg Biomembr. 2005;37:207–225. doi: 10.1007/s10863-005-6631-3. [DOI] [PubMed] [Google Scholar]
- 2.Andrade ZA. Pathogenesis of Chagas disease. Res Immunol. 1991;142:126–129. doi: 10.1016/0923-2494(91)90021-a. [DOI] [PubMed] [Google Scholar]
- 3.Bilate AM. Salemi VM. Ramires FJ. de Brito T. Russo M. Fonseca SG. Fae KC. Martins DG. Silva AM. Mady C. Kalil J. Cunha-Neto TNF blockade aggravates experimental chronic Chagas disease cardiomyopathy. Microb Infect. 2007;9:1104–1113. doi: 10.1016/j.micinf.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Báez AL. Lo Presti MS. Rivarola HW. Pons P. Fretes R. Paglini-Oliva P. Trypanosoma cruzi: cardiac mitochondrial alterations produced by different strains in the acute phase of the infection. Exp Parasitol. 2008;120:397–402. doi: 10.1016/j.exppara.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Brasseur G. Saribas AS. Daldal F. A compilation of mutations located in the cytochrome b subunit of the bacterial and mitochondrial bc1 complex. Biochim Biophys Acta. 1996;1275:61–69. doi: 10.1016/0005-2728(96)00051-5. [DOI] [PubMed] [Google Scholar]
- 6.Brener Z. Gazzinelli RT. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int Arch Allergy Immunol. 1997;114:103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- 7.Bustamante JM. Rivarola HW. Fretes R. Paglini-Oliva PA. Weekly electrocardiographic pattern in mice infected with two different Trypanosoma cruzi strains. Int J Cardiol. 2005;102:211–217. doi: 10.1016/j.ijcard.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Chomczynski P. Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.de Souza AP. Tang B. Tanowitz HB. Araujo-Jorge TC. Jelicks EL. Magnetic resonance imaging in experimental Chagas disease: a brief review of the utility of the method for monitoring right ventricular chamber dilatation. Parasitol Res. 2005;97:87–90. doi: 10.1007/s00436-005-1409-4. [DOI] [PubMed] [Google Scholar]
- 10.di Rago JP. Netter P. Slonimski PP. Intragenic suppressors reveal long distance interactions between inactivating and reactivating amino acid replacements generating three-dimensional constraints in the structure of mitochondrial cytochrome b. J Biol Chem. 1990;265:15750–15757. [PubMed] [Google Scholar]
- 11.Floyd RA. Hensley K. Forster MJ. Kelleher-Anderson JA. Wood PL. Nitrones as neuroprotectants and antiaging drugs. Ann N Y Acad Sci. 2002;959:321–329. doi: 10.1111/j.1749-6632.2002.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 12.Garg N. Bhatia V. Gerstner A. deFord J. Papaconstantinou J. Gene expression analysis in mitochondria from chagasic mice: alterations in specific metabolic pathways. Biochemical J. 2004;381:743–752. doi: 10.1042/BJ20040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg N. Popov VL. Papaconstantinou J. Profiling gene transcription reveals a deficiency of mitochondrial oxidative phosphorylation in Trypanosoma cruzi-infected murine hearts: implications in chagasic myocarditis development. Biochim Biophys Acta. 2003;1638:106–120. doi: 10.1016/s0925-4439(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 14.Harper AE. Glucose-6-phosphatase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press Inc.; 1965. pp. 788–792. [Google Scholar]
- 15.Jafri MS. Dudycha SJ. O'Rourke B. Cardiac energy metabolism: models of cellular respiration. Annu Rev Biomed Eng. 1963;3:57–81. doi: 10.1146/annurev.bioeng.3.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi AT. Sugimachi M. Sunagawa K. Bergsland J. Koide S. Batista RJ. Improved left ventricular contraction and energetics in a patient with Chagas' disease undergoing partial left ventriculectomy. J Card Surg. 2001;16:30–33. doi: 10.1111/j.1540-8191.2001.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 17.Kokoszka JE. Coskun P. Esposito LA. Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci U S A. 2001;98:2278–2283. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemasters JJ. The ATP-to-oxygen stoichiometries of oxidative phosphorylation by rat liver mitochondria: an analysis of ADP-induced oxygen jumps by linear nonequilibrium thermodynamics. J Biol Chem. 1984;259:13123–13130. [PubMed] [Google Scholar]
- 19.Leonardo MR. Dailly Y. Clark DP. Role of NAD in regulating the adhE gene of Escherichia coli. J Bacteriol. 1996;178:6013–6018. doi: 10.1128/jb.178.20.6013-6018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loke KE. Laycock SK. Mital S. Wolin MS. Bernstein R. Oz M. Addonizio L. Kaley G. Hintze TL. Nitric oxide modulates mitochondrial respiration in failing human heart. Circulation. 1991;100:1291–1297. doi: 10.1161/01.cir.100.12.1291. [DOI] [PubMed] [Google Scholar]
- 21.Lui NS. Roels OA. Trout ME. Anderson OR. Subcellular distribution of enzymes in Ochromonas malhamensis. J Protozool. 1968;15:536–542. doi: 10.1111/j.1550-7408.1968.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 22.Palacios-Pru E. Carrasco H. Scorza C. Espinoza R. Ultrastructural characteristics of different stages of human chagasic myocarditis. Am J Trop Med Hyg. 1989;41:29–40. [PubMed] [Google Scholar]
- 23.Parada H. Carrasco HA. Anez N. Fuenmayor C. Inglessis I. Cardiac involvement is a constant finding in acute Chagas' disease: a clinical, parasitological and histopathological study. Int J Cardiol. 1997;60:49–54. doi: 10.1016/s0167-5273(97)02952-5. [DOI] [PubMed] [Google Scholar]
- 24.Rassi A., Jr Rassi A. Little WC. Chagas' heart disease. Clin Cardiol. 2000;23:883–889. doi: 10.1002/clc.4960231205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rassi A., Jr Rassi A. Little WC. Xavier SS. Rassi SG. Rassi GG. Hasslocher-Moreno A. Sousa AS. Scanavacca MI. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med. 2006;355:799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 26.Rocha NN. Garcia S. Gimenez LE. Hernandez CC. Senra JF. Lima RS. Cyrino F. Bouskela E. Soares MB. Ribeiro dos Santos R. Campos de Carvalho AC. Characterization of cardiopulmonary function and cardiac muscarinic and adrenergic receptor density adaptation in C57BL/6 mice with chronic Trypanosoma cruzi infection. Parasitology. 2006;133:729–737. doi: 10.1017/S0031182006001193. [DOI] [PubMed] [Google Scholar]
- 27.Rossignol R. Faustin B. Rocher C. Malgat M. Mazat JP. Letellier T. Mitochondrial threshold effects. Biochem J. 2003;370:751–762. doi: 10.1042/BJ20021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossignol R. Malgat M. Mazat JP. Letellier T. Threshold effect and tissue specificity: implication for mitochondrial cytopathies. J Biol Chem. 1999;274:33426–33432. doi: 10.1074/jbc.274.47.33426. [DOI] [PubMed] [Google Scholar]
- 29.Schagger H. Native electrophoresis for isolation of mitochondrial oxidative phosphorylation protein complexes. Methods Enzymol. 1995;260:190–202. doi: 10.1016/0076-6879(95)60137-6. [DOI] [PubMed] [Google Scholar]
- 30.Schapira AH. Mitochondrial involvement in Parkinson's disease, Huntington's disease, hereditary spastic paraplegia and Friedreich's ataxia. Biochim Biophys Acta. 1999;1410:159–170. doi: 10.1016/s0005-2728(98)00164-9. [DOI] [PubMed] [Google Scholar]
- 31.Sharov VG. Goussev A. Lesch M. Goldstein S. Sabbah HN. Abnormal mitochondrial function in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1998;30:1757–1762. doi: 10.1006/jmcc.1998.0739. [DOI] [PubMed] [Google Scholar]
- 32.Sharov VG. Todor AV. Silverman N. Goldstein S. Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:361–367. doi: 10.1006/jmcc.2000.1266. [DOI] [PubMed] [Google Scholar]
- 33.Sheeran FL. Pepe S. Energy deficiency in the failing heart: linking increased reactive oxygen species and disruption of oxidative phosphorylation rate. Biochim Biophys Acta. 2006;1757:543–552. doi: 10.1016/j.bbabio.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Sims NR. Anderson MF. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat Protoc. 2008;3:1228–1239. doi: 10.1038/nprot.2008.105. [DOI] [PubMed] [Google Scholar]
- 35.Slater EC. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teixeira ARL. Nascimento RJ. Sturm N. Evolution and pathology in Chagas disease: a review. Mem Inst Oswaldo Cruz. 2006;101:463–449. doi: 10.1590/s0074-02762006000500001. [DOI] [PubMed] [Google Scholar]
- 37.Towbin JA. Bowles KR. Bowles NE. Etiologies of cardiomyopathy and heart failure. Nat Med. 1999;5:266–267. doi: 10.1038/6474. [DOI] [PubMed] [Google Scholar]
- 38.Uyemura SA. Albuquerque A. Curti C. Energetics of heart mitochondria during acute phase of Trypanosoma cruzi infection in rats. Int J Biochem Cell Biol. 1995;27:1183–1189. doi: 10.1016/1357-2725(95)00073-x. [DOI] [PubMed] [Google Scholar]
- 39.Uyemura SA. Jordani MC. Polizello AC. Curti C. Heart FoF1-ATPase changes during the acute phase of Trypanosoma cruzi infection in rats. Mol Cell Biochem. 1996;165:127–133. doi: 10.1007/BF00229474. [DOI] [PubMed] [Google Scholar]
- 40.Ventura B. Genova ML. Bovina C. Formiggini G. Lenaz G. Control of oxidative phosphorylation by Complex I in rat liver mitochondria: implications for aging. Biochim Biophys Acta. 2002;1553:249–260. doi: 10.1016/s0005-2728(01)00246-8. [DOI] [PubMed] [Google Scholar]
- 41.Vyatkina G. Bhatia V. Gerstner A. Papaconstantinou J. Garg N. Impaired mitochondrial respiratory chain and bioenergetics during chagasic cardiomyopathy development. Biochim Biophys Acta. 2004;1689:162–173. doi: 10.1016/j.bbadis.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Wen J-J. Bhatia V. Popov VL. Garg NJ. Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas disease. Am J Pathol. 2006;169:1953–1964. doi: 10.2353/ajpath.2006.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen J-J. Garg N. Oxidative modifications of mitochondrial respiratory complexes in response to the stress of Trypanosoma cruzi infection. Free Radic Biol Med. 2004;37:2072–2081. doi: 10.1016/j.freeradbiomed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Wen J-J. Vyatkina G. Garg N. Oxidative damage during chagasic cardiomyopathy development: role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic Biol Med. 2004;37:1821–1833. doi: 10.1016/j.freeradbiomed.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Wen J-J. Dhiman M. Whorton EB. Garg NJ. Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microbes Infect. 2008;10:1201–1209. doi: 10.1016/j.micinf.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen J-J. Garg NJ. Mitochondrial generation of reactive oxygen species is enhanced at the Q(o) site of the complex III in the myocardium of Trypanosoma cruzi-infected mice: beneficial effects of an antioxidant. J Bioenerg Biomembr. 2008;40:587–598. doi: 10.1007/s10863-008-9184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. Control of Chagas disease: second report of the WHO expert committee. Geneva, Switzerland: UNDP/World Bank/WHO; 2002. [Google Scholar]
- 48.Wibom R. Lundin A. Hultman E. A sensitive method for measuring ATP-formation in rat muscle mitochondria. Scand J Clin Lab Invest. 1990;50:143–152. doi: 10.1080/00365519009089146. [DOI] [PubMed] [Google Scholar]